Abstract
Background: Inhibitor development to factor VIII (FVIII) hemophilia therapy results in increased complications and substantial economic costs. The SIPPET study, the first randomized controlled trial to compare the immunogenicity of plasma-derived FVIII (pdFVIII)/von Willebrand factor (VWF) and recombinant-DNA-derived FVIII (rFVIII), demonstrated higher inhibitor rates in previously untreated patients (PUPs) treated with rFVIII than in PUPs treated with pdFVIII/VWF.
Objective: To quantify the economic impact of treating PUPs with pdFVIII/VWF vs rFVIII.
Methods: An Excel-based clinical and economic model was developed from a US healthcare payer perspective and run over a 5-year period. The analysis utilized a cohort approach to model patient treatment and outcomes over a monthly cycle to quantify differences in costs of FVIII, bypassing agents, and hospitalizations for serious bleeds. Rates of high-titer inhibitor development were obtained from the SIPPET study. Patients developing high-titer inhibitors were treated with immune tolerance induction (ITI). Patients who developed low-titer inhibitors and those who did not develop inhibitors continued their usual FVIII treatment. Patients who were successfully treated with ITI returned to FVIII treatment, while unsuccessfully treated patients received bypassing agents. Total costs per treated patient were estimated and a one-way sensitivity analysis was conducted to quantify the impact of parameter uncertainty on the model outcomes.
Results: Total cumulative costs per patient over 5 years were $834,621 for pdFVIII/VWF patients and $1,237,163 for rFVIII patients, representing a total saving of $402,542 per patient over the 5-year period, for an average annual saving of $80,508 per patient.
Conclusions: Based on data from the SIPPET study, this analysis found that initiating FVIII treatment in severe hemophilia A PUPs with pdFVIII/VWF has the potential to offer substantial cost savings to healthcare payers, amounting to a one-third reduction in costs.
Introduction
Hemophilia A is a rare, recessive, X-linked genetic disorder characterized by inadequate levels of clotting factor VIII (FVIII) that occurs in approximately one in every 5,000 male births. Patients with severe hemophilia A (FVIII levels less than 1% of pooled adult normal control) are at risk of frequent and potentially serious spontaneous bleeding episodes and require FVIII replacement for treatment of bleeds or prophylaxis.
Previously untreated patients (PUPs) with hemophilia A are at high risk of developing inhibitors, alloantibodies to FVIII therapy. The first 50 exposure-days to FVIII are when the risk of inhibitor development appears to be the highest, although inhibitors can develop over the life of the patient, rendering FVIII treatment ineffective and requiring bypassing agents to treat or prevent bleedsCitation1–3. Development of an inhibitor is the most serious complication of hemophilia A treatment, and can result in increased bleeds, reduced efficacy of treatment, worse patient quality-of-life, greater caregiver burden, and increased risk of patient mortalityCitation4–7. In addition, inhibitors are associated with a substantial economic burden due to the high cost of immune tolerance induction (ITI) (requiring large quantities of FVIII to eradicate the inhibitor) and bypassing agents to treat or prevent bleeds, resulting in costs over 3-times higher than for patients without inhibitorsCitation8–11.
Individual prospective and retrospective observational studies have offered conflicting results regarding the role of FVIII products on the rates of inhibitor development, with some finding no difference in inhibitor rates between plasma-derived and recombinant productsCitation12–14, while others found higher inhibitor rates for recombinant productsCitation15,Citation16. Three large systematic reviews and meta-analyses of observational studies were conducted, with two finding a higher pooled incidence rate of inhibitors in PUPs treated with recombinant factor VIII (rFVIII)Citation17,Citation18, while the other found no differenceCitation19. The Survey of Inhibitors in Plasma-Products Exposed Toddlers (SIPPET) study, the first prospective randomized controlled trial comparing the incidence of inhibitors in PUPs treated with plasma-derived factor VIII (pdFVIII)/von Willebrand factor (VWF) vs those treated with rFVIII, was conducted to address the confounds and limitations of these observational studiesCitation20. As a randomized controlled trial, the results of the SIPPET study provide the most compelling evidence to date that patients treated with rFVIII products are at higher risk of developing an inhibitor than patients treated with pdFVIII.
