Abstract
Aims: This study explored the association between medication adherence to oral atypical antipsychotics (AAP) and both psychiatric hospitalization and associated costs in bipolar I disorder (BD-I) in a real-world setting.
Materials and methods: This retrospective study used the Truven Health MarketScan Medicaid, Commercial, and Medicare Supplemental Claims Databases. Adults were identified if they had BD-I and initiated an AAP treatment during the study identification period (July 1, 2015–June 30, 2016 for Medicaid, July 1, 2015–March 31, 2016 for Commercial and Medicare Supplemental) and had ≥6-month continuous enrollment before (baseline) and after (follow-up) the first day of treatment. Medication adherence was measured by the proportion of days covered (PDC) and grouped as: fully-adherent (PDC ≥80%), partially-adherent (40% ≤ PDC <80%), and non-adherent (PDC <40%). Logistic and linear regression models were conducted to estimate the risk of psychiatric hospitalization and costs during the 6-month follow-up period.
Results: The final sample consisted of 5,892 (32.0%) fully-adherent, 4,246 (23.1%) partially-adherent, and 8,250 (44.9%) non-adherent patients. The adjusted rate of psychiatric hospitalization during the follow-up period was lower in the fully-adherent (6.0%) vs partially- (8.3%) or non-adherent (8.8%) groups (p < 0.001). Using the fully-adherent cohort as the reference group, the odds of psychiatric hospitalization were significantly higher for the partially-adherent (OR = 1.42; 95% CI = 1.23–1.64) and non-adherent (1.51; 1.33–1.71) cohorts. The mean adjusted psychiatric hospitalization cost over 6 months among hospitalized patients was lower for the fully-adherent cohort ($11,748), than the partially-adherent ($15,051 p = 0.002) or non-adherent cohorts ($13,170, not statistically significant).
Limitations: The medication adherence measures relied on prescription claims data, not actual use.
Conclusions: In the treatment of BD-I, better medication adherence to AAP was associated with fewer psychiatric hospitalizations. Among hospitalized patients, fully-adherent patients had statistically significantly lower psychiatric costs than partially-adherent ones. These findings suggest that improving adherence to AAP in BD-I may be a valuable goal from both clinical and economic perspectives.
Introduction
Bipolar disease (BD), a chronic, relapsing mood disorder characterized by episodes of major depression and mania, causes patients to suffer enormouslyCitation1. Compared with other mood and anxiety disorders, patients with BD have a lower level of functioning, more disability, worse productivity, and more absenteeismCitation2,Citation3. The prevalence of BD variesCitation4–6. A survey of over 60,000 adults in 11 countries reported a lifetime prevalence rate of bipolar spectrum of 2.4%Citation5. The prevalence in the US adult population has been reported to be slightly higher, at 2.8%Citation7. Internationally, costs associated with BD are substantialCitation8,Citation9; a systematic review of 22 studies from eight European, North American, and Asian countries found the direct healthcare cost of BD care to range from $2,500–$5,000 per patient per yearCitation10. In the US, the direct cost is more than $46 billion per year, and the indirect cost may be over $146 billionCitation11.
The American Psychiatric Association recommends initiation of a mood stabilizer, in combination with an atypical antipsychotic as first-line pharmacological treatment for acute treatment of severe manic or mixed bipolar episodes and as second-line in patients with milder symptomsCitation12. Antipsychotic medications have been approved for bipolar depression, mania, and mixed symptoms, and are increasingly used either as monotherapy or as adjunctive therapy in treating patients with BD in the USCitation13,Citation14. In other countries, monotherapy with mood stabilizers, atypical antipsychotics, or anticonvulsants tends to be the mainstay of therapy for BD, with combinations utilized in severe episodesCitation4,Citation15–17. In the UK, lithium is utilized less often than antipsychotic and anticonvulsant medicationsCitation18.
