Abstract
Background: Autonomic nervous system (ANS) testing with heart rate variability (HRV) has been shown in early research to predict 52-week outcomes in rheumatoid arthritis (RA). HRV testing could be combined with putative ANS biologic pathways to improve treatment response for RA patients. This study explored potential costs and health outcomes of introducing HRV testing into RA treatment, without and with ANS optimization.
Methods: A decision tree exploratory economic model compared HRV testing to standard care in moderate-to-severe biologic-eligible patients over a 10-year time horizon. HRV data was derived from an observational study of RA patients (n = 33). Patients were stratified into treatment groups based on HRV test scores indicating “low probability of response” and “moderate to high probability of response”. This study explored adding ANS optimization based on HRV score followed by clinically-appropriate treatment. Costs and quality-adjusted life-years (QALYs) for the US population were estimated.
Results: HRV testing in biologic-eligible patients decreased non-effective biologic use, reducing US healthcare costs by $34.6 billion over 10 years with QALYs unchanged. When combined with ANS optimization in biologic-eligible patients, HRV testing could increase costs by $3.6 billion over 10 years but save over 350,000 QALYs. Among all RA patients, HRV testing with ANS optimization could save over $8 billion and over 100,000 QALYs over 10 years, depending on the positive predictive value (PPV) of the HRV test.
Conclusions: The potential economic impact of introducing HRV testing and ANS optimization into RA treatment appears substantial and cost-effective based on the exploratory analysis. Additional rigorous studies are warranted in larger patient samples to better inform decision-making.
Background
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting up to 1.8 million people in the US, with annual healthcare costs to payers estimated at $8.4 billionCitation1,Citation2. RA causes stiffness and joint swelling, can lead to permanent joint damage and deformity, and can significantly impact social and physical function among afflicted individualsCitation3,Citation4. RA is commonly treated using conventional disease modifying anti-rheumatic drugs (cDMARDs). However, most patients do not have a sufficient reduction in symptoms with cDMARDs alone. Biologic treatments, used in combination with a cDMARD, can greatly improve quality-of-life and survival for some patientsCitation5. Unfortunately, biologics are not effective for everyoneCitation6. Additionally, biologics are associated with a high risk of infection, some intolerabilty, and much greater cost than cDMARDsCitation7.
Heart rate variability (HRV) analysis has emerged in recent years as a practical, reproducible, and non-invasive method to detect early cardiovascular autonomic impairment by assessing the effects of the sympatho-vagal balance on the heartCitation8. Reduced HRV is an expression of an increase in sympathetic nervous system and a reduction in parasympathetic nervous system activity, being associated with a high risk of cardiovascular events, ventricular arrhythmias, and sudden cardiac death in the general populationCitation9–11. Several studies and reviews have discussed connections between the autonomic nervous system (ANS) and autoimmunity associated with RACitation12–19. Early research has shown that testing HRV, through a standardized clinical test, can predict response to biologic treatment for RA with high predictive accuracyCitation20. In a double-blind proof-of-concept study, higher parasympathetic HRV scores were strongly correlated with greater improvement in RA disease controlCitation20. This small observational trial, conducted in 2008 (n = 33), found that a patient’s ANS state, measured by 5-min, frequency domain HRV, holds potential to predict 52-week anti-tumor necrosis factor (TNF) American College of Rheumatology (ACR) 70Citation20,Citation21 outcome for patients with RACitation21. ACR70 is a commonly used measure of sufficient disease control. Larger studies are needed to better understand the value of HRV testing for RA; however, preliminary study outcomes suggest that HRV testing could be used to improve clinical treatment decisions based on a patient’s predicted likelihood of response to biologics.
Three putative models of complementary ANS optimization (vagal nerve stimulationCitation22, obstructive sleep apneaCitation23, and restless legs [RLS] treatmentsCitation24) have established plausibility for significantly enhancing immunosuppressive disease suppression in RA. While augmenting immunosuppressive treatment with medications used to treat RLS and periodic leg movement (PML) is novel, RLS and PML have been postulated as hyper-sympathetic dysautonomic statesCitation25. As such, PML and RLS may be potential cardiovascular risk factors at nightCitation26 and during wakefulnessCitation27. Given the correlations observed between parasympathetic HRV scores and disease control, we expect that patients with low HRV may have a lower likelihood of responding to biologicsCitation20. However, for these patients, it may be feasible to optimize the ANS, which may improve response to treatment with biologics and increase the likelihood of achieving disease remissionCitation20.
