?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Alemtuzumab and natalizumab are approved as second-line therapies for relapsing-remitting multiple sclerosis (RRMS) patients in Iran who have shown an inadequate response to other disease-modifying therapy (DMT). In the absence of head-to-head trials, evaluations based on decision analytic modeling may be a suitable alternative to compare alemtuzumab and natalizumab in RRMS.
Purpose: To evaluate the cost-effectiveness of alemtuzumab compared with natalizumab in RRMS in Iran, based on an indirect comparison of clinical trial data.
Methods: A cost-utility analysis was conducted using a cohort-based Markov model to analyze cost-utility in a cohort of 1,000 RRMS patients treated with alemtuzumab or natalizumab who had failed at least one previous DMT. Costs were measured in 2018 US Dollars, and were estimated from both the societal and National Healthcare Service (NHS) perspective over a 20-year time horizon in Iran. One-way deterministic sensitivity analyses were carried out to investigate the impact of individual variables on model results.
Results: Alemtuzumab dominated natalizumab in both NHS and societal perspective analyses. From the NHS perspective, the total discounted costs per patient were estimated at $147,417 and $150,579 for alemtuzumab and natalizumab, respectively, over 20 years. The discounted quality-adjusted life years were estimated to be 7.07 and 6.05, respectively. Results were similar for the societal perspective analysis. Results were most sensitive to acquisition costs and the time horizon, while no sensitivity was observed for Expanded Disability Status Scale (EDSS) health-states utility, relapse relative risk, adverse event or EDSS-related costs, and laboratory/monitoring costs.
Conclusion: Alemtuzumab was dominant in the treatment of RRMS compared with natalizumab due to lower total cost, greater efficacy and slowing of disease progression, and lower rate of relapses over a 20-year time horizon in Iran. Comparative head-to-head trials and long-term follow-up are needed to confirm these results.
Introduction
Multiple sclerosis (MS) is a chronic disease involving both autoimmune and neurodegenerative mechanisms that causes physical and cognitive disability and thereby negatively impact a patient’s quality-of-life (QoL). An estimated 2.5 million people worldwide have MS, but prevalence ranges from 5–189 per 100,000 across countries and geographical regionsCitation1,Citation2. MS is one of the most widespread disabling neurological disorders of young adults, with an onset during the most productive years of life (ages 15–45). The most common type of MS is relapsing-remitting MS (RRMS), in which patients experience intermittent episodes of new or increased neurological symptoms (relapses) which are separated by periods of full or partial recoveryCitation3,Citation4, but which ultimately lead to increased disability.
According to published studies, MS results in a high economic burden, with indirect costs greatly exceeding direct costs; productivity losses were the main contributor to indirect costsCitation5,Citation6. Indeed, MS poses a substantial economic challenge for public health systems, notably in middle-income countriesCitation7. Based on the Kurtzke classificationCitation2, Iran has a relatively high incidence of MS, at 5.7/100,000, with a prevalence of 54.5 per 100,000 in 2013Citation8, Citation9. The annual direct and indirect costs per patient with MS in Iran ranged from $27,095 to $31,662 in 2012Citation10,Citation11.
Various immunomodulatory medications, including the interferons beta, glatiramer acetate, dimethyl fumarate, and teriflunomide, have been approved as first-line disease-modifying therapies (DMTs) in Iran for the treatment of RRMS12. Despite the relatively high cost of these drugs, their ability to reduce relapses, slow disease progression, and improve QoL may reduce disease-associated resource utilization and, therefore, offset the initial cost of therapyCitation13,Citation14. Subsequent approval in Iran of the monoclonal antibody therapies natalizumab (NTZ) and alemtuzumab (ALM) has changed the treatment landscape as they offer physicians and patients a higher efficacy option, albeit with an increased risk profile compared to platform injectable and oral therapies.
NTZ binds to the α4β1 and α4β7 integrins on the surface of leucocytes, and blocks the interaction of the α4-integrin sub-unit of α4β1 with VCAM-1 and of α4β7 with mucosal MAdCAM-1, preventing translocation of α4β1-expressing immune cells across the blood–brain barrier and into the brainCitation15. ALM binds to CD52, a glycoprotein expressed primarily on mature T- and B-cells in the peripheral circulation, thereby targeting them for depletion. Following depletion, a distinct pattern of repopulation begins within weeks, including a relative increase of regulatory T-cells and a decrease in pro-inflammatory cytokines; these pharmacological effects potentially lead to a rebalancing of the immune systemCitation16.
Both ALM and NTZ have been approved in Iran as second-line therapy for the treatment of RRMS patients who have shown an inadequate response to other DMTsCitation12,Citation17. Given increasing constraints on healthcare resources and cost, there is a need to assess the relative value of high efficacy therapies for RRMS. In addition, decision-makers in middle-income countries also need to manage certain therapeutic categories, including MS, in an attempt to control costs and give access to new, often more expensive, therapies within limited healthcare budgetsCitation18. For countries with limited resources, economic evaluations based on decision analytic modeling may be a suitable alternative to extensive trial-based economic evaluationsCitation19.
Here we report an evaluation of the cost-effectiveness of ALM in comparison to NTZ for the treatment of RRMS, based on an indirect comparison of the clinical trial data of these therapies. The analysis focuses on both the National Healthcare System (NHS) in Iran and the wider societal perspective.
Methods
Overview and model structure
A cohort-based Markov model was developed using TreeAge Pro 2018 software to analyze cost-utility in a cohort of 1,000 patients with RRMS treated with ALM or NTZ, who had failed at least one previous treatment with another DMT. The measurement of effectiveness was based on Quality-adjusted life years (QALYs), which capture health state utilities associated with good health and disutilities associated with relapses, disability worsening, and adverse events (AEs). The costs were measured in 2018 US Dollars (US$). The specified outcome of the model was the incremental cost-effectiveness ratio (ICER) that was determined by comparing each of the treatment strategies according to the following formula (Cost of ALM – Cost of NTZ)/(Effectiveness of ALM – Effectiveness NTZ). The resulting ICER described the relative cost of one additional QALY (cost per QALY gained).
The Markov model was developed based on natural history of MS, comprising 11 health states representing disability status according to the EDSSCitation20 and a single state for death from all causes (). The baseline transition probabilities between EDSS scores were based on the British Columbia MS database reported in Palace et al.Citation21, and representing likelihood of disability worsening and relapse status of RRMS patients receiving best supportive care (BSC) (). RRMS patients might progress to secondary progressive MS (SPMS) over their lifetime; therefore, SPMS states were also included in the model. The transition probabilities for RRMS to SPMS were based on information from the London Ontario data setCitation21,Citation22. The transition probabilities within SPMS were also based on RRMS data reported in Palace et al.Citation21, assuming no possibility of improvement in EDSS score (). The models were adapted from previously published cost-effectiveness studies of MS treatmentsCitation23–28.
