Abstract
Aim: Progel Pleural Air Leak Sealant (Progel) is currently the only sealant approved by the FDA for the treatment of air leaks during lung surgery. This study was performed to determine whether Progel use improves hospital length of stay (LOS) and hospitalization costs compared with other synthetic/fibrin sealants in patients undergoing lung surgery.
Methods: The US Premier hospital database was used to identify lung surgery discharges from January 1, 2010 to June 30, 2015. Eligible discharges were categorized as “Progel Sealant” or “other sealants” using hospital billing data. Propensity score matching (PSM) was performed to control for hospital and patient differences between study groups. Primary outcomes were hospital LOS and all-cause hospitalization costs. Clinical outcomes, hospital re-admissions, and sealant product use were also described.
Results: After PSM, a total of 2,670 discharges were included in each study group; baseline characteristics were balanced between groups. The hospital LOS (mean days ± standard deviation, median) was significantly shorter for the Progel group (9.9 ± 9.6, 7.0) compared with the other sealants group (11.3 ± 12.8, 8.0; p < .001). Patients receiving Progel incurred significantly lower all-cause hospitalization costs ($31,954 ± $29,696, $23,904) compared with patients receiving other sealants ($36,147 ± $42,888, $24,702; p < .001).
Limitations: It is not possible to say that sealant type alone was responsible for the findings of this study, and analysis was restricted to the data available in the Premier database.
Conclusions: Among hospital discharges for lung surgery, Progel use was associated with significantly shorter hospital LOS and lower hospitalization costs compared with other synthetic/fibrin sealants, without compromising clinical outcomes.
Introduction
Approximately 50% of all patients undergoing lung surgery experience an air leak. The majority are minor and resolve without intervention within a few hours or daysCitation1. However, for 18–26% of patients, air leaks develop into prolonged air leaks (PALs) lasting more than 5 or 7 days post-operativelyCitation2–4. Patients at higher risk of air leaks are those with low forced expiratory volume in 1 s (FEV1), underlying lung disease, incomplete or fused fissures, low body mass index (<18.5), and male genderCitation1,Citation4–6. Upper lobectomy and surgical technique are also significant risk factorsCitation7. While air leaks of any nature are undesirable and can be associated with greater economic burdenCitation8, PALs in particular result in a more complicated post-operative course, increased cardiorespiratory or infectious morbidity, increased mortality, decreased quality-of-life, prolonged hospital stay, and increased costsCitation9–12.
Control of air leaks is paramount to successful lung surgery, and preventative measures are recommended for patients at high risk for air leaksCitation9. Approaches for treating PALs include physiotherapy, application of various agents including tetracycline, talcum, or silver nitrate through the chest tube, outpatient management with a chest tube and a one-way valve, or surgical revisionCitation1. Measures for preventing air leaks include pleural tenting, prophylactic intra-operative pneumoperitoneum, buttressing of staple lines, and use of surgical sealants or glues, such as fibrin glue, synthetic materials, and collagen patches coated with fibrinogen and thrombinCitation1,Citation13.
At the time of this publication, Progel Pleural Air Leak Sealant (Progel) is the only lung sealant indicated and approved by the Food and Drug Administration (FDA) for treatment of air leaks. Progel has been shown in clinical studies to be safe and effective at treating intraoperative air leaks in patients undergoing thoracic surgeryCitation13,Citation14. A multi-center, prospective, randomized clinical trial of patients undergoing lung surgery also found that Progel use significantly reduced hospital length of stay (LOS) compared to use of sutures or staples aloneCitation13.
The purpose of this study was to determine whether Progel use outside of the controlled conditions of a clinical trial improves LOS and hospitalization costs compared with other synthetic/fibrin sealants in patients undergoing lung surgery.