Randomized clinical trials are essential to further determining the clinical and biological plausibility of use of HRV testing, but would require a large commitment of time and financial resources. Prior to investing limited health resources into this research, we developed an economic model to better understand the potential value of HRV testing for RA. Through this exploratory analysis, we aimed to assess the costs and health outcomes of introducing HRV testing prior to development of a treatment plan for RA compared to the standard care without HRV testing, and project these results to the US RA population. Additionally, we explored the potential economic effects of hypothetical scenarios of implementing HRV testing combined with ANS optimization.
Methods
Base case analysis of HRV testing
In the base case, we used a decision tree model from a US payer perspective to compare HRV testing to standard care over a 10-year time horizon. A payer perspective was selected because payers will be the primary decision-makers for HRV testing reimbursement and will bear much of the economic burden. Similarly, a 10-year time horizon was selected for its relevance to payer decision-making, as uncertainty around availability and price of treatments is high in longer time horizons. Additionally, our model was based on observational data from a small sample size with limited follow-up time. Therefore, projecting outcomes to a longer time-horizon would have significant limitations.
Characteristics of patients included in the model were based on current knowledge of RA populations in the US. Eligible patients for HRV patients are expected to be adults with moderate-to-severe RA who have not reached disease control using standard treatment with cDMARDs alone. We defined disease control by ACR70. Among 321.4 million people in the US population, we assumed an RA prevalence of 0.6%, of whom 50% are moderate-to-severe casesCitation28. Of the moderate-to-severe sub-set, we assumed that 50% of cases were not sufficiently controlled on cDMARDsCitation28. Mean age was 55, and 79% were femaleCitation29.
In the standard care arm, patients not sufficiently controlled with cDMARDs were offered biologics to reflect current practice (). In practice, it is estimated that ∼50% of RA patients are eligible for biologics, yet a slightly smaller proportion (48%) are actually treated with biologics, since some patients reject biologic treatment for various reasons, including potential adverse events and side-effectsCitation30. We applied these same proportions to the biologic-eligible population in our model, leading to the model assumption that 96% of patients eligible for biologics would actually take them. For patients who received biologics, serious infection was considered a potential adverse event with a 1.8% probability, based on published experience. Adverse events associated with biologics are expected to cost $13,747 for treatment and decrease a patient’s health-related quality-of-life by 0.156 for 1 monthCitation28.
Figure 1. Decision tree of RA patient journey with current standard care (A), and with HRV testing for biologic-eligible patients (B). *Disease control defined as ACR70. References: (a) Ollendorf et al.Citation28, (b) Kim et al.Citation30, (c) Holman and NgCitation21.
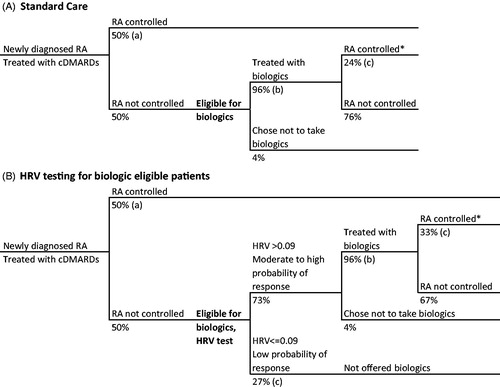
In the HRV testing arm, diagnostic and subsequent treatment patterns were assumed to follow the correlation patterns and HRV prediction outcomes observed in the 2008 pilot studyCitation21. In this group, patients not sufficiently controlled with cDMARDS were stratified into two categories based on HRV score: “low probability of response” (parasympathetic HRV ≤0.09; 27% of patients) and “moderate to high probability of response” (parasympathetic HRV >0.09; 73% of patients), with a positive predictive value (PPV) of 33% and a negative predictive value (NPV) of 100% ()Citation21. Patients in the “moderate to high” group were offered biologics, and those who took a biologic were assumed to have a 33% probability of reaching disease control based on preliminary study outcomesCitation21. Those in the “low” group were not treated with biologics, since a low HRV-test outcome suggests that biologic treatment would likely be ineffective in these individuals. While this is unlikely to occur in clinical practice and is not currently standard care, the base case model was developed to simulate a potential clinical decision-making environment that relied on HRV-testing to determine a patient’s treatment plan.