Figure 1. Schematic representation of the Markov model. EDSS, expanded disability status scale; RRMS, relapsing remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
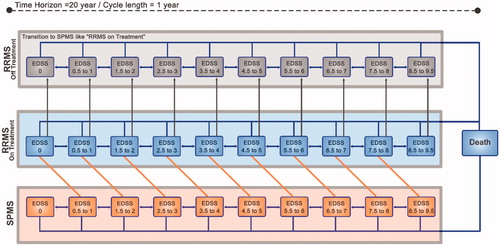
Table 1. Annual transition probability of moving between EDSS states for patients with RRMS or SPMS (age ≥28 years).
Demographics and initial EDSS score distribution were derived by pooling patients from the phase 3 CARE-MS I (NCT00530348)Citation29 and CARE-MS II (NCT00548405)30 studies (age =34 years, disease duration =4 years).
The model was run utilizing an annualized cycle period over a time horizon of 20 years from NHS and societal perspectives. In each cycle, patients could remain in the same EDSS state, progress to a higher EDSS state, regress to a lower EDSS state, transition to SPMS, or die. In SPMS, EDSS scores could increase or remain the same, but were assumed not to decreaseCitation31. Patients were also at risk of one or more acute relapse events during each cycle.
The probability of relapse was based on findings in Patzold and PocklingtonCitation32, who stated that the relapse rate was dependent neither on the severity of the disease, nor on the progression of the illness. However, based on Ontario cohort data, the mean relapse rate for patients in EDSS 0–2 is different compared to EDSS 3Citation33. The annualized relapse rate in different EDSS states was estimated using the following formulaCitation34:
Costs were estimated from both the societal and NHS perspective separately, and were discounted at 7.2%Citation35. QALYs were discounted 3% per annumCitation36. Furthermore, the willingness-to-pay (WTP) thresholds were set to $5,200 and $15,600 per QALY, which represent ∼1- and 3-times the Iranian gross domestic product (GDP) per capita in 2016, respectively, based on the World Health Organization (WHO) recommendation for developing countries (ICER <1 GDP/capita is considered very cost-effective, 1 GDP/capita < ICER <3 GDP/capita is cost-effective, and ICER >3 GDP/capita is not cost-effective)Citation37,Citation38. The model utilized a half-cycle correction.
Clinical inputs
Although the efficacy of NTZ was reported vs placebo in its published randomized clinical trial (RCT) (AFFIRM)Citation39, the clinical trials of ALM (CAMMS223, CARE-MS I, and CARE-MS II) reported the efficacy of ALM vs SC IFN β-1aCitation29,Citation30,Citation40. As there is no reported head-to-head RCT comparing the efficacy of ALM and NTZ, the efficacy inputs for NTZ and ALM vs placebo were obtained from published network meta-analysis. Although there are several published network meta-analyses comparing NTZ and ALM vs placebo indirectly with different results ()31,Citation41–43, it has been noted that Fogarty et al.Citation42 is the most reliable, and was selected to be used in the base case scenario. The other studies were investigated in scenario analysis. In addition, there is a retrospective cohort study published in 2017 in which the authors concluded that alemtuzumab and natalizumab were equally effective in reducing relapse frequency and preventing confirmed disability accumulation over 4 years of follow-up44. This assumption was also investigated in sensitivity analysis.
Table 2. The result of network meta-analysis estimating ALM and NTZ effectiveness vs placebo.
To estimate the treatment-adjusted probabilities, the natural history transition probability matrix was combined with data on the comparative efficacy of treatment vs placebo. Following previous NICE appraisals, treatment effects were assumed to wane over time, with 100% of effect maintained in years 0–5, 75% of effect in years 6–9, and 50% of effect from year 10 onwardsCitation45, assuming no waning effect was explored in sensitivity analysis.
The projected disability worsening and relapse manifestations of RRMS patients while receiving ALM or NTZ (i.e. “RRMS On Treatment”) were modeled in the following way:
After discontinuing ALM or NTZ due to serious adverse events or lack of efficacy, patients were assumed to no longer receive any DMTs and to transition between EDSS states based on the natural history data (i.e. “RRMS Off Treatment”).
Transition from RRMS to SPMS was assumed to be dependent only on the EDSS scores in RRMS, and did not vary with treatment received. Patients transitioning from RRMS to SPMS were assumed to progress by one EDSS point, with the exception that EDSS score 9 in RRMS transitioned to EDSS score 9 in SPMS.
Health states utilities
EDSS-related utilities in RRMS were obtained from Orme et al.Citation46. The SPMS utility decrement, compared to RRMS in the same EDSS score, was 0.04546. Disutility for a non-hospitalized relapse was 0.07, and disutility for a hospitalized relapse was 0.2446,Citation47. It was assumed that the average length of mild/moderate relapse and severe relapse would be 45 and 90 days, respectivelyCitation34,Citation47. EDSS-related utility loss for caregivers was also included, based on Acaster et al.Citation48 For patients who experience relapses, 18.7% were assumed to be severe, with the remainder being mild/moderate in severityCitation49. We assumed a disutility of 0.4 (0.3–0.5) assigned to the year a patient experienced progressive multifocal leukoencephalopathy (PML)Citation50,Citation51. Specific health-state utility weights are presented in .
Table 3. Specific health-state utility weights.
Due to lack of data in the literature, we used adjusted infusion-associated utility for the treatment of patients with bone metastases reported by Matza et al.Citation52 The disutility of ALM (1st year), ALM (other years), and NTZ administration were assumed to be 0.018, 0.011, and 0.016 per year, respectively.
Costs
The model accounted for all direct medical costs, including drug acquisition costs, EDSS state-related costs, hospitalization, AEs, rehabilitation, and other direct medical costs from an NHS perspective (). The cost of lost productivity, morbidity, mortality, and direct non-medical costs such as transportation, wheelchair, and investments to adapt modifications to the house or car were included in the societal perspective. Data on laboratory tests, physicians’ visits, hospitalization, and rehabilitation costs were derived from the official list prices, national tariff book, and the literatureCitation19,Citation53,Citation54. Additionally, the costs of common prescriptions such as anti-depressants, anti-spastics, anti-convulsants, and NSAIDs were considered in the model. The calculation of costs was based on current clinical practice in Iran or published MS cost-of-care studiesCitation19. All costs were reported in 2018 US dollars (exchange rate: $1 = 42,000 Iranian Rial).
