Abstract
Objective: To evaluate the accessibility of essential medicines in China through a new system which integrated the structure, process, and effect of the National Essential Medicine Policy (NEMP).
Methods: A structural equation model was built to verify the reliability of the evaluation system. This study utilized the Delphi method to obtain the structure and process index data, and used the WHO/HAI standard method for the effect index data to evaluate the NEMP. Six regions were selected for empirical analysis so that suggestions for optimization could be put forward.
Results: The structural equation model consisted of three parts: organization structure, executive process, and effect. The factor loading of the three indicators exceeded 0.5, indicating that this model complied with the preliminary fit criteria. In the organizational structure, rules and regulations and resource investment accounted for a large proportion, indicating that they had a great impact on the effect. As for the executive process, the weight of the seven indicators were similar, and they accounted for a large proportion, indicating that each indicator had a non-negligible impact on the effect. The “p” of all the three hypothesizes was less than .01, especially the “p” of hypothesis 3 was less than .001, indicating that the structure and process of NEMP affected the accessibility of the essential medicines, and three components of the model were positively correlated.
Limitations: Some errors may exist in achieving appropriate expert selection because of potential researcher bias in the Delphi approach. The results from only six provinces in China may not be generalized nationwide.
Conclusion: The structural indicators and process indicators have a significant impact on outcome indicators, and they also have correlations. That is, the formulation and implementation of the national drug policy and related supporting measures play an important role in improving the accessibility of essential medicines.
Introduction
The concept of essential medicines was first proposed by the World Health Organization (WHO) at the 28th World Health Assembly, defining “essential medicines” as drugs that give priority to meeting the healthcare needs of the public. In China, the selection of essential medicines was initiated in 1992, and the concept of “essential medicine” has been widely adopted since then. The up-to-date definition of “essential medicine” in China refers to drugs that fulfill basic medical and health needs of the public, with suitable dosage forms, reasonable price, and guaranteed supply, and the public can obtain them fairlyCitation1.
A field survey, conducted in 2007, showed that Chinese people cannot obtain adequate drugs. For example, in the hospital pharmacy, only 19–69% of the types of essential medicines are accessibleCitation2. A survey using WHO/HAI methods conducted in 36 countries and regions (including China) found that some drugs were low in accessibility, including cardiovascular drugs, anti-diabetic drugs, anti-psychotic drugs, asthma drugs, and anti-epileptic drugsCitation3. Against the low accessibility of essential medicines, the national essential medicine policy (NEMP) has been implemented in China since 2009Citation4.
Availability of essential medicines is a significant measure of the effectiveness of the essential medicine policyCitation5. In recent years, a considerable number of studies have explored the accessibility of drugs, but have devoted less research to the accessibility of essential medicinesCitation6–8. Relevant studies have shown that, with the implementation of the essential medicine policy, the accessibility of essential medicines in China remains unsatisfying. Specifically, accessibility to essential medicines does not meet the requirements of official documentsCitation2; it does not meet the needs of certain groups of people, such as femalesCitation9,Citation10; and the national essential medicines list does not cover the actual needs at provincial level, etc.Citation11. In addition, studies on the accessibility of essential medicines in China have mainly focused on the descriptive discussion on essential medicine regimens and evaluation of accessibility to essential medicinesCitation12–19. The existing studies assessing the accessibility of essential medicines are mostly concentrated on the current effects of national essential medicine policy. Few studies evaluate the impact of differences in organizational structure and implementation process on the effect of essential medicine policy, and no systematic and comprehensive evaluation system for essential medicine policy implementation has been established.
Therefore, developing a comprehensive assessment method of the accessibility of essential medicines in China is valuable for the improvement of the national essential medicine policy. Improving the accessibility of essential medicines is one of the important goals of national drug policy. According to Dunn’sCitation20 theory of policy performance research, China’ essential medicine accessibility assessment is a part of policy performance evaluation. Therefore, policy performance assessment methods and “Structural-process-outcome (SPO)” 3-dimensional performance assessment theory, which is the most commonly applied evaluation paradigm in the field of health project performance assessment, can be used to more systematically evaluate essential medicine accessibility in ChinaCitation21. This study builds a structural equation model based on the use of 3-dimensional performance evaluation theory for evaluation of the system structure, implementation process, and implementation results of the policies related to the accessibility of essential medicines, by which accessibility of essential medicines and the impact of various factors can be more clearly and completely evaluated.