For patients in both arms, we assumed that all relevant responses to testing and treatment occurred within the first 6 months. Mortality was expected to occur throughout the time horizon of the model based on all-cause and RA-specific mortality rates as identified through US life tables and mortality ()Citation36. Finally, we calculated results for an individual patient over 10 years and projected those results to the existing US moderate-to-severe RA population.
Table 1. Costs data inputs and sources.
Given the limitation of our base case assumption that patients in the “low” predicted HRV group would not be offered biologic treatment, we conducted a secondary analysis that modeled a scenario involving ANS optimization, described below, in which these patients were all offered biologics plus ANS optimization.
Finally, we conducted an exploratory scenario analysis that used the results of the base case decision tree to assess the potential costs and health outcomes associated with a hypothetical scenario in which HRV testing was used in combination with ANS optimization (RLS methodCitation24). In this scenario, we identified patients with low predicted response to biologics based on HRV score. Rather than no treatment, these patients were modeled to receive RLS medications that have been shown to improve the receptivity of biologic treatment by modifying a patient’s ANS (“HRV testing + ANS optimization”)Citation24. We compared HRV testing + ANS optimization () to standard care () for moderate-to-severe RA patients eligible for biologics in the US. In the HRV testing + ANS optimization arm, patients were stratified into two categories: “low probability of response” (parasympathetic HRV ≤0.19, 76% of patients) and “moderate to high probability of response” (parasympathetic HRV >0.19, 24% of patients) with PPV = 0.63 and NPV = 0.88 ()Citation21. A higher parasympathetic threshold is used here than in the base case, because it would be a more conservative treatment plan to maximize the number of patients getting ANS optimization; in the base case described above, it would be more conservative and clinically appropriate to minimize patients in the “low” response group receiving no treatment. The higher parasympathetic threshold leads to a higher PPV but lower NPV compared to the base case decision tree.
Figure 2. RA patient journey with HRV testing and ANS optimization for biologic-eligible patients. *Disease control defined as ACR70. References: (a) Ollendorf et al.Citation28, (b) Holman and NgCitation21, (c) Kim et al.Citation30, (d) Holman and NgCitation20,Citation24.
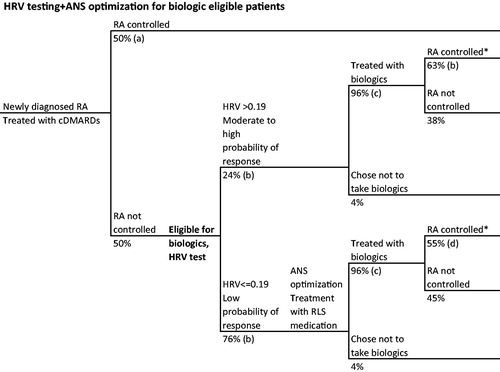
In this scenario, patients in the “moderate to high” response group were offered biologics to reflect current practice. Those in the “low” response group received ANS optimization with RLS medication followed by biologics. After ANS optimization, the probability of reaching disease control was estimated at 55% when taking a biologic, calculated as the proportion of patients achieving disease control after RLS therapy (79%)Citation24 minus the expected proportion of patients achieving disease control without HRV testing or subsequent ANS optimization (24%)Citation20.
Hypothetical scenario
Finally, we conducted an entirely hypothetical scenario analysis in which we adapted the HRV testing + ANS optimization model to compare HRV testing + ANS optimization () to standard care for all severity levels of RA patients, including those newly-diagnosed, in the US. For this scenario, those in the HRV testing + ANS optimization arm were stratified into treatment response groups as above, with a cut point of parasympathetic HRV of 0.19. Patients in the “moderate to high” group were treated with cDMARDs, then biologics, as relevant. Because a study of test performance has not yet been conducted in all newly-diagnosed patients, we do not know the potential test performance characteristics for this population. Therefore, we conducted the analysis over a range of PPV estimates from 40–60% to estimate a range of potential cost and health outcomes for providing HRV testing + ANS optimization to all RA patients in the US.
Figure 3. Hypothetical RA patient journey with HRV tasting and ANS optimization for all patients. *Disease control defined as ACR70. References: (a) Holman and NgCitation21, (b) Holman and NgCitation20,Citation24, (c) Kim et al.Citation30.
