Abstract
Background: Regular molecular monitoring with reverse-transcription quantitative PCR (RT-qPCR) analysis of BCR-ABL1 transcripts is associated with reduced disease progression among patients with chronic myeloid leukemia (CML). Molecular monitoring assists in the timely detection of primary or secondary resistance to tyrosine kinase inhibitor (TKI) therapy and is a recommended practice by the National Comprehensive Cancer Network guidelines. An economic model was developed to estimate the potential impact of CML monitoring vs lack of monitoring on patient healthcare costs.
Methods: An Excel-based decision-analytic economic model was developed from a US payer perspective. The model was used to estimate the expected healthcare cost differences between regular molecular monitoring of CML patients and lack of monitoring. CML progression rates among patients with vs without monitoring, the annual cost of CML progression, the average number of monitoring tests per year, and the average cost per RT-qPCR monitoring test were incorporated into the model. Univariate and multivariable sensitivity analyses were conducted.
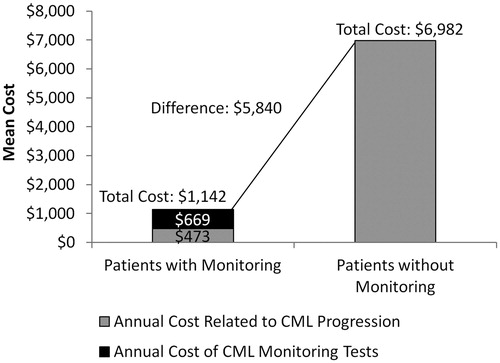
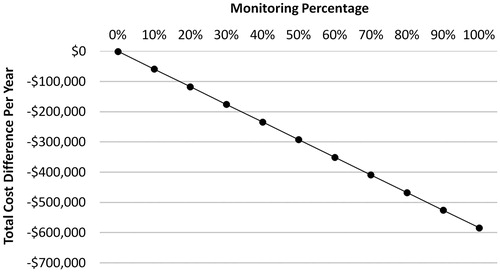
Results: Based on estimates in published literature, disease progression to the accelerated/blast phase occurs among 0.35% of patients with monitoring and 5.12% of patients without monitoring, and the annual cost of CML progression is $136,308 per patient year. The analysis found that total healthcare costs, including the costs associated with CML progression and RT-qPCR monitoring tests (three tests per year), were $1,142 for patients with monitoring and $6,982 for patients without monitoring (difference = $5,840). In a hypothetical cohort of 100 patients with CML, achieving a 100% monitoring rate was associated with a total cost-savings of $584,005 compared to a 0% monitoring rate. This cost-savings remained consistent under both univariate and multivariable sensitivity analyses.
Conclusion: Regular CML monitoring was associated with improved outcomes among CML patients and, consequently, reduced healthcare costs.
Introduction
Chronic myeloid leukemia (CML) has an incidence of 1.8 cases per 100,000 adults in the US based on data from years 2011 through 2015Citation1. CML is a multi-phase disease that usually presents in the chronic phase (CP) and can progress into the more severe accelerated (AP) and blast phases (BP)Citation2,Citation3. It is caused by a reciprocal chromosomal translocation that leads to formation of the tyrosine kinase BCR-ABL1 oncoprotein, which plays a key role in the onset and progression of CMLCitation2,Citation3. In clinical trials, treatment of CML patients in the CP with targeted tyrosine kinase inhibitors (TKIs) has been shown to prolong the chronic phase and improve patient survival. TKIs are recommended by the National Comprehensive Cancer Network (NCCN) guidelines as standard therapies for patients with CMLCitation2,Citation4.
Disease progression among patients with CML is associated with poor outcomes and is costlyCitation2,Citation5. Molecular monitoring, with reverse-transcription quantitative PCR (RT-qPCR) analysis of BCR-ABL1 transcripts, of patients with CML is recommended by the NCCN guidelines to allow for timely detection of loss of response to TKI therapy or failure to achieve certain milestones and to inform treatment decisionsCitation2. Regular molecular testing according to the NCCN guidelines (as often as every 3 months) has been shown to be associated with a lower risk of disease progression and mortalityCitation6. Although shown to be beneficial for patient outcomes, real-world studies indicate that only approximately one-third to one-half of patients with CML receive any molecular testing in the first year of TKI therapy and up to 79% of patients do not receive any testing within the first 3 months of starting treatmentCitation6–12. Currently, there are little data that directly link CML monitoring with economic outcomes, thus we developed an economic model to estimate the potential impact of CML monitoring vs lack of monitoring on patient healthcare costs from a US payer perspective.