Decreasing depression episodes is an important goal of BD treatment, and more episodes predict poor outcomesCitation19,Citation20. Yet adherence to antipsychotic medications in patients with BD has been reported to be less than 60%Citation21–23. In people with mental illness overall, medication non-adherence is associated with more hospitalizationsCitation24,Citation25, violence, arrests, suicide, and with reduced quality-of-lifeCitation26–28. Many prior studies in BD have focused on differences in full adherence (usually meaning 80% or above)Citation21,Citation22,Citation29 among users of specific medicationsCitation22–24, or have not looked at BD specificallyCitation25. We chose to focus exclusively on BD and to examine partially as well as fully adherent patients in order to develop a more complete picture of the relationship between medication use and outcomes. Thus, the aim of the current study was to evaluate psychiatric hospitalization and associated costs for bipolar I disorder (BD-I) patients with different levels of medication adherence to oral atypical antipsychotic (AAP) medications.
Methods
Data source and study design
We conducted a retrospective cohort study using the Truven Health Analytic MarketScan Medicaid, Commercial, and Medicare Supplemental Claims databases to identify patients with BD-I who were newly-treated with an AAP. The Medicaid database includes demographic and clinical information, inpatient and outpatient utilization data, and outpatient prescription data for 40 million Medicaid enrollees from multiple geographically dispersed states. The MarketScan Commercial Database includes medical and pharmacy claims for ∼65 million individuals and their dependents who are covered through employer-sponsored private health insurance plans. The MarketScan Medicare Supplemental Database contains records on ∼5.3 million retired employees and spouses older than 65 years who are enrolled in Medicare with supplemental Medigap insurance paid by their former employers. To ensure complete medical claims histories, in the Medicaid database, patients with Medicare dual-eligibility, with capitated health insurance, and those without mental health coverage were excluded.
The study used medical, pharmacy, and enrollment claims from January 1, 2015 through December 31, 2016 for Medicaid data and January 1, 2015 through September 30, 2016 for Commercial and Medicare Supplemental data. All data were compliant with the Health Insurance Portability and Accountability Act of 1996, and institutional review board approval was not required for this study.
Sample selection
Patients with a diagnosis of BD-I were identified if they had either one inpatient or at least two outpatient medical claims for BD-I (International Classification of Disease–Clinical Modification [ICD-CM]: ICD-9-CM [296.0x, 296.1x, 296.4x–296.8x, excluding 296.82]; ICD-10-CM [F30.x–F31.x, excluding F31.81]) in any diagnosis field of a claim between January 1, 2015 and December 31, 2016 (Medicaid) or January 1, 2015 through September 30, 2016 (Commercial and Medicare Supplemental). Patients must also have had at least one pharmacy claim for any oral AAP (aripiprazole, asenapine, brexpiprazole, cariprazine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone; not all indicated for treatment of BD-I) during the identification period (July 1, 2015 through June 30, 2016 for Medicaid; July 1, 2015 through March 31, 2016 for Commercial and Medicare Supplemental). The first date of oral AAP use was considered the index date. The AAP used on the index date was the index therapy. Patients using more than one antipsychotic medication, including typical antipsychotics and both typical and atypical long-acting injectables (LAIs), on the index date were excluded. LAIs were excluded, as we felt a 6-month period would be inadequate to determine adherence to these medications. To ensure that patients were newly starting the index therapy, we did not allow patients to have any evidence of the index therapy 6 months prior to the index date (baseline period), although use of non-index therapy in the baseline period was allowed. We excluded patients initiating therapy with cariprazine, iloperidone, paliperidone, and clozapine. Together, the first three medications comprised 1.7% of the sample, and, in order to optimize the adjusted analysis, which included index medications as a covariate, those with small sample sizes were excluded. Patients prescribed clozapine were excluded, because this is usually reserved for those who fail to respond adequately to standard antipsychotic treatmentCitation30.
Eligible patients were ≥18 years of age on the index date, had their first diagnosis of BD-I on or before the index date, and fulfilled the requirement of 6 months of continuous enrollment both prior to the index date (baseline period) and after the index date (follow-up period) (). Patients were excluded if they had at least one diagnosis of schizophrenia (ICD-9-CM codes: 295.xx, excluding 295.4x and 295.7x; or ICD-10-CM codes: F20x, excluding F20.81).
Figure 1. Study Timeline for Patients Bipolar I Disorder Treated with Oral Atypical Antipsychotics. Abbreviations. MC, Medicaid; C, Commercial; SUP, Medicare Supplemental.