Conceptual model
SEM is a rapidly growing statistical techniqueCitation22. The model, based on a variety of traditional statistical analysis methods, is usually built to find the inherent structural relationships between variables, and to verify whether the structure of the relationship or the assumptions of the model is reasonable and correct, by analyzing a large number of objective data. The structural equation model can also be used to analyze equations that have inter-relationships, especially causal relationships, and evaluate the impact of each factor. The advantages of applying the structural equation model to the analysis presented in this paper are mainly in the following four aspects: First, there are many basic drug-related policies involved in this study, whose contribution to the accessibility of essential medicines are multi-factorial, and commonly used regression analysis or path analysis can process multiple independent variables simultaneously, while processing multiple dependent variables is infeasible, but the structural equation just solves this problem. Second, the structural equation models allow independent variables and dependent variables to contain measurement errors, and there are a large number of policies with various attributes and functions, which increases the difficulty of measurement, and the structural equation models use multiple indicators to measure variables, and thereby greatly reduce the impact of errors on the statistical results; Third, based on intrinsic logical connections and impact on the path coefficients in the computational model of large amounts of data, the structural equation models can objectively weigh the indicators. Fourth, the structural equation models must be built on a certain theoretical basis, and this study, based on the previous theoretical studies, explores relevant assessment indicators, and then uses SEM to finalize the weight of each indicator. In social science research, purely subjective research is easily limited by the researcher’s own background. The results are very different and may be biased. The research of objective data alone is entirely dependent on the quality of data, which is difficult to guarantee. The structural equation model method perfectly embodies the idea of combining subjectivity and objectiveness. In summary, this study will use a structural equation model to establish a quantitative model that is compatible with the characteristics of multi-level and multi-factor access to basic medicines, in order to build an assessment system that meets practical needs.
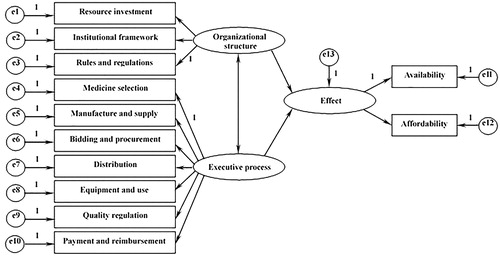
This NEMP is characterized by a fundamental organizational structure that consists of three indicators, namely, resource investmentCitation23, institutional frameworkCitation24,Citation25, and rules and regulationsCitation26,Citation27. First, the inherent exhaustibility and uneven distribution of health resources are likely to affect the NEMP’s outcomes significantly. Hence, government investments are mandatory for achieving the successful enforcement of public policies. In addition, institutional frameworks can be crucial for the success of any essential medicine project. The policies’ executive processes necessitate routine regulations and managers’ investigations across various institutions to obtain current statuses. Similarly, once a policy has been applied, the ideas need to be transformed into corresponding rules or regulations so that more specific guidelines can be providedCitation28, and the policies should be compulsory. Files such as the national essential medicine list and standard treatment guidelines are essential for the NEMP.
NEMP implementation is supported by an effective executive process, which comprises the following seven dimensions: medicine selection, manufacture and supplyCitation25, bidding and procurementCitation29, distributionCitation30, equipment and useCitation25,Citation31, quality regulationCitation2, and payment and reimbursementCitation2,Citation32. Selection, manufacture, supply, and distribution ensure the basic provision of the essential medicines. Whether the selected medicines are sufficient to meet the public’s primary medical needs influences their status as essential medicines. In addition, pharmaceutical manufacturing enterprises are major sources of medicine, but a majority of them do not respond actively to the NEMP. Pharmaceutical trading companies are not willing to distribute essential medicines, because the meager profits result in drug supply shortages. Concerning both bidding and procurement and the equipment and use of essential medicines in medical facilities, health administrative departments play significant roles in regulating these organizing processes. However, China’s province-based, centralized essential medicines procurement system has exposed many defectsCitation33. Some provincial governments established entry barriers for either manufacturing or trading companies from other regions. Local market shares were protected, even though this impeded the full play of the pharmaceutical market. In addition, supplying healthcare facilities with these essential medicines is the basis for ensuring secure access. Furthermore, physicians’ prescribing behaviors and patients’ medication self-care habits are strongly related to the universal use of essential medicinesCitation34. Throughout the entire NEMP enforcement process, quality supervision and payment and reimbursement are of identical importance. Government departments at all levels should be responsible for ensuring the quality of the essential medicines and the reimbursements to incentivize their preferential use in medical institutions.