Table 4. Summary of base case cost input parameters.
NTZ-related costs
The NTZ administration protocol is 300 mg intravenously (IV) once every 4 weeks. Therefore, the annual cost of NTZ was calculated as the drug costs (13 packages annually), the costs for IV administration at a neurology clinic, supervision by a healthcare provider, laboratory tests, and MRI scan for PML monitoring (prior to initiating therapy, during treatment, every 6 months, and for 6 months after discontinuation).
ALM-related costs
The ALM administration protocol is 12 mg/day IV for 5 consecutive days at baseline and for 3 consecutive days 12 months laterCitation30. Following the initial two courses of ALM, patients could receive additional ALM treatment as needed (12 mg/day on 3 consecutive days ≥1 year after the most recent course), based on either relapses or MRI activity. The rates of ALM re-treatment was assumed to be 40.2%, 11.4%, and 1.5% in years 3, 4, and 5, respectively, according to long-term follow-up studiesCitation55–59.
The annual cost of ALM was calculated as the drug cost, IV administration at a neurology clinic and supervision by a healthcare provider, premedication with methylprednisolone (1,000 mg for 3 days), and prophylactic acyclovir until 28 days after infusion (a part of the treatment protocol to reduce herpes infection risk)Citation60. Additionally, the cost of laboratory tests included CBC, SrCr, and UA during treatment and for the following 48 months after the last treatment course; Quarterly TSH, ECG prior to each course, annual HPV screening, and annual skin exams during treatment were also consideredCitation60.
Relapse-related costs
The relapse-related costs were derived from a cost-of-care study in Iran and patient records archived in hospitalsCitation19.
Other direct medical costs
Neurologist visits were considered at 2- and 4-times per year for EDSS <6 and EDSS >6, respectively. Psychiatrist visits were considered twice per year for all health states. Twice weekly rehabilitation cost was considered for EDSS >6.
Direct non-medical cost
The costs of direct non-medical resources, including walking sticks, wheelchairs, house and car modifications, in-house nurse service, and transportation were derived from a previous study in IranCitation19. Home modification costs and car adaptation costs were considered once for each patient upon EDSS >6. In-home nurse service costs were assumed to be higher in EDSS 9–9.5 than in EDSS 6–7.5.
Worker productivity
Monthly working activity losses were assumed to be 10.9 and 14.4 days per patient for EDSS <3.0 and EDSS 3.0–5.5 respectively, based on Orlewska et al.Citation5, and were multiplied by a mean daily wage in IranCitation61. It was assumed that only 52.1% of patients are employed, based on a study by Bell et al.Citation7, and patients with EDSS >6 were assumed to be unemployed. In addition, the number of working days missed due to ALM and NTZ administration were taken into account.
Adverse events
Only the cost of serious AEs of ALM and NTZ were included in this model; these included PML for NTZCitation62,Citation63 and immune thrombocytopenia (ITP) and autoimmune thyroid disorders for ALMCitation64–68. Costs associated with autoimmune glomerular nephropathy were not considered, as the reported incidence is rare (clinical trial incidence: 0.4%; post-marketing frequency: 0.13%)Citation69.
Progressive multifocal leukoencephalopathy
PML is an infection of the CNS caused by the John Cunningham virus (JCV) that results in damage to the brain at multiple locations, and usually leads to severe disability or deathCitation70. The estimated overall risk of PML with NTZ therapy has been reported to be ∼4.1/1,000 patientsCitation71. Anti-JCV antibody status, prior immunosuppressant treatment, and duration of NTZ exposure are risk factors for NTZ-associated PMLCitation72,Citation73. The incidence of PML was included in the model as a dynamic probability from ∼0.13:1,000 to ∼23:1,000, depending on risk factors (such as cycle number) based on published NTZ-associated PML risk stratification studies in MS patientsCitation72,Citation74,Citation75 that are presented in . The probabilities were adjusted per reported JCV seroprevalence (48.7%) among MS patients in the Middle EastCitation76.
Table 5. Calculated risk of NTZ-related PML according to risk factors.
The cost of PML was considered within the model, including diagnosis (a gadolinium-enhanced MRI scan and a cerebrospinal fluid analysis for JCV) and treatment. There is no consensus on how to treat PML; the most common approach is to restore the host adaptive immune responseCitation77. However, based upon limited observational data found in the literature, a course of plasma exchange every other day for a total of five treatments, accompanied by oral mirtazapine for 3–5 weeks, is recommendedCitation78. The survival rate in MS patients who developed NTZ-associated PML is more than 80% at 1 year after PML diagnosis, but almost all survivors have moderate-to-severe disabilityCitation79,Citation80. Thus, the mortality rate of 20% for patients who develop PML was included in the model. NTZ therapy will also be discontinued and patients will be switched to “RRMS Off Treatment”.
Immune thrombocytopenia and thyroid disorders
ITP has been observed in ∼2% of patients treated with ALMCitation57. Glucocorticoids and intravenous immune globulin (IVIG) are used as first-line therapy for the initial treatment of ITPCitation81. The cumulative proportion of patients experiencing autoimmune thyroid disorders over a follow-up of 5 years was 39%, with the most common types comprising Graves’ hyperthyroidism (65.8%), hypothyroidism (20.5%), and sub-acute thyroiditis (12.3%)Citation82. Therefore, an average cost of conventional therapies of thyroid disorders with anti-thyroid drugs, radioactive iodine, thyroid hormone, or surgery based on the proportion of different observed types of thyroid disorders were calculated and used in the model.
Discontinuation of treatments
Discontinuation rates of ALM and NTZ were based on the CARE MS II and AFFIRM long-term follow-up studiesCitation57,Citation83. The annual AE-related discontinuation probability for NTZ was 5%, and that for ALM was 2.1%, 1.2%, 0.2%, 0.8%, and 0.5% in years 1–5, respectively, and were applied while patients were in EDSS scores of 0–6 in “RRMS On treatment”. Patients were forced to discontinue treatment once they reached an EDSS score ≥7 in RRMS or transitioned to SPMS and switched to best supportive care (efficacy similar to placebo)Citation31.
Mortality
Background mortality rates were based on age-sex specific Iran life tablesCitation84, and were adjusted for MS-specific mortality using an EDSS-specific mortality multiplier calculated from PokorskiCitation85 using the following equation: Multiplier =0.0219*EDSS3-0.1972*EDSS2 + 0.6069*EDSS +1
Sensitivity analysis
One-way deterministic sensitivity analyses were carried out to investigate the impact of individual variables on the results of the model. Variables were changed over a credible range of probabilities, parameters, and assumptions extracted from the literature or an assumed variation of ±25% around the base case. Parameters analyzed included drug costs, relative risk reductions of ALM as well as NTZ on relapse and disease progression rates; withdrawal rate of ALM, as well as NTZ, EDSS-states distribution, PML incidences, PML mortality, discount rates, and model time horizon. Sensitivity results were plotted on a Tornado diagram by ranking parameters from most sensitive to least sensitive.