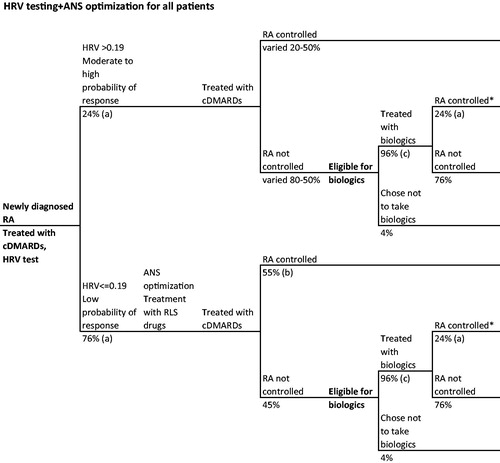
Those in the “low” group received ANS optimization followed by cDMARDS, and then biologics, as relevant. Data on treatment response to RLS therapy combined with biologics is not available. In lieu of appropriate evidence, we assumed that the response to combining RLS therapy with biologics could reasonably be applied to RLS therapy with cDMARDs, yielding a 55% probability of reaching disease control ().
Data inputs
Relevant cost and health outcomes data inputs are listed in . HRV testing occurred quarterly. Costs and health outcomes were discounted at 3% annuallyCitation37.
Total and component costs and quality-adjusted life-years (QALYs) per patient and for the US population were estimated. Cost-effectiveness was defined as an incremental cost-effectiveness ratio (ICER) below $150,000/QALYCitation37.
Sensitivity analyses
We ran one-way sensitivity analyses to identify the key drivers of model outcomes, using plausible ranges for each input derived from 95% confidence intervals from data sources. Probabilistic sensitivity analyses were also performed by jointly varying all model parameters over 5,000 simulations to evaluate the joint model uncertainty. We used normal distributions for population, age, and costs, and beta distributions for utilities and probabilities.
Results
The base case analysis estimated that, compared to standard care, conducting HRV testing to inform the RA treatment plan would save $71,800 for a patient with moderate-to-severe RA over 10 years, with no change in health outcomes (). Although there would be an additional cost of $3,700 for the HRV testing itself, these costs were offset by a projected $75,500 saved from reduction in inefficient use of biologics. When extrapolated to the US population, HRV testing was projected to save $34.6 billion over 10 years compared to standard care, with QALYs unchanged.
Table 2. Results for HRV testing compared to standard care for patients with moderate-to-severe RA eligible for biologic treatment.
Providing HRV testing + ANS optimization to patients eligible for biologics in the US was projected to cost $3.6 billion compared to standard care over 10 years (). This cost increase would be driven by ∼ $2.4 billion in costs for HRV testing itself and $700 million for RLS medication for ANS optimization. We estimate that HRV testing + ANS optimization in this scenario would lead to an addition of 378,000 QALYs over 10 years, resulting in an ICER of $9,600/QALY. This would be considered a highly cost-effective intervention based on our a priori definition of a $150,000/QALY willingness-to-pay threshold.
Table 3. Results for HRV testing + ANS optimization compared to standard care for patients with moderate-to-severe RA in the US.
Providing HRV testing + ANS optimization for all RA patients in the US was projected to cost 3.4 billion when a 40% PPV was assumed and to save $8.8 billion when a 60% PPV was assumed (). Although there would be increased costs for HRV testing itself ($5 billion) and RLS medication for ANS optimization ($1.4 billion), we estimate that $3.0–$15.2 billion could be saved by reducing inefficient use of biologics. In addition, this strategy was projected to save 23,000–115,000 QALYs. This would lead to an ICER of $150,700/QALY at a PPV of 40%, and be a cost-saving intervention at a PPV of 50% or 60%.