The changes in the health system are reflected by the current effect of the NEMP, which can be measured by two indicators, availability and affordability. Availability is defined as the ability for people at all levels in different regions to enjoy equal access to their desired medicines in either public or private pharmacies, and affordability refers to the people’s capacity to pay for their expected medicines in different healthcare facilitiesCitation2,Citation35.
For the purpose of this article, organizational structure and executive process are considered as latent-independent (exogenous) variables, and effect is used as the latent-dependent (endogenous) variable. Three hypotheses are tested, as follows:
H1: Organizational structure influences the effect significantly.
H2: Executive process influences the effect significantly.
H3: Organizational structure and executive process are significantly correlated.
shows the conceptual framework was constructed on the basis of a literature review and the research hypotheses. Each latent variable comprises 3, 7, and 2 constructs, and each construct consists of a set of measurement items, all of which are from a new and comprehensive evaluation system with a hierarchical structure for essential medicine access (see ).
Table 1. Multivariate scales of evaluation system for accessibility of essential medicines.
Methods
Questionnaire survey
Six regions in China with different economic development levels, namely, Shanghai, Jiangsu, Shandong, Ningxia, Jiangxi, and Henan, were surveyed. The Delphi method was used in the survey. The Delphi method is an expert investigation method that selects a number of experts in related fields to evaluate the survey data and reach a unified conclusionCitation36. First, the researchers analyze the research project to collect data, and then select suitable experts in relevant fields and ask them to evaluate the data. The Delphi method usually takes more than two rounds. The experts would not know about each other at first, and are only allowed to exchange views on the data anonymously until a unified conclusion is reachedCitation36. The influence of expert authority on the conclusion is eliminated because of this anonymous form of expert consultation. Many Delphi methods were used to evaluate the effectiveness of drugs, and detailed and convincing results were obtainedCitation37–39.
Choosing experts is the key to Delphi's success or failure. To fully ensure the veracity of the investigation, we selected experts in the fields of pharmacy, medicine, public health and social security, and management. Clinicians in medicine should have at least 10 years of working experience in this field; for academics, at least three high-quality articles related to the medical background have to be published; in order to ensure the sustainability of the investigation, we have chosen experts who are more motivated and able to continue participating in two rounds of interviews. In 2017, a total of 50 experts participated in the consultation, and the basic situation is shown in .
Table 2. The statistical features of experts of the Delphi method.
In round 1, we obtained the opinions of experts on the establishment and selection of indicators through open-ended questions, and set up a panel of interviewers to analyze, screen, and compile the results. In the round 2 surveys, experts evaluated each of the questions on the questionnaires and then the panel was consulted by the interview panel. After six rounds of consultation, we obtained the specific indicators of the structure and process of measurement of content and identified the assessment of the Chinese basic drug accessibility index system.
Based on the results of the Delphi research, we invited experts to rate the metrics. The composition of the expert group adopts a dichotomy which is selecting 25 experts as a national expert group and trying to avoid experts from the sample area when selecting. Selected experts score the metrics of each region. When selecting specific areas as the sample areas, we selected five more experts from local areas to eliminate geographical bias and to ensure the fairness of the assessment.
For the selected sample areas, each member of the expert group is required to score the performance of the structural indicators and process indicators of the region on the second-level index items independently, with the full-scale score of each level 1 being 25 points, and the full score of each level 2 indicator being 5 points. According to the number of secondary indicators, each expert can determine the weight of the specific weight ratio, and then the weight is calculated according to the sum of the authors to get with the regions at all levels of indicators on the score.
The split-half method was used to test the internal consistent reliability of the measurement modelCitation40. Cronbach’s alpha coefficient was used for construct selection. In general, constructs with Cronbach’s alpha coefficients over 0.70 are highly reliable, and those with Cronbach’s alphas of 0.35–0.7 have acceptable reliability. In addition, factor analysis was employed to test the construct validity of the measurement model according to Kerlinger’s recommendationCitation41. The KMO and Bartlett’s test of sphericity were first used to test the sampling adequacy of the scale. For indicators at the same level, a greater factor loading (normally above 0.5) demonstrated higher convergent validity. Meanwhile, for correlations between constructs of the same variable, only one factor loading could be greater than 0.5. The more items conform to the qualification, the higher the scale’s discriminant validity.