Furthermore, in order to assess the joint uncertainty of all parameters concurrently, a probabilistic sensitivity analysis (PSA) was carried out by running 1,000 iterations based on a second order Monte-Carlo simulation. As a consequence, a cost-effectiveness acceptability curve was determined over a range of willingness-to-pay (WTP) thresholds, from $0–$16,500 per QALY. The disease progression hazard ratios, utilities, and healthcare costs were assumed to have log-normal, beta, and gamma distributions, respectivelyCitation86. Relapse rate relative risk, age, and average salary were assumed to be normally distributed.
To investigate the stability of the base case results, scenario analyses was conducted to test the robustness of the model to variations in underlying model assumptions and to the use of alternative input parameters (e.g. different time horizons, different Hazard ratios (network meta-analysis), and no waning effect).
Results
Base-case analysis
ALM dominated NTZ in both NHS and societal perspective analyses. From the NHS perspective, the total discounted costs per patient were estimated at $147,417 and $150,579 for ALM and NTZ, respectively, over the 20-year time horizon (). Drug acquisition costs were the largest component of total costs (∼ 97% and 95% for ALM and NTZ, respectively). The discounted QALYs were estimated to be 7.07 and 6.51, respectively. ALM and NTZ cumulative costs and effectiveness during 20 cycles from the NHS perspective are presented in .
Figure 2. ALM and NTZ cumulative costs and effectiveness (NHS perspective). ALM, alemtuzumab; NTZ, natalizumab; NHS, national health system; QALYs, quality adjusted life years.
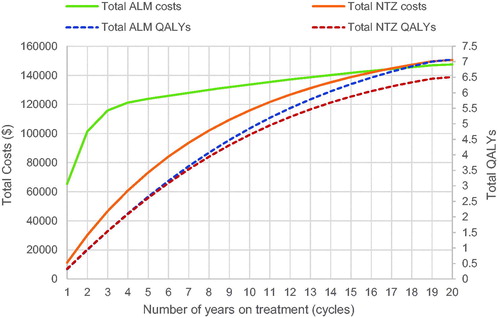
Table 6. Base-case results and sensitivity analyses of the effect of key input parameters on ICER.
From a societal perspective, costs were estimated at $163,044 and $166,767 and QALYs were estimated to be 6.40 and 5.77, respectively. In both perspectives, NTZ was associated with a higher cost due to more progression and productivity loss, as well as EDSS-related non-medical costs. Drug acquisition costs were also the largest component of total cost in the societal context. The productivity loss and direct non-medical costs accounted for only 11.5% and 12.2% of the total costs in the societal perspective, respectively.
One-way sensitivity analyses
Tornado diagrams, plotted using the results of one-way sensitivity analysis of changes in the 20 most sensitive parameters on the ICER, are shown in .
Figure 3. Results for one-way sensitivity analysis. (a) Effect of parameter variation on the incremental cost per QALY in the NHS perspective. (b) Effect of parameter variation on the incremental cost per QALY in the societal perspective. ALM, alemtuzumab; NTZ, natalizumab; NHS, national health system; QALYs, quality adjusted life years.
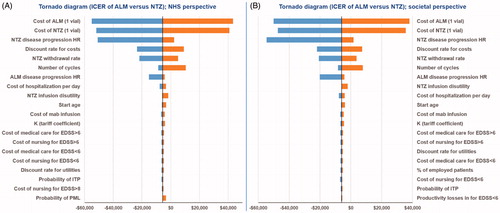
Results were most sensitive to both ALM and NTZ acquisition costs and the time horizon used in the model. Further evaluation showed that changes in withdrawal rate, relapse, and infusion-associated disutility values, state distributions, and discount rates had minimal effect on the results. Moreover, no sensitivity was observed for EDSS health-states utility, start age, relapse rate relative risk, AE-related costs, EDSS-related costs, PML incidence, and laboratory and monitoring costs. Changes in other parameters had no impact on the results. Results for the sensitivity analyses are presented in .
As mentioned, the model was most sensitive to variation in the annual costs for ALM and NTZ acquisition. ALM ceased to be cost-effective if the price of ALM (1 vial) exceeded $14,200, while a decrease in NTZ price to less than $1,218 per vial made NTZ more cost-effective. As expected, decreases in time horizon (≤ 15 years) also altered the cost-effectiveness results. Our sensitivity analysis showed that ALM would not be cost-effective if the retreatment rates increased to more than 76.5% in years 3–5.
Probabilistic sensitivity analysis (PSA)
The PSA resulted in a mean cost for ALM of $147,387 (95% CI = ±$936) and for NTZ of $150,624 (95% CI = ±$4,523) for the NHS perspective. Considering the societal perspective, the PSA results showed a mean cost of $163,116 for ALM (95% CI = ±$3,313) and $167,148 for NTZ (95% CI = ±$5,898). Effectiveness was also robust, with the PSA resulting in 7.07 QALYs for ALM (95% CI = ±0.42) and 6.50 for NTZ (95% CI = ±0.38) for the NHS perspective as well as 6.41 (95% CI = ±0.51) and 5.80 (95% CI = ±0.44), respectively, for the societal perspective. Other results from the PSA found that, in most of the simulations, ALM was dominant compared to NTZ (less costly and more effective) in both the NHS and societal perspectives (64.7% and 68.3%, respectively). The results of Monte Carlo simulation regarding the NHS and societal perspectives are shown in , as an incremental cost-effectiveness scatter plot.
Figure 4. Incremental cost-effectiveness scatter plot for ALM vs NTZ with a willingness-to-pay line at 1 and 3 GDP/capita.