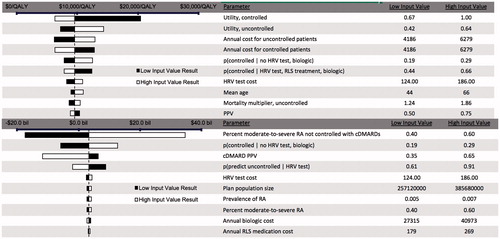
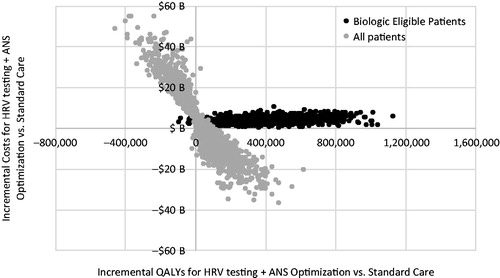
We found that ICER for HRV testing + ANS optimization compared to standard care was most sensitive to the underlying utility values, as well as the annual underlying costs of care for RA patients (). The total population incremental costs were most sensitive to the percentage of patients not sufficiently controlled with cDMARDs, followed by the probability of being controlled without HRV testing, and the PPV of HRV testing for cDMARDs. In the probabilistic analysis (), a wide range of results for incremental QALYs was observed. For biologic eligible patients, HRV testing + ANS optimization had a 99.8% probability of being cost-effective at a threshold of $150,000/QALY. For the total population, HRV testing + ANS optimization had a 41% probability of being cost-saving and increasing QALYs, but also a 40% probability of being cost increasing and decreasing QALYs. This finding depends primarily on the percentage of the population estimated to be controlled with cDMARDs, or population size, which is inversely proportional with the incremental results for costs and QALYs.
Discussion
In this exploratory analysis, we estimate that HRV testing could save $34.6 billion in healthcare costs in the US over 10 years, with no change in health outcomes among patients with moderate-to-severe RA. If ANS optimization were implemented in combination with HRV testing, we estimate that costs for moderate-to severe RA patients in the US would increase by $3.6 billion over 10 years, but health outcomes would improve with over 378,000 QALYs gained. If the model assumptions hold, our findings suggest that conducting HRV testing with RA patients prior to initiating a treatment strategy would be a highly cost-effective intervention, with an ICER of only $9,500/QALY.
When HRV testing + ANS optimization was modeled to be expanded to all diagnosed RA patients in the US, we estimated that cost could range from $3.8 billion to saving 8.8 billion, depending on PPV, while at the same time gaining over 22,000 QALYs. As ANS optimization in RA is a novel area of research, additional studies will be required to confirm how and to what extent ANS therapeutic approaches—whether vagal nerve stimulation, continuous positive airway pressure for co-morbid obstructive sleep apnea (OSA), RLS options, or those yet to be discovered—may enhance immunosuppressive therapeutic outcomes and health economic impact in autoimmune disease.
Recently, biosimilar medications for biologics in the RA space have entered the drug approval process. These products are typically priced lower than the existing drugs. If biosimilars replace existing drugs, the quantitative outcomes of our analysis would change. Lower-priced biosimilars would lead to fewer cost savings when biologic use was avoided due to HRV testing or ANS optimization. We expect that biosimilars would be discounted up to 20–25% from existing biologic prices. If that occurred, we would expect any cost savings from biologics in the above results to be 20–25% lowerCitation38.
Our study had several limitations. First, we based this analysis on data from a small, prospective, double-blind, observational trial (n = 33), which poses challenges for reliability of our estimates and the expected uncertainty around our base case estimates. This trial did include patients with psoriatic arthritis, as well as RA, and combined the results given the significant overlap between these autoimmune, inflammatory diseases with respect to joint activity, and outcomes did not vary significantly with respect to specific disease. In addition, the trial did not stratify treatment by HRV status: therefore, our modeled treatment stratification was hypothetical using assumptions based on opinions of clinical experts and current practice. Furthermore, scenario analyses were based on evaluating a hypothetical future state in which HRV testing and ANS optimization were integrated into treatment plans and available to all RA patients across the US. Since both initiatives are still in the proof-of-concept stage, all parameters for these analyses were extrapolated from current data and based on assumptions. Additionally, we treated biologics as a group, rather than modeling individual therapies. In the future, it may be possible to predict the most beneficial biologic for each patient, but, in the absence of this capability, we used the simplification of modeling the group as a whole. We have generalized results for anti-TNF biologics to other classes; therefore, results should be interpreted cautiously, as further work is completed comparing classes. Finally, our model was a simplified and aggregate representation of a complex disease and treatment pattern. As such, the results presented here should be considered cautiously.
To address these limitations, additional studies are warranted in larger patient samples, as well as investigation into non-biologic therapeutic applications. Importantly, rigorous clinical trials are needed to validate the potential biological plausibility and assumptions of clinical effectiveness and safety. To improve the underlying data informing our economic model, larger non-observational studies should be conducted that follow treatment long-term response, biologic uptake, and health outcomes. Potential variation in treatment response rates, HRV testing accuracy, and health and cost outcomes should be studied among patients when stratified by HRV status, disease severity, treatment history, and other clinically-relevant biomarkers. Additionally, clinical practice implications should be evaluated to identify potential feasibility and any barriers to implementation.