The objective of the present analysis was to model the potential economic impact of treating PUPs with pdFVIII/VWF vs rFVIII from the perspective of a US managed care health plan, based on the clinical findings from the SIPPET study.
Methods
Model design
An Excel-based model was developed from the perspective of a US managed care payer over a 5-year time horizon. The analysis utilized a cohort approach to model patient treatment and outcomes over a monthly cycle to quantify anti-hemophilic agent utilization and costs, in addition to costs of hospitalizations for serious bleeds.
In the model, patients could initiate prophylactic or on-demand treatment with either rFVIII or pdFVIII/VWF (). Patients from either cohort who developed high-titer inhibitors were assumed to be treated with ITI. Patients who developed low-titer inhibitors and those who did not develop inhibitors continued their FVIII treatment. Patients who were successfully treated with ITI returned to FVIII treatment, while unsuccessfully treated patients received prophylaxis with bypassing agents.
Figure 1. Model structure. Abbreviations. ITI, immune tolerance induction; FVIII, factor VIII; pdFVIII, plasma-derived factor VIII; PUP, previously untreated patient; rFVIII, recombinant factor VIII; VWF, von Willebrand factor.
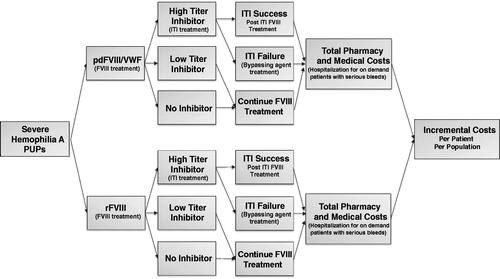
Costs captured in the model included pharmacy costs for FVIII treatment and bypassing agents and hospitalization costs for on-demand patients with serious bleeds. Costs of initial inhibitor testing were not included in the model as they were anticipated to be equivalent in both arms. Although ongoing testing would add additional costs to the recombinant arm, we remained conservative and did not add this to the comparison between groups. A one-way sensitivity analysis was conducted to quantify the impact of parameter uncertainty on the total estimated costs (savings) by varying the model inputs by ±10% of base case values, with the exception of the high-titer inhibitor rates where the range was based on the confidence intervals from the SIPPET study. Additionally, a breakeven analysis was conducted to assess the cost of rFVIII at which treatment with rFVIII would equal the cost of pdFVIII/VWF, and a scenario analysis was conducted to assess the potential impact of switching patients from pdFVIII/VWF to rFVIII after 1 year of treatment (a time at which the risk of inhibitor development has decreased).
Patients
Patients entering the model were assumed to be 1-year-old PUPs at the start of FVIII treatment, reflecting the early age of diagnosis in patients with severe hemophilia A. Annual patient weight for males was obtained from the Centers for Disease Control and Prevention growth charts, and monthly patient weights over the 5-year time horizon were interpolated between yearsCitation21. For the US population-level analysis, the annual number of PUPs with severe hemophilia A who would initiate FVIII treatment was estimated based on population data from the US Census, the crude US birth rate and male/female birth ratios from the National Center for Health Statistics, the number of hemophilia cases per live male births (1:5,000), and the proportion of patients with severe hemophilia A (60%) from the National Hemophilia FoundationCitation22–24.
Factor VIII treatment
To reflect current US real-world treatment practices, it was assumed that 80% of PUPs were treated with FVIII prophylaxis and 20% received on-demand FVIII based on expert opinion. Patients treated with FVIII prophylaxis received three FVIII infusions of 40 IU/kg per weekCitation25,Citation26. It was also assumed that patients receiving FVIII prophylaxis will not have bleeds. Patients treated with on-demand FVIII had an annualized bleed rate (ABR) of 18.0 bleeds, with 10% being serious bleeds (i.e. requiring hospitalization)Citation27,Citation28. On-demand patients with serious bleeds received an average of 1.5 infusions of 50 IU/kg, while on-demand patients with minor/moderate bleeds received an average of 1.0 infusion of 25 IU/kg to stop the bleeds (also based on expert opinion). Inhibitor patients successfully treated with ITI returned to FVIII treatment and were assumed to receive FVIII prophylaxis consisting of three infusions of 40 IU/kg per week25,Citation26.