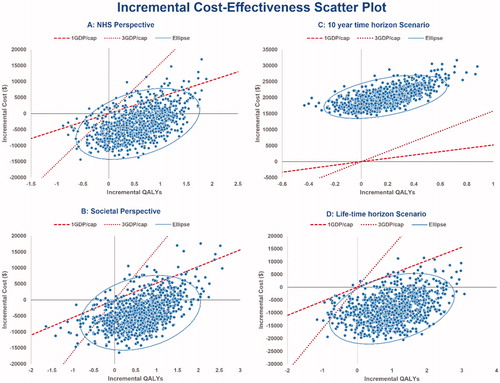
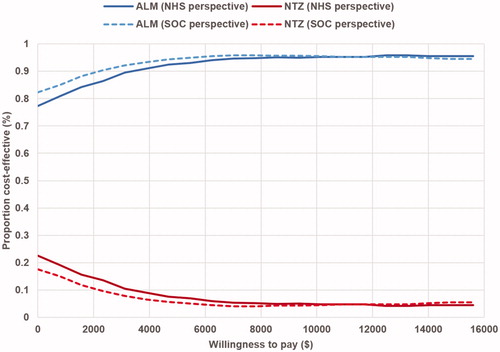
The PSA results showed that the ICER of ALM vs NTZ was < $5,500 (WTP threshold of 1 GDP/capita) in 92% of the simulations, and < $15,600 (WTP threshold of 3 GDP/capita) in 95.6% with regard to the NHS perspective (). Similarly, with respect to the societal perspective, the ICER of ALM vs NTZ was < $5,500 in 93.8% of the simulations and < $16,500 in 95.4% ().
The probability of ALM being cost-effective is presented in an acceptability curve () over a range of WTP thresholds from $0–$15,600 per QALY regarding NHS and societal perspective.
Scenario analysis
Assuming a 10-year time horizon, it was found that ALM ceased to be cost-effective with an ICER of $109,071 () and was dominated in all iterations (). However, considering a lifetime horizon, the results indicated that ALM tended to be cost-effective as a dominant strategy (), with the probability of being accepted in more than 99% at the WTP threshold of < $5,500 ().
In addition, taking different results of network meta-analysis estimating the hazard ratio and relative risk of ALM and NTZ vs placebo into accountCitation31,Citation41–43, it was shown that the results are robust and ALM would remain as a dominant strategy ().
Furthermore, implementing the similar hazard ratio for relapse or disability worsening in the model, as was found in the Kalincik et al.Citation44 cohort study, showed that ALM would remain as a dominant strategy due to NTZ infusion associated disutility as well as PML associated disutility and different discontinuation rate.
Discussion
To the best of our knowledge, this is one of the first health-economic studies undertaken to compare ALM with NTZ in the treatment of MS. Indeed, this is the first cost-effectiveness analysis evaluating ALM in the treatment of RRMS in developing countries. In the absence of head-to-head studies, a decision analysis such as this may provide healthcare decision-makers with the requisite insights to make informed choices.
Although the drug acquisition cost of ALM is higher than for NTZ, the results of the model indicated that the total costs for ALM were lower compared to NTZ over a 20-year time horizon, considering ALM posology and lower treatment cost after year 2. Patients receiving ALM also benefited from more QALYs in comparison with NTZ. Thus, this cost-effectiveness analysis showed that, in RRMS patients, ALM is more cost-effective than NTZ over the time horizon, due to its higher QALYs (+0.57) and lower costs (–USD3,162). The costs saved vs NTZ were mostly due to avoiding long-term expenditure on drug acquisition and administration because of the unique short-course administration and durable effects of ALM.
As has been noted, the model was most sensitive to acquisition costs for both ALM and NTZ, as well as the time horizon. Minimal effects on the results were observed for other key parameters, such as ALM re-treatment, relapse and infusion-associated disutility values, state distributions, and discount rates.
The PSA indicated that the model is robust, based on the uncertainty and distribution of parameters such as clinical efficacy hazard ratio, costs, and utilities. In addition, taking both NHS and societal perspectives into account, the PSA demonstrated that ALM therapy was the optimal strategy, as it was highly cost-effective in 90% of cases (WTP threshold of 1 GDP/capita) and cost-effective in more than 97% of cases (WTP threshold of 3 GDP/capita).
As well as the aforementioned sensitivity analysis, scenario analysis found that both the estimated disease progression hazard ratio and relapse rate relative risk of ALM and NTZ (vs placebo) were not significant drivers of the model, as changes in these parameters (i.e. using data reported in different network meta-analysis or the Kalincik et al.Citation44 cohort study) did not produce a significant change in the ICER. This result affirms that the indirect comparison of the model was not subject to bias.
In addition, investigating the time horizon effect, it was shown that assuming horizons less than 15 years would result in major change in ICER, making NTZ cost-effective. However, in view of the lifetime horizon, ALM would persist to be dominant with lower cost and higher QALY gained compared to NTZ.
As there is a dearth of literature comparing NTZ with ALM in terms of cost-effectiveness, this study thus contributes new knowledge to the body of literature. At the 19th ISPOR Annual European Congresses in 2016, Smith et al.Citation87 reported a cost-utility analysis of ALM vs NTZ from a US payer perspective, over a 20-year time horizon. ALM was dominant over NTZ in RRMS patients with an inadequate response to prior treatment, owing to its lower cost (USD 398,905 vs USD 560,578) and greater effectiveness (8.82 vs 8.79 QALYs). This result is similar to that reported in the current study in terms of cost-effectiveness, but there are some differences between the studies regarding calculated costs and utilities, due to differences in applied methodology and assumptions; specifically, the efficacy of ALM, and the sources used for the extraction of costs and utilities. Similar studies presented at the 20th ISPOR Annual European Congresses in 2017 showed ALM as a dominant strategy with lower cost and higher efficacy compared to NTZCitation88–90.
Only one published full text article was found comparing ALM to NTZ, with the objection of assessing the cost-effectiveness of cladribine tablets in high disease activity RRMS compared with ALM and NTZ, from the perspective of the NHS in EnglandCitation23. The study indicated that cladribine tablets were dominant vs ALM and NTZ in pairwise comparisons.
The model structure used in this analysis has some differences from previous MS models. The health state structure used is a completed version of the 21-health state structure used in previous RRMS studies, which included 10 EDSS states for RRMS, 10 EDSS states for SPMS, and a single state for death. Because of the significant withdrawal rate of ALM and NTZ leading to treatment discontinuation and, therefore, different transition probabilities, 10 EDSS states for “RRMS Off Treatment” were included separately, capturing cost and utilities in patients resistant to either interferons β, ALM, or NTZ. However, pooling the RRMS and SPMS states is consistent with the approach taken by Palace et al.Citation21, since they did not consider MS course (i.e. RRMS vs SPMS) as a covariate in the analysis, because SPMS is “simply a later stage of the relapsing-remitting form of the disease and the transition has considerable overlap”.
Limitations
This study is subject to several limitations. Most notable of these, and in common with many cost-effectiveness studies, was the absence of direct head-to-head clinical trial data comparing ALM and NTZ. In addition, it is clear that the differences in study designs and baseline characteristics of ALM and NTZ phase 3 RCTs may have had an impact on clinical efficacy and outcomes. To address this concern, the authors made substantial efforts to control for these differences through the use of placebo-adjusted rates.