Methods
This was a retrospective, observational analysis of lung procedures in the US using the Premier hospital database. The Premier database contains data starting from the year 2000 for a consortium of US community, teaching, non-profit, and non-governmental hospitals, covering ∼6 million discharges annually and nearly 25% of all US hospital discharges. The hospitals included are nationally representative in terms of geographic location, region (urban vs rural), teaching hospital status, and bed count. Data elements in the database relate to hospital characteristics, payer characteristics, patient demographics, department details, diagnoses and procedures, physician services, laboratory tests, medications, outcomes (LOS, hospital re-admissions, mortality, etc.), and cost of care. Cost variables include all supplies, labor, and depreciation of equipment, and represent the sum of the fixed (overhead) and variable (direct) costs. Premier data are fully de-identified and fully compliant with Health Insurance Portability and Accountability Act (HIPAA) privacy and security requirements. Institutional Review Board (IRB) approval for this study was not required.
Data elements of interest from the Premier database included hospital characteristics (i.e. census region, urban or rural setting, teaching hospital status, and bed count), patient demographics (i.e. age, gender, race, marital status, body mass index [BMI], comorbidities, All Patient Refined Diagnosis Related Groups [APR-DRG] severity level), diagnoses and procedures, surgery time, outcomes (LOS, hospital re-admissions, mortality), use of synthetic/fibrin sealant products, and cost of care.
Data for adult patients (18 years of age or older) with a hospital discharge between January 1, 2010 and June 30, 2015 were evaluated for inclusion in the analysis. Eligible discharges were those with a primary International Classification of Diseases, Ninth Edition (ICD-9) procedure code for lung procedures summarized in (Surgical procedure and ICD-9 code section). The use of Progel or other synthetic/fibrin sealants during hospital admission was captured through text mining the Premier hospital billing data. Discharges identifying Progel use were included in the Progel group, while discharges identifying use of other synthetic/fibrin sealants (e.g. FLOSEAL Hemostatic Matrix, EVICEL Fibrin Sealant, TISSEEL Fibrin Sealant, BIOGLUE Surgical Adhesive, COSEAL Surgical Sealant, DURASEAL Dural Sealant System) were included in the “other sealants” group. Discharges reporting no use of Progel or other synthetic/fibrin sealants, and discharges reporting use of both Progel and another synthetic/fibrin sealant were excluded from the analysis.
Table 1. Hospital and patient characteristics of discharges reporting Progel use vs other sealants, after propensity score matching.
Primary end-points for the study were hospital LOS (days) and all-cause hospitalization cost (US$). LOS and cost for each hospital discharge were taken from the database as supplied. LOS is defined in the Premier database as the time from hospital admission to the time of hospital discharge. A patient with two discharges (i.e. two inpatient stays) contributed to the analysis of LOS separately; LOS was not aggregated across separate discharges for the same patient. Total hospitalization costs include all billable costs (e.g. procedures, medications, room and board, and hospital costs) incurred during the hospitalization. Hospitalization costs were not adjusted for inflation.
Secondary end-points included surgery time, respiratory complications, infections, re-admission to hospital, in-hospital mortality, total number of sealant product units, and sealant product units per discharge. Surgery time was taken from the database, as supplied. Respiratory complications and infections defined in the literature as being associated with PALs and occurring within the hospital stay of the index procedure were identified. ICD-9 codes were used to identify all relevant respiratory events, including pneumonia and empyema. A detailed list of the included respiratory events and their corresponding ICD-9 code is provided in Supplementary Table 1. A list of infection-related ICD-9 codes and Current Procedural Terminology (CPT) codes developed through prior researchCitation15 was used to identify relevant post-operative infections in the database. Codes were categorized into high priority and moderate/low priority infection groups, according to published criteriaCitation16,Citation17. A detailed list of included infections and their corresponding ICD-9/CPT code and priority group is provided in Supplementary Table 2. Patients with at least one high priority code and patients with at least one moderate/low priority infection were reported.
Table 2. Results of secondary end-points for dischargesTable Footnotea reporting Progel use vs other sealants.
Hospital re-admissions, defined as a hospital admission in either the same or subsequent month to the index hospitalization, were identified. Only re-admissions to the same institution in which the index admission took place are captured in the Premier database. In-hospital mortality was determined using discharge status. Finally, the total number of sealant product units was determined using the Premier hospital billing data.
Statistical analysis
Statistical analysis was conducted using SAS 9.4 (SAS Institute, Cary, NC). The level of statistical significance for all comparisons was set to α = 0.05. No adjustment for multiplicity was performed. Descriptive statistics were calculated for hospital, patient, and clinical characteristics, and cohorts were compared for differences in variables. Continuous data were expressed using mean, standard deviation (SD), median, and interquartile range. Numeric counts and percentages were used to summarize categorical data.