Inhibitors, immune tolerance induction, and bypassing agents
Rates of inhibitor development were based on the findings from the SIPPET study. SIPPET compared the cumulative incidence of inhibitors in PUPs with severe hemophilia A between pdFVIII/VWF and rFVIII, which resulted in 18.6% of pdFVIII/VWF patients and 28.4% of rFVIII patients developing high-titer inhibitorsCitation20. It was assumed in the model that patients’ inhibitors developed over the initial 6-month treatment period (to approximate roughly 50 exposure-days)Citation29. Patients who developed high-titer inhibitors initiated ITI 3 months after the inhibitor diagnosis, reflecting a common treatment practice to wait for the inhibitor titer to fall prior to initiation of ITI treatment. Half of the patients received low-dose ITI, while the other half received high-dose ITI. Regimens and outcomes for ITI are shown in .
Table 1. Model inputs and assumptions.
Patients who developed high-titer inhibitors also received bypassing agents (rFVIIa or activated prothrombin complex concentrate [aPCC]) either prophylactically to prevent bleeds or on demand to treat bleeds. To reflect clinical practice per expert opinion, it was assumed that, prior to initiating ITI, 50% of patients received prophylaxis with bypassing agents; during ITI, all patients received prophylaxis with bypassing agents; and, after unsuccessful treatment with ITI, all patients received prophylaxis with bypassing agents. Bypassing agent regimens are shown in . Patients whose FVIII inhibitor was eradicated via immune tolerance were subsequently treated with FVIII prophylaxis.
Costs
Unit costs for anti-hemophilic agents (FVIII products and bypassing agents) were obtained from Red Book based on prices as of February 2018 (). Costs of hospitalizations for serious bleeds were estimated based on a mean length of stay of 4.5 days for hemophilia A inpatient staysCitation30, and a mean cost per day of $2,816, based on a cost of $2,554 in 2014Citation30 inflated to December 2017 US dollars using the medical care component of the Bureau of Labor Statistics Consumer Price IndexCitation31, representing a total hospitalization cost of $12,672 (exclusive of anti-hemophilic agent costs).
Results
Per-patient costs
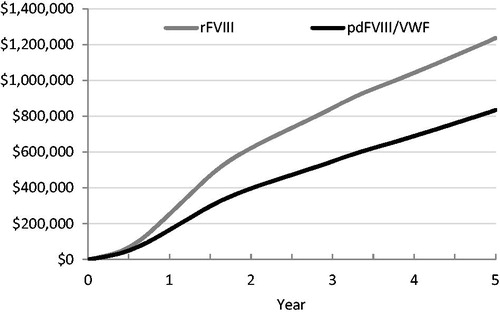
Initiating treatment with pdFVIII/VWF instead of rFVIII in PUPs with severe hemophilia A significantly reduced treatment costs of FVIII replacement. Total cumulative costs per PUP over the 5-year period were $834,621 for pdFVIII/VWF patients and $1,237,163 for rFVIII patients, representing a total saving of $402,542 per patient over the 5-year period, for an average annual saving of $80,508 per patient. This amounted to a savings of over 30%. Total cumulative costs for the 5-year period are shown in .
Figure 2. Cumulative cost per patient. Abbreviations. pdFVIII, plasma-derived factor VIII; rFVIII, recombinant factor VIII; VWF, von Willebrand factor.