The WHO/HAI standardized method
According to the standardized method developed by the WHO/HAI, 50 medicines (including core and supplementary medicines) and a series of representative healthcare facilities in the previously mentioned six regions (see ) were selected. The 50 selected medicines are a relatively large collection that covers multiple types of diseases, and the majority of them are anti-microbial drugs, anti-inflammatory drugs, or cardiovascular system drugsCitation42. Data on the availability and patient prices of both brand name drugs and the lowest-priced generic versions of the 50 medicines were collected in all surveyed healthcare locations (see )Citation43. Prior to the initiation of data collection, 1-week of training was held to provide area supervisors, data collectors, and data entry personnel with the knowledge and skills required to complete the medicine availability, prices, and affordability survey in an accurate and reliable manner. A standardized data collection form was also used to ensure data reliability and consistency. The data collection was completed within 3 months, from July to September 2013. The data were entered twice into the pre-programmed WHO-HAI NoMSG MSH 2012 Excel Workbook provided by the WHO/HAI to ensure accuracy and information on the availability and prices of medicines in different geographic areas, and healthcare sectors were generated with the same software during the data analysis.
Table 3. Characteristics of the six surveyed regions and their healthcare facilities.
Table 4. The rating scale of the availability and the affordability of essential medicines in six areas.
The survey had two primary outcomes, availability and affordability. Availability of medicines was measured as the proportion of pharmacies at which the medicines were available at the time of the survey. In general, the following cut-off points were used to define the availability of medicines:
Absent, 0% of healthcare facilities: the medicines could not be found in any surveyed facilities;
Low, <50% of healthcare facilities: the medicines were only available in a small proportion of facilities;
Fairly high, 50–80% of healthcare facilities: the medicines were available in many facilities; and
High, >80% of healthcare facilities: the medicines enjoy sound availability.
Affordability was estimated by comparing the total cost for a full course of treatment of a particular medication with the average daily wages of the lowest-paid unskilled government worker at the time of the survey. Median medicine unit prices were used to calculate affordability, and the daily wages in different regions were acquired from the China Labor Statistical Yearbook 2013. Generally, medicines were considered affordable if their total cost did not exceed one day’s wages.
Availability in each region was evaluated by the ratio of the medicine’s availability in a specific region to that of the region with the highest availability. Specifically, the region with the highest availability received the maximum 25 points. Affordability in each region was scored in the same manner. Because no human or animal clinical trials were involved in this study, it was not necessary to seek approval from the Institutional Review Board.
After data were obtained on the availability and affordability of medicines in each surveyed area using the standardized WHO/HAI method, the structural model was analyzed by using AMOS 20.0. If the constructs’ factor loadings exceeded 0.5, it indicates that the model complied with the preliminary fit criteria. Moreover, the composite reliabilities of organizational structure, executive process, and effect over 0.7, and the average variances extracted were all above 0.5, which implied a fairly acceptable goodness of fit of the inner structures.
Results
Reliability and validity of the measurement model
Fifty-five experts responded to the current study’s survey. As can be seen in , through a reliability analysis using SPSS 20.0, the Cronbach’s alpha coefficient of all constructs exceeded the cut-off point of 0.7, which supported retaining all of the constructs for further analysis.
Table 5. Reliability of the measurement model.
In addition, the results showed that the KMO value was 0.90 and that the Bartlett’s test of sphericity value was 1477, both with a significance of p < .05, which indicated that factor analysis was suitable for evaluating the measurement model. Then, principal component analysis with varimax rotation was used to determine construct validity, and common factors with an eigenvalue greater than 1 were extracted.
As can be observed in , the factor loadings of the constructs were all above 0.5, indicating the scale’s optimal convergent validity. For correlations between the three constructs of organizational structure, one unique relationship, O-L, was found to have a coefficient greater than 0.5. For the seven constructs of executive process, six items (C-D, S-D, P-Q, D-Q, U-Q, Q-B) met the abovementioned criteria, with correlation coefficients greater than 0.5, suggesting favorable discriminant validity. In general, the measurement scale was of superior construct validity.
Table 6. Validity of the measurement model.