The understanding of specific interactions between the sympathetic and parasympathetic components of the ANS and their effects on immune function, i.e. immuno-autonomics, in infection, autoimmunity, and cancer are often conflicting and evolvingCitation39. Depending on whether sympathetic activity is acute or chronic significantly affects its immunological impact. Maturation of various elements of the immune system affect adrenoreceptors sub-populations, often yielding disparate effects. The most compelling evidence for immuno-autonomics relates to parasympathetic components and, specifically, the cholinergic anti-inflammatory reflex reported by TraceyCitation40. As a natural reflex to reduce inflammation, vagal efferents are thought to reduce pro-inflammatory effects in autoimmune rodentCitation41,Citation42 and humanCitation22 models. Evidence that ANS state drives inflammation is further supported by reports of increased sympathetic profile preceding new onset of inflammatory disease in humansCitation43 and that vagal nerve stimulation reduces rheumatoid arthritis severity, inflammatory cellular pathology, and pro-inflammatory cytokine production, including tumor necrosis factor (TNFα), IL-1, and IL-6Citation22. Restoring sympatho-vagal balance has been proposed with increasing frequencyCitation44–47 to reduce inflammatory joint disease activity and its consequent morbidity.
Many authors have reported increased risk of cardiovascular morbidity and mortality in RACitation48. Assumptions focus on how inflammation prominent in autoimmune disease could spread to invoke cardiovascular consequences. Effective RA control with biologic therapy has been associated with reduced cardiovascular outcomes. However, it has been hypothesized that effective biologic outcome in RA was also expected to correlate with favorable ANS stateCitation49. Additionally, a 53% prevalence of OSA was reported among Japanese patients (85% women) with RACitation23. Effective OSA treatment with continuous positive airway pressure over 5 months resulted in a mean 35% reduction in RA joint activity and C-reactive protein. Of note, cardiovascular studies in patients with RA have failed to note unexpected OSA as a risk factor for increased cardiovascular morbidityCitation50, despite OSA being equivalent to cigarette smoking as a cardiovascular risk factor according to the Sleep Health Heart StudyCitation51. Consequently, while assessing and addressing ANS state may also be beneficial to potentially reduce adverse cardiovascular outcomes, exactly how remains a topic requiring future scrutiny.
Despite the hypothetical and exploratory nature of our analysis, our findings signal important consideration of the directional impact HRV testing could have on RA-related health outcomes and expenditures. Although the economics of HRV testing for RA have been little studied prior to the results presented here, our results for the economics of RA overall are in line with previous estimatesCitation2,Citation32,Citation34,Citation35,Citation52–54. HRV testing, either alone or in combination with ANS optimization, is likely to be a cost-effective or even cost-saving intervention. Use of these techniques in the US RA population could save on the order of $10 billion or more in healthcare costs over 10 years, with no adverse impact on health outcomes. Even in the absence of potential cost-savings, the health benefits for RA patients in the US could be significant, on the order of tens of thousands of QALYs gained over 10 years. Given this potentially positive impact on health and economic outcomes, pursuing further clinical research on HRV testing for RA patients is likely to be a good investment for public health. Given the hypothetical nature of our study, uncertainty in the results is significant. For this reason, we intend these results to be used for hypothesis generation and justification for the direction of future research, rather than a direct quantification of economic impact.
The potential US health economic impact of introducing HRV testing and ANS optimization into RA treatment appears substantial and is possibly cost-effective or cost-saving. Early data shows that the ANS can profoundly affect RA expression, and ANS optimization strategies are beginning to reveal new options for significantly improving patient health outcomes. Although limited by preliminary data on effects of HRV testing and RLS medications on RA activity, this modeling analysis suggests that HRV testing and ANS optimization may also create substantial value for US healthcare payers and patients. HRV testing and ANS optimization may become an important opportunity to improve RA treatment outcomes, while partially mitigating the unsustainable biologic therapeutic costs of patient care.
Transparency
Declaration of funding
This study was funded by Inmedix, Inc.