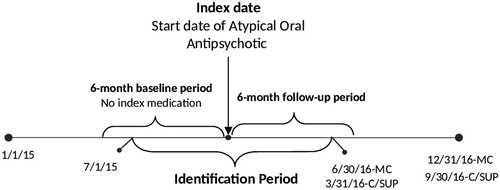
Patients were grouped into three cohorts according to their level of medication adherence to AAP during the 6-month follow-up period, calculated by the proportion of days covered (PDC): (1) fully-adherent (PDC ≥80%), (2) partially-adherent (40% ≤ PDC <80%), and (3) non-adherent (PDC <40%). The 80% threshold for the fully-adherent group is well establishedCitation21,Citation22,Citation29, and some non-mental health studies have used 40% ≤ PDC <80% to define partial adherenceCitation31–33.
Study measures
Baseline measures
Baseline variables potentially related to illness severity were examined using data during the 6-month pre-index period. These included patient demographics (age, gender, and insurance type), Charlson Comorbidity Index (CCI)Citation34,Citation35, number of chronic condition indicatorsCitation36, psychiatric comorbidities (depression, anxiety, personality disorder, and substance abuse disorder), non-index antipsychotic medication use, psychiatric medication use (antidepressants, anti-anxiety medications, sedatives or hypnotics, and mood stabilizers) and non-psychiatric medications (anti-diabetic medications, lipid-lowering medications, and anti-hypertensive medications), and hospitalizations. Race and ethnicity were available only for the minority of patients in the database with Medicaid coverage and were, therefore, not used in this analysis. The Charlson Comorbidity Index predicts the 1-year mortality for patients, incorporating a total of 22 conditions. The scores for each condition range from 1–6, and the summation of these scores represents the final CCICitation34. Unlike our patient identification algorithm (which required one inpatient or two outpatient claims for the target condition), when we identified patients as having psychiatric comorbidities (depression, anxiety, personality disorder, and substance abuse disorder), the presence of a single code during the baseline period for the relevant condition was considered adequate.
Outcome measures
Outcomes of interest comprised psychiatric hospitalization and cost during the 6-month follow-up period. Psychiatric hospitalizations were those with a medical claim with a primary diagnosis of mental illness (ICD-9-CM code: 290.xx–311.xx; ICD-10-CM code: F01.xx–F99.xx). Psychiatric hospitalization costs were calculated for the 6-month follow-up period. All outcomes were compared among study cohorts.
Statistical analysis
Descriptive statistics were performed to assess differences among the three adherence cohorts across all baseline covariates, including means and standard deviations (SD) for continuous variables, and counts and percentages for categorical variables. Chi-square tests were used for categorical variables, and F and Kruskal-Wallis tests were used for continuous variables. Logistic regression was used to examine the likelihood of having a psychiatric hospitalization during the 6-month follow-up period. General linear regression was utilized to estimate the cost of psychiatric hospitalization among patients who were hospitalized during the 6-month follow-up period. Both models were controlled for using baseline covariates, including age, gender, insurance type, CCICitation34,Citation35, number of chronic conditionsCitation36, psychiatric comorbidities, baseline hospitalization, baseline psychiatric and non-psychiatric medication use, and index AAP use. Odds ratios, p-values, and 95% confidence intervals for model covariates were provided. All costs were adjusted to 2016 US dollars using the medical care component of the Consumer Price Index, and all data transformations and statistical analyses were performed using SAS version 9.4 (Cary, NC).
Results
Sample description
Of the 222,498 patients with BD-I identified from the combined dataset (Medicaid, Commercial, and Medicare Supplemental), 18,699 initiated an AAP and met the remaining study criteria. Patients treated with cariprazine (n = 46), iloperidone (n = 46), and paliperidone (n = 219) were excluded due to small sample sizes, leaving 18,388 patients in the study sample. Of those, 44.9% (8,250) patients were non-adherent (PDC <40%), 23.1% (4,246) were partially-adherent (40% ≤ PDC <80%), and 32.0% (5,892) were fully-adherent (PDC ≥80%), during the 6-month follow-up period.
Baseline characteristics
The mean age for the overall sample was 40.3 years, 69.2% of patients were female, 54.9% carried commercial insurance, and 68.3% suffered from at least one psychiatric comorbidity, with anxiety (51.5%) being the most common; 27.8% experienced a baseline hospitalization. Patients at each of the levels of medication adherence differed significantly in age, insurance type, CCI, number of chronic conditions, psychiatric comorbidities, psychiatric and non-psychiatric medication use, and baseline hospitalization (p < 0.05 for all comparisons). The fully-adherent group was older, had the highest percentage of patients being commercially insured, had more chronic conditions, had fewer psychiatric comorbidities and baseline hospitalizations, and included a relatively higher percentage of patients who had taken both psychiatric and non-psychiatric medications (p < 0.05 for all comparisons) ().