The breakdown by cost component over 5 years is shown in . The majority of the cost savings associated with pdFVIII/VWF treatment were due to the reduced rates of inhibitor development seen with pdFVIII/VWF compared to rFVIII. This reduction in inhibitor development resulted in lower utilization of, and therefore costs for, pdFVIII/VWF treatment. This pdFVIII/VWF reduction in inhibitor rates also decreased utilization and costs of bypassing agents to treat bleeds and use of bypassing agent prophylaxis in patients developing inhibitors. In this study, hospitalizations were defined by the presence of serious bleeds. Hospitalization costs for patients with serious bleeds were slightly higher for pdFVIII/VWF patients due to the fact that patients who develop inhibitors were more likely to receive prophylaxis (as opposed to on-demand treatment) and that patients receiving prophylaxis were assumed not to have bleeds, thereby reducing the number of hospitalizations in the rFVIII cohort.
Table 2. Cumulative treatment cost per patient.
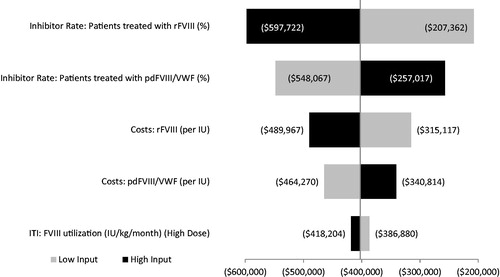
The results of the one-way sensitivity analysis are shown in , showing a minimum cost savings of over $200,000 for patients treated with pdFVIII/VWF. The most impactful parameters include the high-titer inhibitor rates for patients treated with rFVIII, high-titer inhibitor rates for patients treated with pdFVIII/VWF, cost of rFVIII, cost of pdFVIII/VWF, and utilization of high-dose ITI. Other parameters resulted in very small differences in cost savings.
Figure 3. One-way sensitivity analysis (5-year cost difference). Abbreviations. ITI, immune tolerance induction; FVIII, factor VIII; pdFVIII, plasma-derived factor VIII; rFVIII, recombinant factor VIII; VWF, von Willebrand factor. Figures in parentheses reflect cost-savings.
In the breakeven analysis, for costs of patients treated with rFVIII to equal the costs for patients treated with pdFVIII/VWF, the cost of rFVIII would need to be reduced to $0.83 per unit, a cost reduction of 45%. Under a scenario where pdFVIII/VWF patients switch to rFVIII after 1 year, the total costs of treatment over 5 years increases from $834,621 to $921,807; however, this still represents a cost savings of $315,356 compared to treatment with rFVIII.
Population costs
Based on US male birth rates, the number of hemophilia cases per birth, and the proportion of patients with severe hemophilia A as described above, the model estimated that 247 PUPs with severe hemophilia A would initiate FVIII treatment annually in the US. If these patients initiated FVIII treatment with pdFVIII/VWF instead of rFVIII, the cost savings in this cohort of patients would amount to over $99 million over 5 years, an average saving of nearly $20 million per year.
Discussion
Hemophilia A is a rare, costly disease, and the development of an inhibitor is an even costlier complication of treatment, requiring large quantities of FVIII to attempt to eradicate the inhibitor and prevent lifetime use of bypassing agents. Prior studies have compared the costs of patients with and without inhibitorsCitation8–10. Valentino et al.Citation8 conducted a retrospective analysis of patients with hemophilia A in the US, and found median annual costs of $271,357 for patients with inhibitors and $63,935 for patients without inhibitors. Armstrong and MaloneCitation10 conducted a retrospective analysis of US health system costs of patients with hemophilia A with and without inhibitors, and found median annual costs of $325,780 and $98,334, respectively. Guh et al.Citation9 conducted a retrospective analysis of Medicaid-enrolled males with hemophilia and found mean costs of $509,778 (median $55,038) and $83,981 (median $23,697), respectively, for children with and without inhibitors. Avoidance of inhibitors, therefore, has the potential to significantly reduce overall costs of treatment of patients with severe hemophilia A.