Methods
Economic model
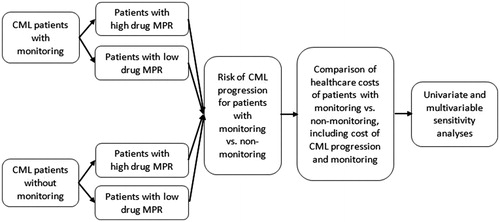
An Excel-based decision-analytic economic model () was developed to compare healthcare costs associated with two scenarios: regular molecular monitoring, defined as 3 RT-qPCR tests per year, vs lack of monitoring, defined as 0 RT-qPCR tests per year.
Model inputs
Model inputs are summarized in . Rates of CML progression among patients with vs without monitoring tests were obtained from Haque et al.Citation6. In this study of 245 CML patients with a median follow-up of 4.6 years, disease progression to the AP/BP occurred in 0.35% of patients with monitoring and 5.12% of patients without any monitoringCitation6. The annual cost of CML progression was obtained from Jabbour et al.Citation5, which was estimated at $136,308 per patient year. The average cost per molecular RT-qPCR monitoring test was defined as $223, which was obtained from Latremouille-Viau et al.Citation10.
Table 1. Values of model parameters.
Healthcare cost estimates
Mean total annual healthcare costs, defined as the sum of molecular monitoring test costs and costs related to CML progression, were estimated in the model for patients with vs without monitoring. The changes in total annual healthcare costs resulting from increasing the proportion of patients with regular monitoring, ranging from 0–100% of a hypothetical cohort of 100 CML, were also evaluated.
Sensitivity analyses
Univariate and multivariable sensitivity analyses were also conducted to test the robustness of the results by varying the following model inputs: level of adherence to TKI therapy, cost of disease progression, number of monitoring tests per year, and cost of each monitoring test.
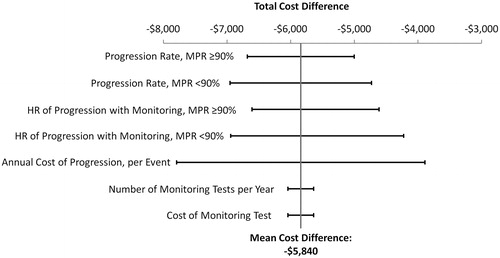
Haque et al.Citation6 assessed CML progression among CML patients with vs without monitoring, and stratified patients by high and low TKI adherence status (Medication Possession Ratio (MPR) ≥ 90% and MPR <90%). For patients with a MPR ≥90%, the study reported a hazard ratio (HR) for disease progression of 0.07 [95% confidence interval (CI) = 0.03–0.19] for patients with vs without monitoring; for patients with a MPR <90%, a HR of 0.07 (95% CI = 0.02–0.21) was reported for patients with vs without monitoringCitation6. Disease progression rates at high and low TKI adherence, as well as the HRs for disease progression, were varied between the ranges of their lower and upper 95% confidence intervals and incorporated into the sensitivity analyses. Other parameters, including the annual cost of disease progression (per event), the number of monitoring tests per year, and the cost of each monitoring test, were varied within ±30% of their mean values. The impact of these variations on the estimated cost difference among CML patients with vs without monitoring was evaluated in the univariate (one-way) sensitivity analysis by varying one model parameter at a time between its lower and upper bounds, as described above.
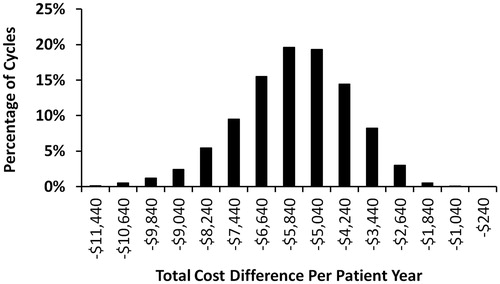
Additionally, 10,000 Monte Carlo simulation cycles were conducted as the multivariable sensitivity analysis, in which all model parameters were allowed to vary simultaneously in each cycle. In the Monte Carlo simulation, each cycle thus represents one scenario where all key model parameters may randomly vary from their default values. Descriptive statistics from the 10,000 cycles of Monte-Carlo simulation were used to evaluate the distribution and robustness of the model results.