Second, the generalizability of these results to other healthcare settings may be limited, and the differences in costs between developed and developing countries should be considered. It should be noted that the costs used in the current study are considered highly relevant to the setting of the Iranian NHS, having been derived from national tariffs, NHS reference costs, and Iranian studies. Both lower productivity and lower wages in developing countries will affect the calculated indirect costs. Furthermore, the cost of medicines in our study (e.g. common prescriptions in MS or best supportive therapy in EDSS >6 patients) are not comparable with countries that are less favorable toward generic regimens. For these reasons, the costs may be under-estimated in this model compared with developed countries.
Third, using the British Columbia dataset as the core component for the estimation of disease progression may have introduced some uncertainty regarding the relevance and generalizability of those datasets to the current Iranian MS patient population. However, the British Columbia dataset provides a more appropriate set of transitions for the natural history of RRMS than the London Ontario dataset that was collected in the 1970s and 1980s, and might not be reflective of the present-day MS patient experience. In addition, the London Ontario dataset did not capture patients moving to a lower EDSS value. However, the data from ALM RCTs and other evidence provided by the clinical specialists indicates that patient’s EDSS states can improveCitation91,Citation92. In this appraisal, the British Columbia dataset was selected as the preferred source of natural history data. However, we explored the uncertainty around the natural history of MS in sensitivity analysis and found that the results were not sensitive to this parameter.
Fourth, the impact of neutralizing antibodies (NAbs) for both ALM and NTZ was not considered. Although the exact role of NAbs in the loss of efficacy is unclear, it seems NAbs to NTZ slightly abrogate the therapeutic responseCitation93. However, NAbs to ALM do not appear to have any effect on efficacy, infusion-associated reactions, or AEsCitation94.
Fifth, there is a concern about the 20 year time horizon considered in this study, such as whether it is reasonable to consider such a long time while we don’t have robust data on long-term evolution of disability in treated/untreated patients, and on the development of possible new and unknown adverse events. Guidelines for cost-effectiveness analyses (e.g. NICECitation95 or Academy of Managed Care PharmacyCitation96 and other guidelinesCitation97) suggest that a longer time horizon in pharmacoeconomic models for chronic diseases such as MS is more appropriate to reflect all relevant costs and outcomes. In addition, similar cost-effectiveness studies in MS have utilized such a long time horizon from 10 year to lifetimeCitation23,Citation24,Citation26,Citation28,Citation98–101. The 20-year time horizon was selected to capture the effect of treatment in the case of a chronic disease with no cure like MS, without extrapolating the effect to a lifetime. However, we investigated a shorter time horizon (5 and 10 years) in our sensitivity analysis (see ).
Finally, the results of the base case analysis are subject to the limitations and uncertainties surrounding the long-term effectiveness of ALM and NTZ in RRMS. Although guidelines for cost-effectiveness analysis suggest that a longer time horizon is more appropriate in health-economic models for chronic disease such as MS, extrapolating clinical efficacy and treatment persistency from short-term RCTs to a 20- or 50-year treatment period carries significant uncertainty and requires additional assumptions that are not well acknowledged within the literatureCitation102. In addition, it is clear that longer time horizons will favor dominance of ALM due to its lower rates of re-treatment post-5 years.
Conclusion
Based on the efficacy estimates from published clinical trials, adjusted to characteristics of MS patients treated in clinical practice in Iran, ALM was a dominant strategy in the treatment of RRMS compared with NTZ because of its lower total cost, greater efficacy, greater impact on slowing of disease progression (EDSS), and lower rate of relapses over a 20-year time horizon. Comparative head-to-head RCTs and long-term follow-up are needed to confirm these results.
Transparency
Declaration of funding
This paper is the outcome of a study approved by the students’ research committee of Shahid Beheshti University of Medical Sciences. This study was funded by Sanofi and Rougine Darou Corporation.
Declaration of financial/other relationships
ST and NY were paid consultants to Rougine Darou Corp. for the purposes of this project. MS has no relevant financial relationships. One peer reviewer on this manuscript discloses serving on advisory boards for Almirall, Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi Genzyme, and TEVA. The remaining peer reviewers have no relevant financial or other relationships to disclose.
Acknowledgements
The authors are grateful to Sanofi and Rougine Darou Corp. for the use of their dossier for ALM for this study.
References
- Multiple sclerosis International federation [Internet]. London, UK. Available at: https://www.msif.org/ [Last accessed January 2017]
- Kurtzke JF. Multiple sclerosis in time and space–geographic clues to cause. J Neurovirol 2000;6(Suppl 2):S134–S40
- Facts about MS [Internet]. Florida, USA. Available at: http://msfocus.org/Facts-About-MS.aspx [Last accessed January 2017]
- Pittock SJ, McClelland RL, Mayr WT, et al. Clinical implications of benign multiple sclerosis: a 20-year population-based follow-up study. Ann Neurol 2004;56:303–6
- Orlewska E, Mierzejewski P, Zaborski J, et al. A prospective study of the financial costs of multiple sclerosis at different stages of the disease. Eur J Neurol 2005;12:31–9
- Naci H, Fleurence R, Birt J, et al. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics 2010;28:363–79
- Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm 2007;13:245–61
- Etemadifar M, Izadi S, Nikseresht A, et al. Estimated prevalence and incidence of multiple sclerosis in Iran. Eur Neurol 2014;72:370–4
- Eskandarieh S, Heydarpour P, Minagar A, et al. Multiple sclerosis epidemiology in East Asia, South East Asia and South Asia: a systematic review. Neuroepidemiology 2016;46:209–21
- Imani A, Rasekh HR, Asefzadeh S, et al. Cost analysis of disease-modifying drugs therapy for patients with multiple sclerosis in Iran. Am J Sci Res 2012;95–102
- Khanizadeh H, Izham M, Akmal A. PND12 the costs analysis of multiple sclerosis at different stages in Iran. Value Health 2012;15:A143
- Yamout B, Alroughani R, Al-Jumah M, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: the Middle East North Africa Committee for Treatment and Research In Multiple Sclerosis (MENACTRIMS). Curr Med Res Opin 2015;31:1349–61