Our analysis found that the costs associated with anti-hemophilic agents and costs of hospitalizations to treat bleeds can be reduced by one-third by initiating treatment with pdFVIII/VWF instead of rFVIII. This reduction in costs is due to two primary factors: a lower unit cost for pdFVIII/VWF compared to rFVIII in the US, and a reduction in the rate of patients developing high-titer inhibitors when treated with pdFVIII/VWF (reducing the need for ITI and bypassing agents). With a unit cost of $1.16 for pdFVIII/VWF vs $1.52 for rFVIII (as of February 2018), pdFVIII/VWF alone represents a 24% reduction in FVIII costs. Thus, even if the inhibitor rates were the same between the two product classes, pdFVIII/VWF would still be cost-saving. Similarly, pdFVIII/VWF would also be cost-saving at parity pricing for pdFVIII/VWF and rFVIII, due to the reduced rate of inhibitor development with pdFVIII/VWF. For instance, when our analysis was adjusted to parity pricing, pdFVIII/VWF resulted in a reduction in costs of 18% due to the reduction in inhibitors. Note that this analysis is based on the average savings incurred in a cohort of patients. For example, based on an absolute risk reduction of ∼10% for high-titer inhibitors from the SIPPET study, it follows that, for every 10 patients treated with pdFVIII/VWF instead of rFVIII, on average, one high-titer inhibitor can be expected to be prevented. Actual cost savings in a particular health plan will, therefore, depend on the number of patients treated and the number of inhibitors prevented, which cannot be predicted ex ante. Nevertheless, cost savings will accrue to a health plan utilizing pdFVIII/VWF, due to the lower product unit cost.
Our cost analysis of anti-hemophilic agents for PUPs with severe hemophilia A is based on data from the SIPPET study, a randomized controlled trial assessing the incidence of inhibitors among PUPs treated with pdFVIII/VWF and rFVIII. Prior to the SIPPET study, perspectives on whether or not there were treatment-related differences in the rate of inhibitor development variedCitation32,Citation33. Several observational studies have yielded inconclusive resultsCitation12–19. The SIPPET study was specifically designed to compare the inhibitor risk between FVIII classes among 251 PUPs to address the potential confounds and limitations of prior observational studies. While not without its own limitations, the SIPPET study represents the strongest available evidence to date regarding the increased immunogenicity of rFVIII vs pdFVIII/VWF, with a hazard ratio (HR) of 1.87 (95% confidence interval [CI] = 1.17–2.96) for all inhibitorsCitation20. The HR for high-titer inhibitors was slightly lower than for all inhibitors (HR =1.69, 95 CI =0.96–2.98), and did not quite reach statistical significanceCitation20. However, the SIPPET study investigators, in a follow-up publication, commented that the absence of statistical significance for high-titer inhibitors is likely due to the wider CI and smaller number of high-titer events, and does not likely represent a differential effect of rFVIII vs pdFVIII/VWF on all inhibitors but not on high-titer inhibitorsCitation34. The authors write (page 356):
Since there is no likely mechanism by which a particular product would increase the risk of all inhibitors but not of the subgroup of high-titre inhibitors, and given the consistency of effect estimates for all and high-titre inhibitors, we feel confident to conclude that rFVIII is associated with an increased rate of high-titre inhibitorsCitation34.
Accordingly, the high-titer inhibitor rate was used in the model, given the clinical and economic significance of managing patients with high-titer inhibitors. Finally, the overall findings of the SIPPET study are consistent with the bulk of prior observational research, and, together with a recent large cohort studyCitation35, provide compelling evidence for a higher inhibitor rate among patients treated with rFVIIICitation34.
Our study represents the first published study to assess the potential economic impact of the findings of the SIPPET study in the US. A prior cost analysis compared rFVIII and pdFVIII in Germany and Austria. That analysis also concluded that, due to the high cost of patients developing high-titer inhibitors, pdFVIII would be cost-saving compared to rFVIIICitation36. The SIPPET investigators reported a budget impact analysis that also assessed costs in previously untreated patients with severe hemophilia A receiving rFVIII or pdFVIII/VWF. That study utilized a Markov model, and the base-case analysis showed a €202,047 cost difference over 15 years per patient, favoring pdFVIIICitation37.