Analysis of the structural model
The results in show that the model’s goodness of fit was relatively favorable. All of the constructs’ factor loadings exceeded 0.5 and were statistically significant, which indicated that the model complied with the preliminary fit criteria. Rules and regulations and resource investment were highly weighted in the evaluation of access to essential medicines, whose numbers are, respectively, 0.86 and 0.89. Moreover, the composite reliabilities of organizational structure, executive process, and effect were 0.80, 0.95, and 0.95, respectively, all of which were over 0.7, and the average variances extracted were all above 0.5 (0.58, 0.73, and 0.91, respectively), which implied a fairly acceptable goodness of fit of the inner structures. The seven dimensions of the executive process-medicine selection, manufacture and supply, bidding and procurement, distribution, equipment and use, quality regulation, and payment and reimbursement had weights of 0.86, 0.84, 0.87, 0.86, 0.83, 0.88, and 0.86, respectively, in the evaluation of access to essential medicines.
Table 7. Evaluation of the goodness of fit of the structural model.
The structural equation model was then utilized to test the research hypotheses. All of the null hypotheses were supported, as shown in . Path hypotheses 1 and 2 were supported at a superior significance level of p < .01, which indicated that both organizational structure and executive process were significantly related to the effect. Path hypothesis 3 was also supported, with an optimal level at p < .001, indicating a strong, significant relationship between organizational structure and executive process. These results are discussed in the next section.
Table 8. Summary results of the structural model.
Discussion
The NEMP’s organizational structure was found to significantly influence its effect, i.e. the changes in access to essential medicines. Rules and regulations and resource investment were highly weighted in the evaluation of access to essential medicines in that both reflected the government’s emphasis on the NEMP and the efforts to construct a healthcare system. National regulations and policies are most effective at constraining the behaviors of individual stakeholders during the enforcement of national policies and are crucial to facilitating the policies, leading to the direct improvement of access to essential medicines. First, the juridical status of the NEMP should be enhanced by incorporating it into the “Drug Administration Law”, and research on corresponding legislation related to essential medicines should be strengthened to supplement the successful practice of NEMPCitation44. Second, because the current legislative documents related to essential medicines are mostly “additional regulatory documents”, their legal force is insufficient. At the national and local levels, legislation research on essential medicines should be intensified, and eventually relevant laws, administrative regulations, departmental rules, local regulations, etc. that can guarantee and promote the coerciveness and constraining force of essential medicine policy in practice. Third, the essential medicine system involves various administrative departments such as health, pharmaceutical administration, and quality inspection. These departments are all important components in the process of implementing and advancing the essential medicine policy. Therefore, it is necessary to correctly recognize the responsibilities that each department should bear and establish a coordination mechanism to reduce the conflict between departments and reduce administrative costs. In addition, based on China’s national conditions, referencing the experience of essential medicine legislation of other countries, and formulate laws, regulations and norms for an effective essential medicine system is also a valid approach. Many countries considered the essential medicine policies in the pharmaceutical legislation practice. For example, “Drug Registration Regulations” in Australia clearly stipulates: The drug is subject to price subsidy by the government to ensure the interests of drug manufacturers or new drug developers so as to ensure market supplyCitation45. As to resource investment, adequate healthcare resources are required to improve people’s access to essential medicines. Given that China is confronted with problems such as an enormous population, an obvious aging trend, and imbalanced regional economic development, whether the medical resource investment is sufficient and the resource allocation is equal may exert great influence on whether superior access to essential medicines is achieved. The government should endeavor to reasonably adjust the range and focus of fiscal expenditures for resource investment if it is to increase the proportion of health expenditures in the regular fiscal expenditures and to ensure that increased medical investments focus on public health, rural hygiene, urban community health, and basic medical security.
Also, executive process was found to significantly influence the effect of the national drug policy. Medicine selection, manufacture and supply, bidding and procurement, distribution, equipment and use, quality regulation, and payment and reimbursement—the seven dimensions of the executive process—all played a major role in the realization of preferable access to essential medicines in the evaluation of access to essential medicines. To improve the provision of essential medicines, the government should regularly update the essential medicine list according to the strict selection principle of “the necessary medicine for preventing and curing diseases, safe and effective, reasonable price, easy to use, and simultaneously valuing the traditional Chinese medicine and Western medicine”Citation46 and establish rational compensation mechanisms for designated manufacturing and trading enterprises.