Results
Healthcare cost estimates
The total annual healthcare costs, including costs associated with CML progression and molecular monitoring tests, were estimated to be $5,840 per-patient-per-year (PPPY) lower for patients with monitoring compared to patients without monitoring ($1,142 vs $6,982; ). When extrapolated to a hypothetical cohort of 100 CML patients, monitoring 100% of patients was associated with a total cost-savings of $584,005 compared to 0% monitoring ().
Sensitivity analyses
The estimated cost reduction for patients with vs without monitoring remained consistent under both univariate () and multivariable sensitivity analyses (), demonstrating the validity of the model calculations. The model parameters with the greatest impact on the estimated cost-savings associated with monitoring vs no monitoring were the annual cost of progression (relative cost change = 67%), progression rates (relative cost change = 29–38%), and hazard ratios of progression (relative cost change = 34–47%). Variation in the number of monitoring tests per year and cost of monitoring tests had relatively small impacts on the estimated cost-savings. The mean cost-savings for patients with vs without monitoring estimated from the 10,000 random cycles of Monte Carlo simulations was $5,708 PPPY (95% CI = –$9,193 to –$2,876) and similar to the mean cost-saving estimated in the default model analysis ($5,840 PPPY). Out of the 10,000 cycles of Monte Carlo simulations, 100% of them showed a cost reduction for patients with vs without monitoring.
Discussion
The annual cost-savings associated with molecular monitoring vs non-monitoring of patients with CML estimated in this economic model was $5,840 per patient. Since there may be other healthcare costs associated with monitoring, such as additional lab tests, annual vascular assessment, and physician visits, the total costs for monitoring services may be higher than just the cost of RT-qPCR tests. As there is no known evidence quantifying such additional costs of monitoring, we can arbitrarily increase monitoring costs by $500 per test (from $223 to $723). This would increase the total PPPY costs associated with monitoring by $1,500 compared to the default analysis, but would still result in cost savings of $4,340 PPPY for patients with vs without monitoring ($2,642 vs $6,982).
Although this analysis was not designed to be a cost-effectiveness assessment, as monitoring is being shown to be associated with increased benefit and reduced costs, regular monitoring is likely to be a dominant care option vs non-monitoring in terms of its cost-effectiveness metric.
This economic analysis further highlights the importance of regular molecular monitoring for improving patient care and clinical outcomes, as well as reducing healthcare costs. The cost-savings associated with monitoring vs lack of monitoring estimated in this model is similar to that reported by Latremouille-Viau et al.Citation10, who conducted a retrospective claims analysis of patients with CML from January 2006 through June 2015. In this study of 1,431 patients with CML, the healthcare savings associated with each additional molecular monitoring test performed was $2,918 per-patient-per-year (2015 USD)Citation10.
The estimate of the cost-savings associated with molecular monitoring in the analysis presented here may be somewhat under-estimated, as we used the incremental cost of CML progression of $136,308, which, according to Jabbour et al.Citation5, may potentially represent the lower estimate of the incremental economic burden of CML progression. The cost of CML progression is influenced by the frequency of use of allogeneic hematopoietic stem cell transplantation (HSCT), which may vary depending on factors such as patient age and treatment response, etc. For this economic analysis we used the mean incremental cost of CML progression derived from a retrospective claims database analysis of 587 patients with CML progression (mean age = 59 years), of which 30% received allogeneic HSCT as index treatmentCitation5; the actual cost of CML progression may vary on a case by case basis. Also, the costs of TKI therapy were not included in this economic model, since Haque et al.Citation6 did not report any differences in TKI drug treatment between patients with monitoring and patients without monitoring. Therefore, this model did not evaluate the differences in TKI drug cost between patients with vs without monitoring. Whether this is true in the real-world setting may require validation in other future studies.
Several studies have provided evidence of the improvement of outcomes of CML patients who receive routine molecular monitoringCitation6–12. Possible explanations of better outcomes associated with regular molecular monitoring of CML patients include, more optimal assessment of treatment response, rapid intervention when treatment resistance or failure is detected, and better adherence to TKI therapyCitation6–12. This study provides further evidence of the value of regular treatment response monitoring in CML patients, and may be informative to stakeholders involved in the delivery of care to patients with CML. Examining the reasons for the lack of monitoring in real-world clinical practice was outside of the scope of this analysis, which may be an area of new research opportunities. However, it should be noted that patients with CML should receive individualized care, and decisions on the intensity of monitoring ultimately depend on the treating physician’s judgment.