- Earnshaw SR, Graham J, Oleen-Burkey M, et al. Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Health Policy 2009;7:91–108
- Whetten-Goldstein K, Sloan FA, Goldstein LB, et al. A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult Scler 1998;4:419–25
- Pucci E, Giuliani G, Solari A, et al. Natalizumab for relapsing remitting multiple sclerosis. Cochrane Database Syst Rev 2011;CD007621
- Fox EJ. Alemtuzumab in the treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother 2010;10:1789–97
- National Institute for Health and Care Excellence. Final appraisal determination alemtuzumab for treating relapsing–remitting multiple sclerosis. London, UK: NICE; 2013. p. 1–62. Available at: http://www.nice.org.uk/nicemedia/live/14059/66001/66001.pdf [Last accessed August 2017]
- Palesh M, Jonsson PM, Jamshidi H, et al. Diffusion of interferon beta in Iran and its utilization in Tehran. Pharmacoepidemiol Drug Saf 2008;17:934–41
- Nikfar S, Kebriaeezadeh A, Dinarvand R, et al. Cost-effectiveness of different interferon beta products for relapsing-remitting and secondary progressive multiple sclerosis: decision analysis based on long-term clinical data and switchable treatments. Daru 2013;21:50
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52
- Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open 2014;4:e004073
- Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis, a geographically based study 10: relapses and long-term disability. Brain 2010;133:1914–29
- Hettle R, Harty G, Wong SL. Cost-effectiveness of cladribine tablets, alemtuzumab and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England. J Med Econ 2018;21(7):676–686
- Hernandez L, Guo S, Toro-Diaz H, et al. Peginterferon beta-1a versus other self-injectable disease-modifying therapies in the treatment of relapsing-remitting multiple sclerosis in Scotland: a cost-effectiveness analysis. J Med Econ 2017;20:228–38
- Melendez-Torres GJ, Auguste P, Armoiry X, et al. Clinical effectiveness and cost-effectiveness of beta-interferon and glatiramer acetate for treating multiple sclerosis: systematic review and economic evaluation. Health Technol Assess 2017;21:1–352
- Yang H, Duchesneau E, Foster R, et al. Cost-effectiveness analysis of ocrelizumab versus subcutaneous interferon beta-1a for the treatment of relapsing multiple sclerosis. J Med Econ 2017;20:1056–65
- Dashputre AA, Kamal KM, Pawar G. Cost-effectiveness of peginterferon beta-1a and alemtuzumab in relapsing-remitting multiple sclerosis. J Manag Care Spec Pharm 2017;23:666–76
- Mauskopf J, Fay M, Iyer R, et al. Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ 2016;6998:1–11
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–28
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–39
- The Institute for Clinical and Economic Review (ICER). Disease-modifying therapies for relapsing remitting and primary-progressive multiple sclerosis: effectiveness and value. Boston, USA: ICER; 2017. p 1–253. Available at: https://icer-review.org/wp-content/uploads/2016/08/CTAF_MS_Final_Report_030617.pdf [Last accessed January 2018]
- Patzold U, Pocklington PR. Course of multiple sclerosis. Acta Neurol Scand 1982;65:248–66
- Tappenden P, Chilcott J, O’Hagan A, et al. Cost effectiveness of beta interferons and glatiramer acetate in the management of multiple sclerosis. Report to the National Institute for Clinical Excellence (NICE); 2001
- Tran K, Milev S, Jabr MF, et al. CADTH therapeutic review comparative clinical and cost-effectiveness of drug therapies for relapsing-remitting multiple sclerosis. Can Agency Drugs Technol Health 2014;1:1–102
- Abdoli G. Estimation of social discount rate for Iran. Economic Research Review 2009;3(34):135–156
- Robberstad B. Estimation of private and social time preferences for health in northern Tanzania. Soc Sci Med 2005;61:1597–607
- I.R. Iran GDP per capita (current US$) [Internet]. Washington, D. C.: World Bank. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD/countries/IR [Last accessed January 12, 2016]
- WHO. Table: Threshold values for intervention cost-effectiveness by Region. Geneva: WHO; 2014
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910
- Coles AJ, Compston DAS, Selmaj KW, et al. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. The CAMMS223 trial investigators. N Engl J Med 2008;359:1786–801
- Hamidi V, Couto E, Ringerike T, et al. A multiple treatment comparison of eleven disease-modifying drugs used for multiple sclerosis. J Clin Med Res 2018;10:88–105
- Fogarty E, Schmitz S, Tubridy N, et al. Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: systematic review and network meta-analysis. Mult Scler Relat Disord 2016;9:23–30
- Filippini G, Del Giovane C, Vacchi L, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane database Syst Rev 2013;6:CD008933
- Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017;16:271–81
- National Institute for Health and Care Excellence. Alemtuzumab for treating relapsing–remitting multiple sclerosis. Technology appraisal guidance TA312. London, UK: NICE; 2014. Available at: www.nice.org.uk/guidance/ta312 [Last accessed November 2016]
- Orme M, Kerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 2007;10:54–60
- Prosser LA, Kuntz KM, Bar-Or A, et al. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health 2004;7:554–68
- Acaster S, Perard R, Chauhan D, et al. A forgotten aspect of the NICE reference case: an observational study of the health related quality of life impact on caregivers of people with multiple sclerosis. BMC Health Serv Res 2013;13:1
- Nickerson M, Cofield SS, Tyry T, et al. Impact of multiple sclerosis relapse: the NARCOMS participant perspective. Mult Scler Relat Disord 2015;4:234–40
- Campbell JD, McQueen RB, Miravalle A, et al. Comparative effectiveness of early natalizumab treatment in JC virus-negative relapsing-remitting multiple sclerosis. Am J Manag Care 2013;19:278–85
- Couto E, Hamidi V, Ringerike T, Odgaard-Jensen J, Harboe I, Klemp M. Medicines used for Multiple Sclerosis – A Health Technology Assessment. Report from Norwegian Institute of Public Health. Oslo: Norwegian Institute of Public Health. Oslo: 2016. Available at: https://www.fhi.no/globalassets/kss/filer/filer/publikasjoner/rapporter/20162/rapport_2016_ms-legemidlerv5.pdf [Last accessed January 2017]
- Matza LS, Cong Z, Chung K, et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Prefer Adherence 2013;7:855–65
- Tariff of healthcare services in public and private sectors in Iran. Tehran: Iran Ministry of Health and Medical Education Press; 2016
- Rasekh HR, Imani A, Karimi, et al. Cost-utility analysis of immune tolerance induction therapy versus on-demand treatment with recombinant factor VII for hemophilia A with high titer inhibitors in Iran. Clin Outcomes Res 2011;207–212