To estimate a treatment effect using observational data obtained from the Premier database, propensity score matching (PSM) was used to balance characteristics between the two study groups. Unconditional logistic regression models were fit to determine those variables associated with Progel and other sealants. All relevant baseline covariates that were collected in the database were included in the propensity model. Covariates included treatment, hospital characteristics (i.e. census region, urban or rural setting, teaching hospital status, and bed count), patient demographics (age, gender, race, marital status, and APR-DRG severity level), payer type (i.e. Medicare, Medicaid, managed care, and other), patient comorbidities (chronic obstructive pulmonary disease [COPD], obesity, and chronic pulmonary disease), admission type (i.e. emergency, urgent, and other), surgical procedure, presence of air leak, and number of risk factors (obesity, COPD, chronic pulmonary disease, cerebrovascular disease, any malignancy, hypertension, diabetes, and peripheral vascular disease); all were considered explanatory variables. Using the regression model, propensity scores (indicating the probability of belonging to the Progel group) were calculated for each discharge. Discharges within the two study groups were sorted randomly before matching, and each discharge in the Progel group was then matched 1:1 to a discharge in the other sealants group with the closest propensity score. Subjects without a match, using the criteria of 0.2-times the standard deviation of Euclidean distance of propensity score, were excluded from further analysis. In the case of more than one match, the first encountered match was used. The balance of measured covariates was assessed using absolute standardized differences, with an absolute standardized difference of ≤0.1 representing an acceptable difference between study groups. Standardized differences for included covariates were calculated for study groups before and after PSM. Using the matched cohorts, differences between study groups were calculated for the primary end-points. Statistical differences in hospital LOS and all-cause hospitalization costs were assessed with a paired samples t-test. Differences between study groups were not calculated for secondary end-points, due to the limitations of available sample size.
As additional supportive analyses, propensity score classification (PSC) and traditional multivariable regression analysis were explored as alternative methods for controlling for confounding factors. In PSC, subjects were classified into five groups based on quintiles of the estimated propensity score for each subject. Within each group, subjects are expected to be homogeneous, and therefore more comparable, with respect to their propensity scores. Treatment differences were estimated within each group, and then the five estimated treatment effects were combined into one overall treatment effect estimate. Multivariable regression analysis was conducted using separate models that included: (1) all covariates, and (2) significant covariates based on a stepwise selection approach. The full regression model included the following covariates: treatment, age, APR-DRG severity level, gender, race, marital status, admission type, payer type, COPD, obesity, chronic pulmonary disease, census region, surgical procedure, presence of air leak, and number of risk factors. The selected regression model for hospital LOS included treatment, age, APR-DRG severity level, race, admission type, bed count, payer type, COPD, chronic pulmonary disease, census region, surgical procedure, and air leak. The selected regression model for all-cause hospitalization cost included treatment, age, APR-DRG severity level, gender, chronic pulmonary disease, admission type, payer type, COPD, hospital teaching status, census region, surgical procedure, and presence of air leak.
Results
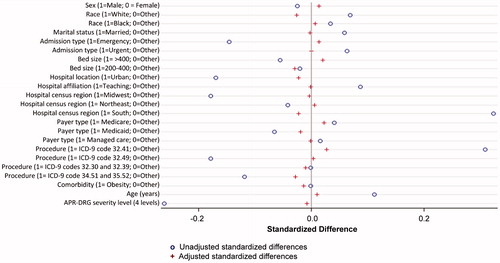
Eligible discharges were identified in the Premier database during the 5.5 year study period. A total of 3,562 discharges reported exclusive Progel use and 3,539 reported use of other synthetic/fibrin sealants. After PSM, there were a total of 2,670 discharges in the Progel group and 2,670 discharges in the other sealants group. presents the hospital and patient characteristics for the study cohorts after PSM. Study groups were well balanced on all characteristics, with standardized differences falling below 0.1 for most variables ().