Table 1. Demographics and baseline clinical characteristics by PDC levels.a
Medication adherence and psychiatric hospitalization
The group of patients in the fully-adherent cohort had the lowest unadjusted mean psychiatric hospitalization rate [mean (SD) = 0.10 (0.4), p < 0.001] during the 6-month follow-up period. Of the fully-adherent cohort, 7.4% experienced ≥1 psychiatric admission. The partially-adherent and non-adherent cohorts had unadjusted rates of 10.0% and 11.0%, respectively (p < 0.001). The fully adherent cohort also had non-significantly different, although numerically fewer, days hospitalized during follow-up (10.0 days vs 10.9 days for the non-adherent cohort and 11.6 days for the partially-adherent cohort) (p = 0.217) ().
Table 2. Unadjusted results: psychiatric hospitalizations and associated costs by PDC levels.
After adjusting for differences in baseline characteristics, the odds of having any psychiatric hospitalization during the 6-month follow-up period were significantly higher for both the partially-adherent (OR =1.51; 95% CI =1.33–1.71) and non-adherent (1.42; 1.23–1.64) cohorts compared to the fully adherent cohort. Adjusted percentages of psychiatric hospitalizations during the follow-up period ranged from 6.0% (fully-adherent cohort) to 8.8% (non-adherent cohort) ().
Table 3. Results from multivariable analyses: association between PDC levels and psychiatric hospitalization and costs during the follow-up period.
Medication adherence and hospitalization cost
For all patients with BD-I (n = 18,388), the fully-adherent cohort showed the lowest psychiatric hospitalization costs [mean (SD) = $883 (4,807)] compared to the partially-adherent [$1,486 (7,140)] and non-adherent [$1,447 (6,706)] cohorts (p < 0.001). Among those hospitalized (n = 1,767), psychiatric hospitalization costs were lowest for the fully-adherent group [$11,905 (13,440) vs $14,845 (17,651) for the partially-adherent cohort and $13,191 (15,978) for the non-adherent cohort, p < 0.024].
Medication adherence to AAP treatment was a significant predictor of psychiatric hospitalization costs. The partially-adherent cohort incurred $3,303 more costs during the 6-month follow-up period (95% CI =1,226–5,380)] than the fully-adherent cohort. The non-adherent cohort had numerically higher costs compared with the fully-adherent cohort, but the difference was not statistically significant (p = 0.118). The adjusted psychiatric hospitalization costs among those with an admission (n = 1,767) ranged from $11,748 for the fully-adherent cohort to $15,051 for the partially-adherent cohort ().
Discussion
About one-third of our combined sample of Medicaid, Medicare, and commercially insured patients with BD-I were fully adherent (defined as PDC of 80% or more) to their index antipsychotic medication. In these patients, there was a 40–50% reduction in the odds of psychiatric hospitalizations compared to those less adherent. The cost of psychiatric hospitalization was also lower in fully adherent patients, both as a group mean and among those with hospitalization. Fully adherent patients had an adjusted mean cost of $1,400–$3,300 lower than less adherent patients over 6 months. Nearly 10% of patients in all cohorts experienced at least one psychiatric hospitalization within 6 months of initiating antipsychotic therapy, possibly related to suboptimal medication adherence.