- Coles AJ, Boyko AN, De Seze J, et al. Alemtuzumab durably improves clinical outcomes in patients with active RRMS in the absence of continuous treatment: 7-year follow-up of CARE-MS-I patients (TOPAZ study). Mult Scler J 2017;23:427–679
- Oreja-Guevara C, Alroughani R, Brassat D, et al. Alemtuzumab demonstrated durable efficacy and safety in CARE-MS I patients switching from SC IFNB-1a: 5-year follow-up after alemtuzumab (TOPAZ study). Mult Scler J 2017;23:427–679
- Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017;89:1117–26
- Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up. Neurology 2017;89:1107 LP–1116
- Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015;86:208–15
- Olek MJ. Treatment of relapsing-remitting multiple sclerosis in adults. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate; 2012. p. 1–18
- Statistical center of Iran. Tehran, Iran. Available at: https://www.amar.org.ir [Last accessed January 2017]
- Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005;353:375–81
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005;353:369–74
- Daniels GH, Vladic A, Brinar V, et al. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J Clin Endocrinol Metab 2014;99:80–9
- Cuker A, Coles AJ, Sullivan H, et al. A distinctive form of immune thrombocytopenia in a phase 2 study of alemtuzumab for the treatment of relapsing-remitting multiple sclerosis. Blood 2011;118:6299–305
- Twyman C, Oyuela P, Palmer J, et al. Thyroid autoimmune adverse events in patients treated with alemtuzumab for relapsing-remitting multiple sclerosis: four-year follow-up of the CARE-MS studies (P2.199). Neurology 2014;82
- Tsourdi E, Gruber M, Rauner M, et al. Graves’ disease after treatment with Alemtuzumab for multiple sclerosis. Hormones 2015;14:148–53
- Willis M, Robertson NP. Drug safety evaluation of alemtuzumab for multiple sclerosis. Expert Opin Drug Saf 2014;13:1115–24
- Selmaj KW, Habek M, Bass A, et al. Efficacy and safety of alemtuzumab in patients with RRMS is durable over 10 years: follow-up from the CAMMS223 study (P5. 338). Neurology 2017;88:P5–338
- Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 2010;9:425–37
- TYSABRI® (natalizumab): PML incidence in patients receiving TYSABRI. Cambridge, MA. Available at: https://medinfo.biogenidec.com/medinfo [Last accessed October 22, 2016]
- Fox RJ, Rudick RA. Risk stratification and patient counseling for natalizumab in multiple sclerosis. Neurology 2012;78:436–7
- Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–80
- Berger JR, Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol 2016;22:533–5
- McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016;87:117–25
- Alroughani R, Akhtar S, Ahmed SF, et al. JC virus seroprevalence and seroconversion in multiple sclerosis cohort: a Middle-Eastern study. J Neurol Sci 2016;360:61–5
- Calabrese L. A rational approach to PML for the clinician. Cleve Clin J Med 2011;78(Suppl 2):S38–S41
- Koralnik IJ. Progressive multifocal leukoencephalopathy: treatment and prognosis. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate; 2016. p. 1–13
- Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry 2013;84:1068–74
- Hunt D, Giovannoni G. Natalizumab-associated progressive multifocal leucoencephalopathy: a practical approach to risk profiling and monitoring. Pract Neurol 2012;12:25–35
- George JN. Immune thrombocytopenia (ITP) in adults: initial treatment and prognosis. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate; 2016. p. 1–11
- Havrdova E, Horakova D, Kovarova I. Alemtuzumab in the treatment of multiple sclerosis: key clinical trial results and considerations for use. Ther Adv Neurol Disord 2015;8:31–45
- O’Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS study. Neurology 2014;83:78–86
- GHO. By category. Life tables by country - Iran (Islamic Republic of). Geneva: WHO. Available at: http://apps.who.int/gho/data/?theme=main&vid=60760 [Last accessed May 27, 2018]
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med 1997;29:101–6
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006
- Smith A, Hashemi L, Ma I. Cost-utility analysis of alemtuzumab versus natalizumab for the treatment of relapsing-remitting multiple sclerosis: US payer perspective. Value Health 2016;19:A431
- Djambazov S, Vekov T. Cost-effectiveness analysis of alemtuzumab for the treatment of multiple sclerosis in Bulgaria, 2016. 22nd Annu. Int. Meet. Int. Soc. Pharmacoeconomics Outcomes Res. poster) abstr. PND31; 2017
- Taheri S, Yousefi N, Sahraian M, et al. Cost-effectiveness analysis of alemtuzumab in comparison with natalizumab, intramuscular interferon beta-1a, subcutaneous interferon beta-1b, and fingolimod for the treatment of relapsing-remitting multiple sclerosis in Iran. Value Health 2017;20:A723
- Chirikov V, Ma I, Joshi N, et al. Cost-effectiveness of alemtuzumab in the treatment of relapsing forms of multiple sclerosis in the United States and societal spillover effects. Value Health 2017;20:A722
- Willis M, Harding K, Pickersgil LT, et al. Alemtuzumab for multiple sclerosis: long term follow-up in a multi-centre cohort. Mult Scler 2015;22(9):1215–1223
- Dubey D, Cano C, Stuve O. Intractable and highly active relapsing multiple sclerosis &ndash; role of alemtuzumab. Neuropsychiatr Dis Treat 2015;11:2405
- Fox EJ, Vartanian TK, Zamvil SS. The immunogenicity of disease-modifying therapies for multiple sclerosis: clinical implications for neurologists. Neurologist 2007;13:355–62
- Coyle PK. Current evaluation of alemtuzumab in multiple sclerosis. Expert Opin Biol Ther 2014;14:127–35
- National Institute for Clinical Excellence (NICE). Guide to the methods of technology appraisal. London: National Institute for Clinical Excellence; 2004
- Academy of Managed Care Pharmacy. AMCP format for fomulary submissions, version 4. Virginia, USA: ACMP; 2016. Available at: www.amcp.org/FormatV4/ [Last accessed November 1, 2018]
- Canadian Coordinating Office for Health Technology Assessment. Guidelines for economic evaluation of pharmaceuticals: Canada. 2nd ed. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCHOTA); 2014
- Bozkaya D, Livingston T, Migliaccio-Walle K, et al. The cost-effectiveness of disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis. J Med Econ 2017;20:297–302
- Montgomery SM, Maruszczak MJ, Slater D, et al. A discrete event simulation to model the cost-utility of fingolimod and natalizumab in rapidly evolving severe relapsing-remitting multiple sclerosis in the UK. J Med Econ 2017;20:474–82
- Su W, Kansal A, Vicente C, et al. The cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing-remitting multiple sclerosis in Canada. J Med Econ 2016;19:718–27
- Hernandez L, Guo S, Kinter E, et al. Cost-effectiveness analysis of peginterferon beta-1a compared with interferon beta-1a and glatiramer acetate in the treatment of relapsing-remitting multiple sclerosis in the United States. J Med Econ 2016;6998:1–12
- Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics 2008;26:617–27