Declaration of financial/other relationships
MZ, EV, and LPG are consultants for Global Health, LLC, which received fees from Inmedix, Inc., the study sponsor. AH is an employee of Inmedix Inc. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
We would like to acknowledge Dr Robert Ettlinger for his review and critique of this study.
References
- Crane MM, Juneja M, Allen J, et al. Epidemiology and treatment of new-onset and established rheumatoid arthritis in an insured US population. Arthritis Care Res 2015;67:1646-55
- Birnbaum H, Pike C, Kaufman R, et al. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin 2010;26:77-90
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8
- Kristiansen TM, Primdahl J, Antoft R, et al. Everyday life with rheumatoid arthritis and implications for patient education and clinical practice: a focus group study. Musculoskeletal Care 2012;10:29-38
- van Nies JAB, de Jong Z, van der Helm-van Mil AHM, et al. Improved treatment strategies reduce the increased mortality risk in early RA patients. Rheumatology 2010;49:2210-16
- Moreland LW, Weinblatt ME, Keystone EC, et al. Etanercept treatment in adults with established rheumatoid arthritis: 7 years of clinical experience. J Rheumatol 2006;33:854-61
- Hazlewood GS, Barnabe C, Tomlinson G, et al. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 2016;353:i1777
- Stein PK, Bosner MS, Kleiger RE, et al. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J 1994;127:1376-81
- La Rovere MT, Pinna GD, Maestri R. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003;107:565-70
- Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation 2000;102:1239-44
- Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events: the Framingham heart study. Circulation 1996;94:2850-5
- Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum 2007;56:399-408
- di Comite G, Marinosci A, Di Matteo P, et al. Neuroendocrine modulation induced by selective blockade of TNF-alpha in rheumatoid arthritis. Ann N Y Acad Sci 2006;1069:428-37
- Vassilopoulos D, Mantzoukis D. Dialogue between the brain and the immune system in inflammatory arthritis. Ann N Y Acad Sci 2006;1088:132-8
- Aydemir M, Yazisiz V, Basarici I, et al. Cardiac autonomic profile in rheumatoid arthritis and systemic lupus erythematosus. Lupus 2010;19:255-61
- Nejad ZJ, Jamshidi AR, Qorbani M, et al. Cardiovascular autonomic neuropathy in rheumatoid arthritis assessed by cardiovascular autonomic function tests: a cross-sectional survey. Anatol J Cardiol 2015;15:722-6
- Kiselev AR, Karavaev AS, Mironov SA, et al. The possibility of using spectral indices of heart rate variability to improve the diagnostic value of cardiovascular autonomic function tests in rheumatoid arthritis patients. Anatol J Cardiol 2015;15:510
- Syngle V, Syngle A, Garg N, et al. Predictors of autonomic neuropathy in rheumatoid arthritis. Auton Neurosci Basic Clin 2016;201:54-9
- Adlan AM, Veldhuijzen Van Zanten JJCS, Lip GYH, et al. Cardiovascular autonomic regulation, inflammation and pain in rheumatoid arthritis. Auton Neurosci Basic Clin 2017:1
- Holman AJ, Ng E. Heart rate variability predicts anti-tumor necrosis factor therapy response for inflammatory arthritis. Auton Neurosci Basic Clin 2008;143:58-67
- Holman AJ, Ng E. How substantive is heart rate variability as a predictor of anti-TNF treatment outcome for inflammatory arthritis? [abstract]. Arthritis Rheumatol 2015;67:#1571
- Koopman FA, Chavan SS, Miljko S, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci 2016;113:8284-9
- Shimizu M, Tachibana N, Hagasaka YGM. Obstructive sleep apnea (OSA) in RA patients and effect of CPAP on RA activity. [abstract]. Abstr Arthritis Rheum 2003. Available at: https://www.researchgate.net/publication/298302248_Obstructive_sleep_apnea_OSA_in_RA_patients_and_effect_of_CPAP_on_RA_activity
- Holman AJ, Ng E. Use of adjunctive neuroregulatory medication to improve etanercept treatment response for patients with inflammatory arthritis: a pilot study - ACR meeting abstracts. ACR/ARHP Annu Meet 2015:28-9
- Figorilli M, Puligheddu M, Congiu P, et al. The clinical importance of periodic leg movements in sleep. Curr Treat Options Neurol 2017;19:10