Patient, physician, disease, healthcare system, and social/economic factors all play a role in whether patients adhere to treatment recommendationsCitation37, and our study could not shed light on the reasons for non-adherence. We can, however, estimate the magnitude of the impact adherence has on health outcomes. Consistent with prior research on a broad range of conditions, we found that improving adherence can provide a level of benefit that may be nearly as large as those provided by recent advances in pharmacotherapy. Without high levels of medication adherence, these advances will not realize their full potential. Investments in improving medication adherence are often fully repaid with savings in healthcare utilization, or the improvement in health outcomes fully justifies the investmentCitation37. Improving adherence to therapy is not a simple task, and we believe that, given the level of benefit, systematic efforts to improve adherence (many of which are already underway in various health systems), should be encouraged and advanced in conjunction with continued biomedical research in new treatments. Together, these pathways will be able to reduce the suffering of individuals diagnosed with BD far more than either one alone. In BD, simpler interventions specific to medication adherence, instead of complex ones that combined medication adherence with mood management or lifestyle changes, have been found to be most successfulCitation38. Two LAIs are FDA approved for maintenance treatment of BD (risperidone microspheres and aripiprazole monohydrate)Citation36,Citation37, and LAIs are associated with higher medication adherence rates in BDCitation39,Citation40. These medications could be particularly beneficial for patients who intend to be adherent, as opposed to those who refuse medication.
The current study adds to the literature in several ways. First, most prior studies of adherence to atypical antipsychotics have focused on comparisons among various agentsCitation22,Citation24,Citation25,Citation41 or have studied a combination of psychiatric illnesses, rather than focusing on BD-I aloneCitation25. Second, this study combines three real-world data sources, unlike others that examined MedicaidCitation22 or commercial claims aloneCitation23–25,Citation41. Third, we stratified medication adherence into three categories: non-adherent, partially-adherent, and fully-adherent, rather than focusing on the dichotomy of adherent vs non-adherent. When compared to the fully-adherent cohort, both the partially-adherent and non-adherent cohorts had 1.42 and 1.51 higher odds of a psychiatric hospitalization, respectively. There is clearly value in improving the adherence of partially-adherent patients.
Our findings are largely consistent with prior research. Estimates of full adherence to antipsychotic medication in this population have ranged from 15–58% of patientsCitation21–23. Psychiatric hospitalization rates in this study ranged from 7.4% in the fully adherent cohort to 11.0% in the non-adherent cohort (6.0% and 8.8% after adjustment), consistent with prior studies.
Hospitalization is a significant cost driver, which is confirmed in this studyCitation11,Citation42. Other covariates associated with higher cost in this study included age (55+ vs 35–44 years), being commercially insured (vs Medicaid), having a higher number of chronic conditions, having experienced a baseline hospitalization, and being partially adherent to antipsychotic medication (vs fully adherent). Other than the type of insurance and age, which are not considered to be modifiable risk factors, being partially adherent was associated with the highest increase in cost. There were 425 patients in the partially-adherent cohort with at least one psychiatric hospitalization during the follow-up period. If each of these patients became fully adherent, our estimated $3,303 cost savings per patient (statistically significant difference between the partially and fully adherent cohorts) would amount to total savings of ∼ $1.4M on psychiatric inpatient costs within a 6-month period.
This study had several limitations. First, the administrative claims data used to identify patients and outcomes are not clinically detailed and are primarily designed for reimbursement, not research. They lack measures of disease severity, and key variables may be absent, miscoded, or under-reported. For example, the number of patients with claims for suicide or suicide attempt during the 6-month follow-up period was 0.3%, much lower than would be expected. We did, however, use a variety of measures to adjust for severity, including baseline non-psychiatric comorbidities, psychiatric comorbidities, medication usage and hospitalizations, but all are based on insurance claims, rather than being clinically derived. Second, the follow-up period was only 6 months, and we plan to extend the follow-up period in a future study. Third, we found that the psychiatric hospitalization cost for the non-adherent cohort was not significantly different from the cost for the fully adherent cohort. The explanation for this finding is unclear. It may be that non-adherent patients avoid hospitalization because they are unable to access the healthcare system, are treated in the correctional system, or have moved to a different state, which may mitigate costs, or at least our ability to identify these costs. There is likely unmeasured between-group variance in our study, and these unmeasured confounders could also explain the finding. Fourth, adherence measures used prescription claims data, not actual use. We could not account for medication samples, which may have been provided differentially for different drugsCitation43. Most experts recommend using objective measures such as pill counts, pharmacy records, serum levels, combined with self-report to help improve accuracy; we were unable to supplement claims with any other data due to privacy restrictionsCitation29. Fifth, although our definition of partial adherence had been used previously, it has not been used in the context of BDCitation31–33. We did not account for medication co-payments, which may affect medication adherence (although perhaps less in mental illness)Citation44, as we were studying the relationship between adherence and outcomes, rather than determinants of adherence.