With the development of society and changes in the spectrum of diseases, the government should promptly adjust and update the national essential medicine list, so as to constantly improve the basic drug system. In addition, in the selection of essential medicines in our country, we must not only consider “the necessary medicine for preventing and curing diseases, safety and effectiveness”, but also consider “reasonable prices”. For such economic problems, we can introduce pharmacoeconomical evaluations by adopting cost-effectiveness, cost-benefit, cost-utility, or minimum cost analysis to find the optimal cost-effectiveness of essential medicines, and, ultimately, under the pre-condition of “assuring basic drug use”, achieve the best economical efficiency of drugs, and reduce medical costs to a certain extent, releasing the burden on the patients.
The government should take measures to ensure the quality and supply of essential medicines. First, implement preferential policies to encourage production and circulation, and encourage the production and circulation of essential medicines through subsidies or tax relief. Second, overcome the concept of local protectionism, adopt effective competition methods, and gradually establish a public supervision mechanism for the procurement process. In addition, local regulatory authorities must conduct effective long-term supervision of drugs and reduce counterfeit drugs, unqualified drugs, and altered drugsCitation47,Citation48. Advisable bidding and procurement systems are required to ensure the fairness and transparency of the process. The equipment proportion of essential medicines in the medical facilities in small towns and rural areas must also be enhanced to expand the coverage of essential medicines.
To change physicians’ prescribing behaviors, mechanisms for the preferential use of essential medicines in healthcare facilities and for government reimbursements for using essential medicines are indispensableCitation49. In the meantime, the regulations on the production, operation, and use of essential medicines are expected to be strengthened to ensure their quality and accessibility.
Hypothesis 3, i.e. organizational structure and executive process are significantly correlated, was strongly supported as well. Continuous improvement of rules and regulations, rational institution settings, and optimized resource allocation would exert a positive impact on the standardization and promotion of the NEMP’s executive process. At the same time, the successful implementation of the NEMP would provide guidance for additional reforms to the healthcare system’s organizational structures. The two variables interact with and promote each other.
In summary, these results are noteworthy in that they verify that the NEMP’s effect is significantly influenced by its organizational structure and executive process within a reliable and valid evaluation system with favorable goodness of fit. These findings demonstrate that the central government should not only focus on the macro improvement of NEMP’s organizational structures, but also pay attention to the micro progress of its executive processes so that the two can be perfectly coordinated to increase people’s access to essential medicines.
The analysis outlined in this manuscript is subject to some limitations. First, the Delphi approach has been criticized due to its potential for researcher biasCitation50. Although some quality control measures were taken during this investigation, some individual errors may inevitably exist in achieving appropriate expert selection. Second, since our study included only six provinces in China, the results may not be generalized nationwide. Despite the limitations and difficulties inherent in evaluation of national medicine policy, our results still have significant policy implications. Additionally, since our study was a typical empirical research rather than theoretical research, it is hoped that the results can provide empirical evidence for the further development of theoretical research.
Conclusions
In conclusion, our results showed that the structural indicators and process indicators have a significant impact on outcome indicators, while structural indicators and process indicators also have significant correlations. That is, the formulation and implementation of the national drug policy and related supporting measures play an important role in improving the accessibility of essential medicines. The fundamental process of implementing the essential medicines system is to increase the availability of essential medicines, and organize, conduct, and monitor them. In order to ensure the availability of essential medicines, the relevant government departments need to establish and improve the rational drug bidding and procurement system, improve the proportion of essential medicines in medical institutions, and foster the environment in which medical institutions give priority to the use of essential drugs. At the same time, we should also strengthen the quality of the essential medicine system sampling, and establish a sound essential medicine supervision system.
In short, through the construction of the evaluation index system of “structure–process–result”, this paper argues that the essential medicine system in China has just been established for a short time and has not been improved in terms of legislation, law enforcement, or supervision and administration. Hence, the availability and affordability of essential medicines need to be further improved.
Transparency
Declaration of funding
There is no funding to support this research.
Declaration of financial/other relationships
The authors have no financial/other relationships.
Acknowledgements
Authors of this manuscript acknowledge that this article could not have been finished without the help of many people involved in the course of data generation and major revision.