Some clinicians have indicated that they may start a PUP on a pdFVIII product and then switch to an rFVIII product after 50 exposure-days. The assumption is that, by using a plasma-derived product for the first 50 exposure-days, one can likely get through the high-risk period for inhibitor development. While the development of inhibitors in the SIPPET study occurred within the first 50 exposure-days, inhibitors can develop in any patient at any timeCitation2,Citation3. In addition, there is no evidence that switching after 50 exposure-days will indeed protect the patient from developing an inhibitorCitation38. From a US cost perspective, as our analysis shows, initiating treatment with pdFVIII/VWF and then switching to rFVIII is more costly than leaving the patient on pdFVIII due to the higher acquisition cost of rFVIII in the US, but is still less costly than treatment with rFVIII. However, there may be cost savings associated with switching in countries where rFVIII is priced lower than pdFVIII.
Opportunities for cost savings in the US are substantial, as the majority of US hemophilia A patients are currently treated with rFVIII. This is a consequence of the significant historical risk of viral transmission of HIV and hepatitis infection from plasma-derived blood products. In the late 1970s and early 1980s, an estimated 70% of hemophilia patients were infected with HIV, and many died of AIDSCitation39. The resulting introduction of rFVIII products in the early 1990s essentially eliminated this risk since recombinant products are not plasma-derived. In addition, this medical catastrophe led to numerous safety improvements in screening and viral-inactivation methods among plasma-derived products, and, since their introduction over 25 years ago, there have been no documented cases of blood-borne transmission of hepatitis or HIVCitation39. Nonetheless, rates of inhibitors over the past two decades have increased. Clinicians and patients must, therefore, weigh the extremely small risk of viral transmission with plasma-derived products against the much larger risk of inhibitor development associated with recombinant products when making treatment decisions.
Limitations
This analysis was conducted from a US healthcare payer perspective. Accordingly, the results of the analysis are affected by the relative costs of pdFVIII and rFVIII in the US and the treatment strategies commonly utilized in the US. The results of this study, therefore, may not generalize to other countries with different cost structures and different treatment practices. Additional studies comparing the costs of treating severe hemophilia A patients with pdFVIII and rFVIII are warranted.
This analysis does not include the potentially high indirect costs of severe hemophilia A, such as caregiver burden, missed school or work, and reduced quality-of-life. The analysis does not include costs associated with surgeries (e.g. total knee replacement) and the costs of FVIII for surgical interventions. To the extent that these costs will be magnified in patients with inhibitors, the model, as designed, reflects a conservative perspective on the potential cost differences between rFVIII and pdFVIII. Finally, treatment costs of patients with hemophilia A vary significantly from patient to patient; the cost estimates in this analysis reflect average costs in a cohort of patients and may, therefore, not necessarily reflect the costs incurred by any particular patient in a managed care health plan, particularly in light of the small numbers of patients with severe hemophilia A initiating treatment in a health plan in a given year.
The modeling presented here cannot yet take into account the introduction of non-factor products such as emicizumab for inhibitor patientsCitation40. The long-term effect of emicizumab on treatment paradigms in hemophilia patients with or without inhibitors cannot yet be predicted, but the costs are expected to be high, with estimates of $482,000 for the first year of treatmentCitation41.
Conclusions
Based on data from the SIPPET study, the first randomized controlled trial to compare the immunogenicity of pdFVIII/VWF and rFVIII, this analysis found that initiating FVIII treatment in PUPs with severe hemophilia A with pdFVIII/VWF has the potential to offer substantial cost savings to US healthcare payers, amounting to a one-third reduction in costs. Further research is warranted to corroborate these findings in real-world analyses and to quantify the potential wider direct and indirect clinical and economic benefits of preventing inhibitors in patients with severe hemophilia A.
Transparency
Declaration of funding
This research was supported with funding by Grifols.