The healthcare costs estimated in this model may not precisely reflect costs in specific US health plans. However, this model may still be useful for evaluating the impact of monitoring on healthcare costs of patients with CML. Additionally, the input of the annual cost of CML progression was at the 2015 US cost level and was not adjusted for inflation. This is a more conservative approach, as the cost of CML progression might be even greater after the potential inflation changes since 2015. Hence, the numerical value of the economic benefit associated with regular monitoring in reality may be greater than what was reported here. The inputs of CML progression rates were from an observational study that had a relatively small sample sizeCitation6. However, these and most other model inputs were derived from real-world evidence, which represent the main strength of this analysis. Although we incorporated real-world evidence derived from Haque et al.Citation6 on the impact of monitoring frequency and adherence on CML progression, other patient variables, such as baseline risk of progression, treatment compliance, usage of different TKI drugs, etc., were not incorporated in the model. Further real-world study of these patient and provider factors on outcomes of CML patients may be helpful for estimating the value of regular monitoring.
Conclusions
Optimal molecular monitoring of CML patients treated with TKIs as recommended by the NCCN guidelines may reduce CML progression rates and reduce the healthcare economic burden associated with CML. To improve clinical and economic outcomes among CML patients, healthcare providers, payers, and other decision-makers should strategize to develop measures that promote better adherence to the NCCN recommended molecular testing guidelines.
Transparency
Declaration of funding
This study was funded by Bristol-Myers Squibb.
Declaration of financial/other relationships
EJ is a consultant to Bristol-Myers Squibb. LRS and DM are full time employees of Bristol-Myers Squibb. JL, MLS, and BM are employees of Novosys Health, which has received research funds from Bristol-Myers Squibb in connection with conducting this study and development of this manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- SEER. Cancer Stat Facts: Chronic Myeloid Leukemia [Internet]. Bethesda, MD: National Cancer Institute. Available at: https://seer.cancer.gov/statfacts/html/cmyl.html [Last accessed August 8, 2018]
- National Comprehensive Cancer Network Guidelines. Chronic myeloid leukemia. v.3.2018. Fort Washington, PA: NCCN; December 20, 2017
- Jabbour EJ, Hughes TP, Cortés JE, et al. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia. Leuk Lymphoma 2014;55:1451-62
- Jain P, Kantarijian H, Alattar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. Lancet Haematol 2015;2:e118-e128
- Jabbour EJ, Lin J, Siegartel LR, et al. Evaluation of healthcare resource utilization and incremental economic burden of patients with chronic myeloid leukemia after disease progression to blast phase. J Med Econ 2017;20:1007-12
- Haque R, Shi J, Chung J, et al. Medication adherence, molecular monitoring, and clinical outcomes in patients with chronic myelogenous leukemia in a large HMO. J Am Pharm Assoc (2003) 2017;57:303-10e.2
- Guérin A, Chen L, Dea K, et al. Association between regular molecular monitoring and tyrosine kinase inhibitor therapy adherence in chronic myelogenous leukemia in the chronic phase. Curr Med Res Opin 2014;30:1345-52
- Di Bella NJ, Bhowmik D, Bhor M, et al. The effectiveness of tyrosine kinase inhibitors and molecular monitoring patterns in newly diagnosed patients with chronic myeloid leukemia in the community setting. Clin Lymphoma Myeloma Leuk 2015;15:599-605
- Saleh MN, Haislip S, Sharpe J, et al. Assessment of treatment and monitoring patterns and subsequent outcomes among patients with chronic myeloid leukemia treated with imatinib in a community setting. Curr Med Res Opin 2014;30:529-36
- Latremouille-Viau D, Guerin A, Gagnon-Sanschagrin P, et al. Health care resource utilization and costs in patients with chronic myeloid leukemia with better adherence to tyrosine kinase inhibitors and increased molecular monitoring frequency. J Manag Care Spec Pharm 2017;23:214-24
- Goldberg SL, Chen L, Guerin A, et al. Association between molecular monitoring and long-term outcomes in chronic myelogenous leukemia patients treated with first line imatinib. Curr Med Res Opin. 2013;29:1075-82
- Goldberg SL, Cortes JE, Gamacorti-Passerini C, et al. First-line treatment selection and early monitoring patterns in chronic phase-chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol 2017;92:1214-23