References
- Hogerzeil HV, Jing S. Health-sector reform in China and access to essential medicines. Lancet Glob Health 2013;1:e174-5
- Chen W, Tang S, Sun J, et al. Availability and use of essential medicines in China: manufacturing, supply, and prescribing in Shandong and Gansu provinces. BMC Health Serv Res 2010;10:211
- Van Mourik MSM, Cameron A, Ewen M, et al. Availability, price and affordability of cardiovascular medicines: a comparison across 36 countries using WHO/HAI data. BMC Cardiovasc Disord 2010;10:25
- Communist Party of China Central Committee, State Council. Opinions of the Communist Party of China Central Committee and the State Council on Deepening the Health Care System Reform. British Art Journal 2009;66(3):39-46. http://www.china.org.cn/government/scio-press-conferences/2009-04/09/content_17575378.htm
- Zhang Y. Evaluation of the implementation effect of the national essential medicine system—taking Zhenjiang as an example. Doctoral dissertation, Jiangsu University; 2013 (in Chinese)
- Vialle-Valentin C, Serumaga B, Wagner A, et al. Evidence on access to medicines for chronic diseases from household surveys in five low- and middle-income countries. Health Policy Plan 2015;30:1044-52
- Niëns L, Cameron A, Van de Poel E, et al. Quantifying the impoverishing effects of purchasing medicines: a cross-country comparison of the affordability of medicines in the developing world. PLoS Med 2010;7:1-8
- Ferrario A, Chitan E, Seicas R, et al. Progress in increasing affordability of medicines for non-communicable diseases since the introduction of mandatory health insurance in the Republic of Moldova health policy plan 2016;31:793-800
- Xu S, Bian C, Wang H, et al. Evaluation of the implementation outcomes of the essential medicines system in Anhui county-level public hospitals: a before-and-after study. BMC Health Serv Res 2015;15:403
- Leung N-HZ, Chen A, Yadav P, et al. The impact of inventory management on stock-outs of essential drugs in sub-Saharan Africa: secondary analysis of a field experiment in Zambia. PLoS One 2016;11:e0156026
- Feng J, Jia J, Zhang J. Review and discussion on the development of the national essential medicine system: based on the 2012 edition of the national essential medicine catalogues. China Pharm 2014;12:1057-1060
- Barber SL, Huang B, Santoso B, et al. The reform of the essential medicine system in China: a comprehensive approach to universal coverage. J Glob Health 2013;3:010303
- Zhang WY, Li YR, Li YJ, et al. A cross-sectional analysis of prescription and stakeholder surveys following essential medicine reform in Guangdong Province, China. BMC Health Serv Res 2015;15(1):98
- Peng. Effects of essential medicines on primary medical and health institutions. China Pharm 2010;21(32) (in Chinese)
- Kotwani A, Ewen M, Dey D, et al. Prices & availability of common medicines at six sites in India using a standard methodology. Indian J Med Res 2007;125:645-54
- World Health Organization and Health Action International.. Medicine Prices: a new approach to measurement. 2008:13-19
- WHO. Medicine prices surveys and proposed interventions to improve sustainable access to affordable medicines in 6 sub-Saharan African countries. WHO; 2006
- Robertson J, Forte G, Trapsida JM, et al. What essential medicines for children are on the shelf? Bull WHO 2009;87:231-7
- Yang H, Dib HH, Zhu M, et al. Prices, availability and affordability of essential medicines in rural areas of Hubei Province, China. Health Policy Plan 2010;25(3):219-29 (in Chinese)
- Dunn WN. Introduction to public policy analysis. Beijing, China: Renmin University of China Press; 2002. p 436
- Farris KB, Kirking DM. Assessing the quality of pharmaceutical care II. Application of concepts of quality assessment from medical care. Ann Pharmacother 1993;27:215-23
- Hershberger SL. The growth of structural equation modeling: 1994-2001. Struct Equat Model 2003;10:135-46
- Liu WX, Yang JJ. Increase financial input to ensure the smooth implementation of essential medicine policy—investigation on the implementation of essential medicine policy in Hunan and Guangdong provinces. Price Theory Pract 2010;4(5):29-31 (in Chinese)
- Zhao YW, Wu JY, Wang H, et al. A cross sectional study assessing predictors of essential medicines prescribing behavior based on information motivation behavioral skills model among county hospitals in Anhui, China. Chin Med J 2015;128(21):2887-95 (in Chinese)