Declaration of Financial/other relationships
RFS received honoraria for participation in advisory boards from Shire, Genentech, Uniqure, Grifols, Biomarin, CSL Behring, Pfizer, and Bayer. RFS has investigator initiated grants from Shire, Bioverativ, Grifols, and Kedrion. MCR and JS are employees of Grifols, which provided funding for this research. KO and KM are employees of Xcenda, a consulting company that received funding from Grifols to complete this study. EJN has served on advisory boards for CSL-Behring, Grifols, Octapharma, Novo Nordisk, and Shire, and received research funding from Octapharma. He also serves on a DSMB for Bayer. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
An abstract of this manuscript was presented at the 2017 American Society of Hematology’s annual meeting.
Acknowledgments
The authors would like to acknowledge Kylie Matthews for assistance with the final preparation of the manuscript.
References
- DiMichele DM. Inhibitors in Hemophilia: a primer. Hemophilia 2008;7:1-9
- Soucie JM, Miller CH, Kelly FM, et al. A study of prospective surveillance for inhibitors among persons with haemophilia in the United States. Haemophilia. 2014;20:230-7
- Hay CRM, Palmer B, Chalmers E, et al. Incidence of factor VIII inhibitors throughout life in severe hemophilia A in the United Kingdom. Blood 2011;117:6367-70
- Leissinger CA. Prevention of bleeds in hemophilia patients with inhibitors: emerging data and clinical direction. Am J Hematol 2004;77:187-93
- Brown T, Lee W, Joshi A, et al. Health-related quality of life and productivity impact in hemophilia patients with inhibitors. Hemophilia 2009;15:911-17
- DeKoven M, Karkare S, Lee WC, et al. Impact of hemophilia with inhibitors on caregiver burden in the United States. Hemophilia 2014;20:822-30
- Walsh CE, Sourcie JM, Miller CH. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol 2015;90:400-5
- Valentino LA, Pipe SW, Tarantino MD, et al. Healthcare resource utilization among hemophilia A patients in the United States. Hemophilia 2012;18:332-8
- Guh S, Grosse SD, McAlister S, et al. Health care expenditures for Medicaid-covered males with hemophilia in the United States, 2008. Hemophilia 2012;18:276-83
- Armstrong EP, Malone DC. Costs and utilization of hemophilia A and B patients with and without inhibitors. J Med Econ 2014;17:798-802
- Chen SL. Economic costs of hemophilia and the impact of prophylactic treatment on patient management. Am J Manag Care 2016;22:S126-S33
- Kreuz W, Ettingshausen CE, Zyschka A, et al. Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Semin Thromb Hemost 2002;28:285-90
- Gouw SC, van der Bom JG, Auerswald G, et al. Recombinant versus plasma-derived factor VIII products and the development of inhibitors in previously untreated patients with severe hemophilia A: the CANAL cohort study. Blood 2007;109:4693-7
- Gouw SC, van der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med 2013;368:231-9
- Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia 2003;9:418-35
- Goudemand J, Rothschild C, Demiguel V, et al. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood 2006;107:46-51
- Marcucci M, Mancuso ME, Santagostino E, et al. Type and intensity of FVIII exposure on inhibitor development in PUPs with haemophilia A: a patient-level meta-analysis. Thromb Haemost 2015;113:958-67
- Iorio A, Halimeh S, Holzhauer S, et al. Rate of inhibitor development in previously untreated hemophilia A patients treated with plasma-derived or recombinant factor VIII concentrates: a systematic review. J Thromb Haemost 2010;8:1256-65
- Franchini M, Coppola A, Rocino A, et al. Systematic review of the role of FVIII concentrates in inhibitor development in previously untreated patients with severe hemophilia a: a 2013 update. Semin Thromb Hemost 2013;39:752-66
- Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med 2016;374:2054-64