Conclusions
In a mixed population of Medicaid, Medicare, and commercially insured patients with BD-I who initiated treatment with an atypical antipsychotic, high levels of medication adherence to an index antipsychotic treatment are associated with lower psychiatric hospitalization and associated costs. While retrospective studies cannot establish causality, our findings suggest that patients with BD-I taking atypical antipsychotics may benefit from the implementation of programs and/or interventions to encourage high levels of adherence.
Transparency
Declaration of funding
This research was supported by Otsuka Pharmaceutical Development and Commercialization Inc. and Lundbeck.
Declaration of financial/other interests
MG is an employee of Otsuka Pharmaceutical Development and Commercialization Inc., Princeton, NJ. TY, MSB, EC, and IY are employees of Partnership for Health Analytic Research, LLC, Beverly Hills, CA. AH is an employee of Lundbeck, Deerfield, IL. Peer reviewers on this manuscript have received an honorarium from JME for their review work. One reviewer discloses consulting for Acadia, Alkermes, Allergan, Indivior, Intra-Cellular Therapeutics, Janssen, Lundbeck, Merck, Neurocrine, Noven, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva, and Vanda in the past 12 months. The remaining reviewers have no other relevant financial relationships to disclose.
Data availability statement
The data that support the findings of this study are available from Truven Health Analytics. Restrictions apply to the availability of these data, which were used under license for this study. Data are available with the permission of IBM Health.
Acknowledgments
No assistance in the preparation of this article is to be declared. This study was presented at the AMCP Managed Care & Specialty Pharmacy Meeting, April 23–26, 2018, Boston, MA.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association; 2013
- Shippee ND, Shah ND, Williams MD, et al. Differences in demographic composition and in work, social, and functional limitations among the populations with unipolar depression and bipolar disorder: results from a nationally representative sample. Health Qual Life Outcomes 2011;9:90
- Pini S, de Queiroz V, Pagnin D, et al. Prevalence and burden of bipolar disorders in European countries. Eur Neuropsychopharmacol 2005;15:425–34
- Shah N, Grover S, Rao Gp. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry 2017;59:51
- Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch Gen Psychiatry 2007;64:543
- Esan O, Esan A. Epidemiology and burden of bipolar disorder in Africa: a systematic review of data from Africa. Soc Psychiatry Psychiatr Epidemiol 2016;51:93–100
- The National Institute of Mental Health. Bipolar Disorder [Internet]. Bethesda, MD; 2017. Available at: https://www.nimh.nih.gov/health/statistics/bipolar-disorder.shtml [Last accessed January 26, 2017]
- Fagiolini A, Forgione R, Maccari M, et al. Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord 2013;148:161–9
- Young AH, Rigney U, Shaw S, et al. Annual cost of managing bipolar disorder to the UK healthcare system. J Affect Disord 2011;133:450–6
- Kleine-Budde K, Touil E, Moock J, et al. Cost of illness for bipolar disorder: a systematic review of the economic burden. Bipolar Disord 2014;16:337–53
- Cloutier M, Greene M, Guerin A, et al. The economic burden of bipolar I disorder in the United States in 2015. J Affect Disord 2017;226:45–51
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:1–50
- López-Muñoz F, Shen WW, D’Ocon P, et al. A history of the pharmacological treatment of bipolar disorder. Int J Mol Sci 2018;19:2143
- Taylor DM, Cornelius V, Smith L, et al. Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple-treatments meta-analysis. Acta Psychiatr Scand 2014;130:452–69
- Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2016;30:495–53
- Malhi GS, Outhred T, Morris G, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Med J Aust 2018;208:219–25
- Malhi GS, Gessler D, Outhred T. The use of lithium for the treatment of bipolar disorder: recommendations from clinical practice guidelines. J Affect Disord 2017;217:266–80
- National Institute for Health and Care Excellence. Bipolar disorder: the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care [Internet]. Manchester: National Institute for Health and Care Excellence; 2014. Available at: https://www.nice.org.uk/guidance/cg185 [Last accessed September 18, 2018]