- Yang L, Liu C, Ferrier JA, et al. Organizational barriers associated with the implementation of national essential medicines policy: a cross-sectional study of township hospitals in China. Social Sci Med 2015;145:201-8 (in Chinese)
- Wang X, Fang Y, Yang S, et al. Access to paediatric essential medicines: a survey of prices, availability, affordability and price components in Shaanxi Province, China. PLOS One 2014;9:e90365
- Tang RW, Zhao GX. Study on the accessibility of rural essential medicines under the background of new healthcare reform. Expanding Horizons 2010;1:18 (in Chinese)
- Ratanawijitrasin S, Soumerai SB, Weerasuriya K. Do national medicinal drug policies and essential medicine programs improve drug use?: a review of experiences in developing countries. Soc Sci Med 2001;53:831-44
- Kotwani A. Where are we now: assessing the price, availability and affordability of essential medicines in Delhi as India plans free medicine for all. BMC Health Serv Res 2013;13:285
- Bo N. Theory, practice, and effect of circulation system related to essential medicine policy. Dissertation for Doctoral Degree, Shandong University; 2014 (in Chinese)
- Chao J, Gu J, Zhang H, et al. The impact of the national essential medicines policy on rational drug use in primary care institutions in Jiangsu Province of China. Iran J Public Health 2018;47:24-32
- Guan X, Liang H, Xue Y, et al. An analysis of China’s national essential medicines policy. J Public Health Policy 2011;32:305-19
- Yip WC, Hsiao WC, Chen W, et al. Early appraisal of China’s huge and complex health-care reforms. Lancet 2012;379:833-42
- Yang L, Liu C, Ferrier JA, et al. The impact of the national essential medicines policy on prescribing behaviours in primary care facilities in Hubei province of China. Health Policy Plan 2013;28:750-60 (in Chinese)
- Magadzire BP, Budden A, Ward K, et al. Frontline health workers as brokers: provider perceptions, experiences and mitigating strategies to improve access to essential medicines in South Africa. BMC Health Serv Res 2014;14:520
- Suvikas-Peltonen E, Granfors E, Celikkayalar E, et al. Development and content validation of an assessment tool for medicine compounding on hospital wards. Int J Clin Pharm 2016;38:1457-63
- Stewart D, Gibson-Smith K, MacLure K, et al. A modified Delphi study to determine the level of consensus across the European Union on the structures, processes and desired outcomes of the management of polypharmacy in older people. PLoS One 2017;12:e0188348
- Morris C, Dunkley C, Gibbon FM, et al. Core health outcomes in childhood epilepsy (CHOICE): protocol for the selection of a core outcome set. Trials 2017;18:572
- Li W, Zeng L, Li J, et al. Development of indicators for assessing rational drug use to treat community-acquired pneumonia in children in hospitals and clinics. Medicine 2017;96(51):e9308
- Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess 2003;80:99-103
- Haertel EH. A positivist foundation for behavioral research. Psyc Critiques 1987;320(3)
- National Essential Medicine List. Ministry of Health of the People’s Republic of China. Government document; 2012
- Xi X, Chen P, Ma D, et al. An empirical study on evaluation index system of the accessibility of essential medicines in China. Chin J New Drugs 2017;26:620-5 (in Chinese)
- Guo Y, Shibuya K, Cheng G, et al. Tracking China’s health reform. Lancet 2010;375:1056-8
- Liu Y. The Australian drug subsidy program and its inspiration for improving the supervision mode of essential medicines in China. Soft Sci Health 2013;27:756-9 (in Chinese)
- Health Commission of the People’s Republic of China. Management of national essential medicines catalogues. 2018. http://www.nhfpc.gov.cn/yaozss3581/201504/8147002103b741179217eced1ad77efc.shtml (in Chinese) [Accessed 2018]
- Zhao W, Xu L, Yang P, et al. The current situation of national essential medicine system and its countermeasures. Chin Health Serv Manag 2011;9:664-9 (in Chinese)
- Ma J. Thinking on the problems related to the implementation of the essential medicine system. Chin Health Econ 2010;29:51-2 (in Chinese)
- Xi XY, Chu SZ. Analysis of social regulation on the priority of essential medicines. China Pharm 2010;21:3766-70 (in Chinese)
- Vernon W. 2009. The Delphi technique: a review. Int J Ther Rehabil 2009;16:69-76