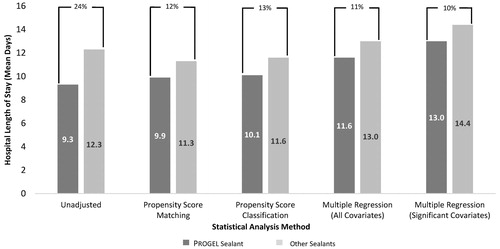
Before PSM, utilization of Progel was associated with a 24% reduction in mean hospital LOS compared with other sealants (9.3 vs 12.3 days; p < .001; ). After PSM, hospital LOS (mean ± standard deviation, median) remained significantly shorter for the Progel group (9.9 ± 9.6, 7.0) compared with the other sealants group (11.3 ± 12.8, 8.0; p < .001); utilization of Progel was associated with a 12% reduction in mean hospital LOS compared with other sealants. Results of the PSC and traditional multivariate regression analyses were similar to those for the PSM (). Hospital LOS results were similar across all procedure types (results not shown).
Figure 2. Mean hospital LOS per discharge for Progel Sealant and other sealants by statistical method. All differences statistically significant at the p < 0.001 level; p-values assessed using paired t-test.
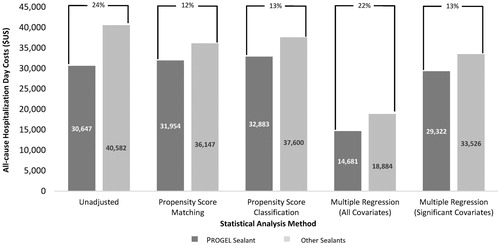
Before PSM, utilization of Progel was associated with a 24% reduction in mean all-cause hospitalization costs compared with other sealants ($30,647 vs $40,582; p < .001; ). After PSM, all-cause hospitalization costs (mean ± standard deviation, median) remained significantly lower for the Progel group ($31,954 ± $29,696, $23,904) compared with the other sealants group ($36,147 ± $42,888, $24,702; p < .001); utilization of Progel was associated with a 12% reduction in mean all-cause hospitalization costs compared with other sealants. As with LOS, the cost results of the PSC and traditional multivariate regression analyses were similar to those for the PSM (), and were similar across all procedure types (results not shown).
Figure 3. Mean all-cause hospitalization cost per discharge for Progel Sealant and other sealants by statistical method. All differences statistically significant at the p < .001 level; p-values assessed using paired t-test.
summarizes the secondary end-point results. Since no statistical analyses were performed on the secondary end-points, these results must be interpreted with caution. Surgeries using Progel were shorter in duration than those using other sealants (248.96 vs 256.95 min). A total of 1,049 (14.8%) discharges reported respiratory complications. Progel use was associated with a lower prevalence of respiratory complications (13.5% vs 16.1%), pneumonia (1.4% vs 2.0%), and emphysema (3.6% vs 4.7%) compared with other sealants. A total of 45 (0.6%) discharges reported high priority infections, and a total of 1,410 (19.9%) discharges reported moderate/low priority infections. The incidence of high priority infections (0.5% vs 0.8%) and moderate/low priority infections (17.2% vs 22.6%) was lower in the Progel group compared with the other sealants group.
A total of 27 hospital re-admissions were reported for the eligible discharges. A greater number of hospital re-admissions were captured for the Progel group compared with the other sealants group (re-admissions were less than 1% in both groups). A total of 259 in-hospital deaths were reported for the eligible discharges. Progel use was associated with fewer in-hospital deaths compared with use of other sealants (3.2% vs 4.1%).
The total number of sealant units across all eligible discharges and the mean number of sealant units per discharge was lower for the Progel group (4,747 total units; 1.3 units per discharge) compared with the other sealants group (5,521 total units; 1.6 units per discharge).