- Fulda S. The role of periodic limb movements during sleep in restless legs syndrome: a selective update. Sleep Med Clin 2015;10:241-8
- Izzi F, Placidi F, Romigi A, et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med 2014;15:1392-7
- Ollendorf DA, Chapman R, Pearson SD, et al. Targeted immune modulators for rheumatoid arthritis: effectiveness & value. 2017. Available at: https://icer-review.org/wp-content/uploads/2016/08/NECEPAC_RA_Draft_Report_012017.pdf
- Curtis JR, Jain A, Askling J, et al. A comparison of patient characteristics and outcomes in selected European and U.S. rheumatoid arthritis registries. Semin Arthritis Rheum 2010;40:2-14.e1
- Kim SC, Yelin E, Tonner C, et al. Changes in use of disease-modifying antirheumatic drugs for rheumatoid arthritis in the United States during 1983–2009. Arthritis Care Res 2013;65:1529-33
- Yang HK, Simoni-Wastila L, Zuckerman IH, et al. Benzodiazepine use and expenditures for Medicare beneficiaries and the implications of Medicare Part D exclusions. Psychiatr Serv 2008;59:384-91
- Lee J, Pelkey R, Gubitosa J, et al. Comparing healthcare costs associated with oral and subcutaneous methotrexate or biologic therapy for rheumatoid arthritis in the United States. Am Heal Drug Benefits 2017;10:42-9
- Wolfe F, Michaud K, Gefeller O, et al. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum 2003;48:1530-42
- Carlson JJ, Ogale S, Dejonckheere F, et al. Economic evaluation of tocilizumab monotherapy compared to adalimumab monotherapy in the treatment of severe active rheumatoid arthritis. Value Health 2015;18:173-9
- Nguyen CM, Bounthavong M, Mendes MAS, et al. Cost utility of tumour necrosis factor-α inhibitors for rheumatoid arthritis. Pharmacoeconomics 2012;30:575-93
- Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 2013;381:1541-50
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093-103
- Mulcahy AW, Predmore Z, Mattke S. The cost savings potential of biosimilar drugs in the United States. Rand Corp 2014:16:1530-1542
- Elenkov IJ, Wilder RL, Chrousos GP, et al. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 2000;52:595-638
- Tracey KJD. The inflammatory reflex. Nature 2002;420:853-9
- Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458-62
- Levine YA, Koopman FA, Faltys M, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One 2014;9:1093-1103
- Koopman FA, Tang MW, Vermeij J, et al. Autonomic dysfunction precedes development of rheumatoid arthritis: a prospective cohort study. EBioMedicine 2016;6:231-7
- Koopman FA, van Maanen MA, Vervoordeldonk MJ, et al. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J Intern Med 2017;282:64-75
- van Maanen MA, Vervoordeldonk MJ, Tak PP. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat Rev Rheumatol 2009;5:229-32
- Adlan AM, Lip GYH, Paton JFR, et al. Autonomic function and rheumatoid arthritis-A systematic review. Semin Arthritis Rheum 2014;44:283-304
- Koopman FA, Stoof SP, Straub RH, et al. Restoring the balance of the autonomic nervous system as an innovative approach to the treatment of rheumatoid arthritis. Mol Med 2011;17:937-48
- del Rincón ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737-45
- Holman AJ. An alternative, autonomic rationale for decreased risk of MI in patients with RA responsive to anti-TNF therapy: comment on article by Dixon [letter]. Arthritis Rheum 2008;58:1886
- Holman AJ. Considering cardiovascular mortality in patients with rheumatoid arthritis from a different perspective: a role for autonomic dysregulation and obstructive sleep apnea. J Rheumatol 2007;34:671-3
- Partinen M, Jamieson A, Guilleminault C. Long-term outcome for obstructive sleep apnea syndrome patients. Mortality. Chest 1988;94:1200-4
- Yazici Y, Shi N, John A. Utilization of biologic agents in rheumatoid arthritis in the United States: analysis of prescribing patterns in 16,752 newly diagnosed patients and patients new to biologic therapy. Bull NYU Hosp Jt Dis 2008;66:77-85
- Mennini FS, Marcellusi A, Gitto L, et al. Economic burden of rheumatoid arthritis in Italy: possible consequences on anti-citrullinated protein antibody-positive patients. Clin Drug Investig 2017
- Jansen JP, Incerti D, Mutebi A, et al. Cost-effectiveness of sequenced treatment of rheumatoid arthritis with targeted immune modulators. J Med Econ 2017:1-12