- Rosa AR, Reinares M, Michalak EE, et al. Functional impairment and disability across mood states in bipolar disorder. Value Health 2010;13:984–8
- Kora K, Saylan M, Akkaya C, et al. Predictive factors for time to remission and recurrence in patients treated for acute mania: health outcomes of manic episodes (HOME) study. Prim Care Companion J Clin Psychiatry 2008;10:114–19
- Berger A, Edelsberg J, Sanders KN, et al. Medication adherence and utilization in patients with schizophrenia or bipolar disorder receiving aripiprazole, quetiapine, or ziprasidone at hospital discharge: a retrospective cohort study. BMC Psychiatry 2012;12:99
- Rascati KL, Richards KM, Ott CA, et al. Adherence, persistence of use, and costs associated with second-generation antipsychotics for bipolar disorder. Psychiatr Serv 2011;62:1032–40
- Sajatovic M, Ng-Mak D, Solem CT, et al. Dosing patterns and medication adherence in bipolar disorder patients treated with lurasidone: a US retrospective claims database analysis. Ther Adv Psychopharmacol 2016;6:355–68
- Gianfrancesco FD, Sajatovic M, Rajagopalan K, et al. Antipsychotic treatment adherence and associated mental health care use among individuals with bipolar disorder. Clin Ther 2008;30:1358–74
- Jiang Y, Ni W. Estimating the impact of sdherence to and persistence with atypical antipsychotic therapy on health care costs and risk of hospitalization. Pharmacotherapy 2015;35:813–22
- Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry 2006;67:453–60
- Novick D, Haro JM, Suarez D, et al. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res 2010;176:109–13
- Hong J, Reed C, Novick D, et al. Clinical and economic consequences of medication non-adherence in the treatment of patients with a manic/mixed episode of bipolar disorder: results from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM) study. Psychiatry Res 2011;190:110–14
- Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 2009;70(Suppl 4):1–46; quiz 47–8
- Hui Poon S, Sim K, Baldessarini RJ. Pharmacological approaches for treatment-resistant bipolar disorder. Curr Neuropharmacol 2015;13:592–604
- Wei L, Wang J, Thompson P, et al. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart 2002;88:229–33
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007;297:177–86
- Choudhry NK, Glynn RJ, Avorn J, et al. Untangling the relationship between medication adherence and post–myocardial infarction outcomes. Am Heart J 2014;167:51–58.e5
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–19
- Agency for Healthcare Research and Quality. HCUP chronic condition indicator [Internet]. Rockville, MD: Healthcare Cost and Utilization Project (HCUP); 2015. Available at: www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp [Last accessed November 30, 2017]
- World Health Organization. Adherence to long-term therapies: evidence for action [Internet]. Geneva: World Health Organization; 2003. Available at: http://books.google.com/books?hl=en&lr=&id=kcYUTH8rPiwC&oi=fnd&pg=PR5&dq=%22VI+%E2%80%93+How+can+improved+adherence+be+translated+into%22+%22XIII+%E2%80%93%22+%22III+%E2%80%93+Disease-specific%22+%22VIII+%E2%80%93+Cancer+(palliative%22+%22XI+%E2%80%93%22+%22X+%E2%80%93%22+%22VII+%E2%80%93%22+%22XII+%E2%80%93%22+&ots = tz5Kir5cv_&sig = c5FxwOS1GP1PLX8P5yKo_CJzhyA [Last accessed March 5, 2013]
- MacDonald L, Chapman S, Syrett M, et al. Improving medication adherence in bipolar disorder: a systematic review and meta-analysis of 30 years of intervention trials. J Affect Disord 2016;194:202–21
- Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ 2018;21:127–34
- Yan T, Greene M, Chang E, et al. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther 2018;35:1612–1625
- Zhu B, Kulkarni PM, Stensland MD, et al. Medication patterns and costs associated with olanzapine and other atypical antipsychotics in the treatment of bipolar disorder. Curr Med Res Opin 2007;23:2805–14
- Bergeson JG, Kalsekar I, Jing Y, et al. Medical care costs and hospitalization in patients with bipolar disorder treated with atypical antipsychotics. Am Health Drug Benefits 2012;5:379–86
- Brown JD, Doshi PA, Talbert JC. Utilization of free medication samples in the United States in a nationally representative sample: 2009–2013. Res Social Adm Pharm 2017;13:193–200
- Doshi JA, Li P, Desai S, et al. Impact of Medicaid prescription copayments on use of antipsychotics and other medications in patients with schizophrenia. J Med Econ 2017;20:1252–60