Discussion
Progel is indicated and approved by the FDA for the treatment of patients with air leaks in both open and minimally invasive thoracic surgery. The occurrence of air leaks may be associated with greater economic burden, including significantly greater LOS, operating room time, and hospital costs, when compared with no air leaksCitation8. In this retrospective study comparing Progel use with use of other synthetic/fibrin sealants, Progel was associated with significantly shorter hospital LOS and lower all-cause hospitalization costs. Overall, the mean hospital LOS was 10.8 days in our study sample. Mean LOS for discharges reporting exclusive use of Progel was 9.9 days compared to 11.3 days for discharges reporting use of other sealants. The 12% reduction in hospital LOS contributed to a similar, significant reduction in mean all-cause hospitalization costs. Although comparative analyses were not conducted on secondary end-points, descriptive analyses confirmed that use of Progel did not compromise the clinical outcomes investigated. Surgeries using Progel were shorter in duration than those using other sealants, and Progel was associated with fewer respiratory complications, infections, sealant products, and deaths. Re-admissions were less than 1% in both groups.
Other clinical studies have investigated the impact of sealants on hospital LOS with mixed resultsCitation13,Citation14,Citation18–37. A randomized controlled trial and a retrospective chart review reported LOS data for Progel compared with use of sutures or staples alone in patients undergoing lung surgeryCitation13,Citation14. In the study by Allen et al.Citation13, the median LOS for Progel sealant use (6 days) was significantly shorter compared with use of sutures or staples alone (7 days). In a single-center, retrospective chart review of 121 consecutive patients who underwent lung surgery with and without Progel, mean (median) LOS was significantly reduced from 4.2 (3.0) days in the control group to 1.7 (1.5) days in the Progel group14. Differences in LOS observed for patients treated with Progel in these studies may be partially explained by differences in severity of air leaks and post-operative complications in each study.
In three other trials, LOS was significantly reduced in patients receiving synthetic/fibrin sealants compared with standard care (sutures, staples, and/or electrocautery)Citation18,Citation24,Citation25. Mean LOS ranged from 4–6.2 days for sealant groups and from 7–8 days for standard care groupsCitation18,Citation24,Citation25. Sixteen additional clinical studies showed no statistically significant difference in LOS between treatment groupsCitation19–23,Citation26–36, including two studies that compared two synthetic/fibrin sealant treatmentsCitation20,Citation36.
Four comparative studies were identified that reported the impact of sealants vs standard care (sutures, staples, and/or electrocautery) on hospitalization costsCitation23,Citation30,Citation37,Citation38. In the RCT reported by Zaraca et al.Citation37, a significant reduction of costs was observed in the sealant group compared with the sutures/staples group, as a result of a significantly shorter hospital stay. The cost of two additional days of hospitalization for patients in the control group easily exceeded the price of the lung sealantCitation37. In a small pilot study evaluating two different surgical techniques for preventing air leaks in patients undergoing lobectomy, Droghetti et al.Citation23 reported significantly lower mean procedure costs for procedures using electrocautery and sealant vs standard care with staples. The mean cost of hospitalization was also lower for patients receiving electrocautery and sealant; however, this difference was not statistically significantCitation23. The remaining two studies reported clinical outcome trends in favor of sealants, but failed to demonstrate a significant difference in hospitalization costsCitation30,Citation38. Although more economic analyses are needed to clarify the impact of sealants on hospitalization costs, the Zaraca et al.Citation37 RCT and the current PSM analysis support the hypothesis that use of a lung sealant like Progel significantly reduces hospitalization costs compared with standard care and synthetic/fibrin sealants, respectively.
Meta-analyses and systematic reviews have determined that sealants reduce post-operative air leak duration and time to chest drain removal compared with no sealant use, but have failed to show a definitive advantage in terms of hospital LOS and hospitalization costsCitation39–41. Importantly, all meta-analyses grouped different sealants together, suggesting that such products could be evaluated as a classCitation42. However, differences in product characteristics and efficacy strongly suggest that sealants should be assessed as individual agents. As a case in point, Progel is the only available sealant at the time of this study that is specifically indicated for use in lung surgery, and approved by the FDA for treatment of air leaks. As sealant materials and technology continue to improve, it is imperative that additional studies are conducted and meta-analyses are updated to reflect these advances.
All observational, retrospective studies have inherent limitations. Specific to our study, we cannot definitively say that sealant type alone was responsible for our findings. Since patients were not randomly allocated to sealant study groups, there may have been underlying differences between study groups in important prognostic factors such as product availability in hospitals, hospital practices, surgical technique, FEV1, and the presence of incomplete or fused fissuresCitation1,Citation5,Citation6. Similarly, only limited information regarding nutritional status, a significant prognostic factor for PALs, was available from the Premier database. We used PSM to balance study groups as best as possible on all available hospital and patient characteristics and confirmed adequate performance of the PSM using established techniques. We also conducted analyses using PSC and traditional multivariable regression analyses, which all supported the results of the PSM. Despite our best efforts, we cannot be certain that no differences existed between treatment groups that may have influenced study outcomes.