- Centers for Disease Control and Prevention, National Center for Health Statistics. Growth charts – data table of weight-for-age charts. 2001. http://www.cdc.gov/growthcharts/html_charts/wtage.htm [Accessed December 2016]
- U.S. Census Bureau, Population Division. Annual Estimates of the Resident Population: April 1, 2010 to July 1, 2015. For the United States, regions, divisions, states, and Puerto Rico Commonwealth, December 2015. https://factfinder.census.gov, [Accessed December 2016]
- Hamilton BE, Martin JA, Osterman MJK, et al. Births: final data for 2014. National vital statistics reports. Hyattsville, MD: National Center for Health Statistics; 2015:64
- National Hemophilia Foundation. Fast facts. 2016. https://www.hemophilia.org/About-Us/Fast-Facts [Accessed December 2016]
- Alphanate [package insert]. Los Angeles, CA: Grifols Biologicals Inc; March 2015
- Advate [package insert]. Westlake Village, CA: Baxter Healthcare Corporation; May 2015
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007;357:535-44
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Hemophilia 2013;19:e1-e47
- Peyvandi F, Cannavo A, Garagiola I, et al. Timing and severity of inhibitor development in recombinant versus plasma-derived factor VIII concentrates: a SIPPET analysis. J Thromb Haemost 2018;16:39-43
- Agency for Healthcare Research and Quality. 2014. Healthcare cost and utilization project. http://hcupnet.ahrq.gov/HCUPnet [Accessed December 2016]
- U.S. Bureau of Labor Statistics. Consumer price index. 2018. http://data.bls.gov/cgi-bin/surveymost?cu [Accessed February 2018]
- Aledort LM. Is the incidence and prevalence of inhibitors greater with recombinant products? Yes. J Thromb Haemost 2004;2:861-2
- Lusher JM. Is the incidence and prevalence of inhibitors greater with recombinant products? No. J Thromb Haemost 2004;2:863-5
- Peyvandi F, Mannucci PM, Palla R, et al. SIPPET: methodology, analysis and generalizability. Haemophilia 2017;3:353-61
- Calvez T, Chambost H, d’Oiron R, et al. Analyses of the FranceCoag cohort support immunogenicity differences among one plasma-derived and two recombinant factor VIII brands in boys with severe hemophilia A. Haematologica 2018;103:179-89
- Wali B, Halimeh S, Male C. Economic considerations on the use of recombinant vs plasmatic factor VIII in the treatment of previously untreated hemophilia A patients. Presented at the ISPOR 19th Annual Conference; October 29–November 2, 2016; Vienna Austria
- Messori A, Peyvandi F, Trippoli S, et al. High-titre inhibitors in previously untreated patients with severe haemophilia A receiving recombinant or plasma-derived factor VIII: a budget-impact analysis. Blood Transfus 2018;16:215-220
- Warren B, Wang M, Young G. Recombinant factor VIII concentrates associated with increased risk of inhibitor development in severe hemophilia a: perspectives on the results of the SIPPET trial. https://www.aap.org/en-us/Documents/SIPPET_Summary.pdf
- Gringeri A. Factor VIII safety: plasma-derived versus recombinant products. Blood Transfus 2011;9:366-70
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med 2017;377:809-18
- Institute for Clinical and Economic Review. Emicizumab for hemophilia A: effectiveness and value. Draft evidence report. Boston, MA. January 26, 2018. https://icer-review.org/wp-content/uploads/2017/08/ICER_Hemophilia_A_Draft_Report_012618.pdf
- Hay CRM, DiMichele DM. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012;119:1334-44
- Konkle BA, Ebbesen LS, Erhardtsen E, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost 2007;5:1904-13
- Leissinger C, Gringeri A, Antmen B, et al. Anti-inhibitor coagulant complex prophylaxis in hemophilia with Inhibitors. N Engl J Med 2011;365:1684-92
- 45. NovoSeven RT [package insert]. Plainsboro, NJ: Novo Nordisk Inc; May 2016
- Feiba [package insert]. Westlake Village, CA: Baxter Healthcare Corporation; November 2013
- IBM Corporation. IBM MicroMedex® 2018. http://www.micromedexsolutions.com/home/dispatch