With respect to hospital re-admissions, it is important to reiterate that only admissions to the same institution as the index procedure are captured as re-admissions in the Premier database. For this reason, the number of re-admissions may be under-estimated in the analysis, and these results must be interpreted with caution.
Finally, the results of this study may not be generalizable to other hospitals not included in the Premier database, to other inpatient lung procedures, or to non-lung surgical procedures.
Conclusion
This study provides further evidence that the clinical benefits of Progel Pleural Air Leak Sealant seen in clinical trials may translate into economic benefits in practice. Among hospital discharges for lung surgery, Progel use was associated with significantly shorter hospital LOS and lower hospitalization costs compared with other synthetic/fibrin sealants, without compromising clinical outcomes.
Transparency
Declaration of funding
Funding was provided by Davol Inc., a subsidiary of C. R. Bard. Bard has joined BD.
Declaration of financial/other relationships
KDM is a paid consultant for Becton Dickinson. Cornerstone Research Group (IS, NF) received funding from Becton Dickinson to conduct the study. MC, XZ, and IB are employees of Becton Dickinson. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
This research was presented at the ISPOR 19th Annual European Congress, Vienna, Austria, October 30–November 2, 2016, and the 30th Annual Meeting of the General Thoracic Surgical Club, Clearwater, Florida, March 9–12, 2017.
Supplementary Material
Download MS Word (37.7 KB)Acknowledgments
The authors would like to acknowledge Ying Wan for assisting in study design and results interpretation, and Lisa Bernard for assisting with drafting of the manuscript.
References
- Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis 2014;6:271-84
- Stephan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000;118:1263-70
- Abolhoda A, Liu D, Brooks A, et al. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest 1998;113:1507-10
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: an analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64
- Thomas PA, Berbis J, Falcoz PE, et al. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg 2014;45:652-9; discussion 659
- Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513-25
- Yoo A, Ghosh SK, Danker W, et al. Burden of air leak complications in thoracic surgery estimated using a national hospital billing database. Clinicoecon Outcomes Res 2017;9:373-83
- Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl 2012;94:422-7
- Bille A, Borasio P, Gisabella M, et al. Air leaks following pulmonary resection for malignancy: risk factors, qualitative and quantitative analysis. Interact Cardiovasc Thorac Surg 2011;13:11-15
- Lackey A, Mitchell JD. The cost of air leak: physicians' and patients' perspectives. Thorac Surg Clin 2010;20:407-11
- Varela G, Jimenez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33
- Allen MS, Wood DE, Hawkinson RW, et al. Prospective randomized study evaluating a biodegradable polymeric sealant for sealing intraoperative air leaks that occur during pulmonary resection. Ann Thorac Surg 2004;77:1792-801
- Klijian A. A novel approach to control air leaks in complex lung surgery: a retrospective review. J Cardiothorac Surg 2012;7:49
- Corral M, Ferko N, Hollmann S, et al. Health and economic outcomes associated with uncontrolled surgical bleeding: a retrospective analysis of the Premier Perspectives Database. Clinicoecon Outcomes Res 2015;7:409-21
- Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg 2012;256:973-81
- Miner AL, Sands KE, Yokoe DS, et al. Enhanced identification of postoperative infections among outpatients. Emerg Infect Dis 2004;10:1931-7
- Lequaglie C, Giudice G, Marasco R, et al. Use of a sealant to prevent prolonged air leaks after lung resection: a prospective randomized study. J Cardiothorac Surg 2012;7:106
- De Leyn P, Muller MR, Oosterhuis JW, et al. Prospective European multicenter randomized trial of PleuraSeal for control of air leaks after elective pulmonary resection. J Thorac Cardiovasc Surg 2011;141:881-7
- Belcher E, Dusmet M, Jordan S, et al. A prospective, randomized trial comparing BioGlue and Vivostat for the control of alveolar air leak. J Thorac Cardiovasc Surg 2010;140:32-8
- Bertolaccini L, Lyberis P, Manno E. Lung sealant and morbidity after pleural decortication: a prospective randomized, blinded study. J Cardiothorac Surg 2010;5:45
- D’Andrilli A, Andreetti C, Ibrahim M, et al. A prospective randomized study to assess the efficacy of a surgical sealant to treat air leaks in lung surgery. Eur J Cardiothorac Surg 2009;35:817-20; discussion 820–1
- Droghetti A, Schiavini A, Muriana P, et al. A prospective randomized trial comparing completion technique of fissures for lobectomy: stapler versus precision dissection and sealant. J Thorac Cardiovasc Surg 2008;136:383-91
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202
- Tansley P, Al-Mulhim F, Lim E, et al. A prospective, randomized, controlled trial of the effectiveness of BioGlue in treating alveolar air leaks. J Thorac Cardiovasc Surg 2006;132:105-12
- Belboul A, Dernevik L, Aljassim O, et al. The effect of autologous fibrin sealant (Vivostat) on morbidity after pulmonary lobectomy: a prospective randomised, blinded study. Eur J Cardiothorac Surg 2004;26:1187-91
- Fabian T, Federico JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg 2003;75:1587-92
- Porte HL, Jany T, Akkad R, et al. Randomized controlled trial of a synthetic sealant for preventing alveolar air leaks after lobectomy. Ann Thorac Surg 2001;71:1618-22
- Wain JC, Kaiser LR, Johnstone DW, et al. Trial of a novel synthetic sealant in preventing air leaks after lung resection. Ann Thorac Surg 2001;71:1623-8; discussion 1628–9
- Macchiarini P, Wain J, Almy S, et al. Experimental and clinical evaluation of a new synthetic, absorbable sealant to reduce air leaks in thoracic operations. J Thorac Cardiovasc Surg 1999;117:751-8
- Wong K, Goldstraw P. Effect of fibrin glue in the reduction of postthoracotomy alveolar air leak. Ann Thorac Surg 1997;64:979-81
- Mouritzen C, Dromer M, Keinecke HO. The effect of fibrin glueing to seal bronchial and alveolar leakages after pulmonary resections and decortications. Eur J Cardiothorac Surg 1993;7:75-80
- Wurtz A, Gambiez I, Chambon JP, et al. Assessment of the effectiveness of fibrin sealant in wedge lung resection. Results of a controlled trial including 50 patients. Chirurgical 1992;88:368-71
- Fleisher AG, Evans KG, Nelems B, et al. Effect of routine fibrin glue use on the duration of air leaks after lobectomy. Ann Thorac Surg 1990;49:133-4
- Wurtz A, Chambon JP, Sobecki L, et al. [Use of a biological glue in partial pulmonary excision surgery. Results of a controlled trial in 50 patients]. Ann Chir 1991;45:719-23
- Kilic B, Ersen E, Demirkaya A, et al. A prospective randomized trial comparing homologous and autologous fibrin sealants for the control of alveolar air leak. J Thorac Dis 2017;9:2915-22
- Zaraca F, Vaccarili M, Zaccagna G, et al. Cost-effectiveness analysis of sealant impact in management of moderate intraoperative alveolar air leaks during video-assisted thoracoscopic surgery lobectomy: a multicentre randomised controlled trial. J Thorac Dis 2017;9:5230-8
- Gundogdu AG, Yazicioglu A, Kara M, et al. [The use of tissue glue and its effect on hospital cost in patients undergoing pulmonary surgery]. Tuberk Toraks 2006;54:157-60
- Malapert G, Hanna HA, Pages PB, et al. Surgical sealant for the prevention of prolonged air leak after lung resection: meta-analysis. Ann Thorac Surg 2010;90:1779-85
- Serra-Mitjans M, Belda-Sanchis J, Rami-Porta R. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2005;(3):CD003051
- Belda-Sanchis J, Serra-Mitjans M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2010;(1):CD003051
- Fuller C. Reduction of intraoperative air leaks with Progel in pulmonary resection: a comprehensive review. J Cardiothorac Surg 2013;8:90