Abstract
Objective: To assess the cost-effectiveness (CE) of the originator follitropin-α (Gonal-F) in patients undergoing a medically assisted reproduction (MAR) program in comparison to its biosimilars Bemfola and Ovaleap in a French context.
Methods: A CE model was developed for France with a National Health Service (NHS) perspective. Clinical, safety, and dosage data were derived from pivotal clinical trials that compared Gonal-F to Ovaleap and Bemfola. Costs pertaining to drugs, hospitalizations, specialist visits, and examinations were retrieved from the French Programme de Médicalisation des Systèmes d'Information (PMSI) hospital database, literature review, and French clinical experts using 2017 Euro tariffs. In order to test the robustness of results, deterministic one-way sensitivity analyses were carried out on the main variables to assess the impact of treatment cost, probability of birth, ovarian hyperstimulation syndrome (OHSS) rates, and dosage.
Results: The average incremental cost per live birth with OHSS and without OHSS was €259.56 and €278.39, respectively, for Gonal-F compared to the pooled biosimilars (i.e. Ovaleap and Bemfola). GONAL-F had an incremental efficacy of 0.06 over the pooled biosimilars. The incremental cost-effectiveness ratio for Gonal-F with OHSS ranged from €3,274.80 to €4,877.76 compared to the pooled biosimilars, owing to the additional live births reported with Gonal-F. Sensitivity analyses also supported results from the base case analyses, with Gonal-F being cost-effective or the dominant strategy in most cases.
Conclusion: Gonal-F seems to be a cost-effective strategy compared to its biosimilars Ovaleap and Bemfola, irrespective of the incidence of OHSS events, but further data are needed to confirm these results.
Introduction
Infertility is a medical condition which is recognized as a global public health issue by the World Health Organization (WHO)Citation1. Infertility is a growing concern in many countries, including the European Union (EU) member states, and fertility rates are steadily declining from the mid-1960s through the turn of the centuryCitation2. The common risk factors include age, smoking, alcohol consumption, obesity, diabetes or thyroid disease, and other occupational and environmental risk factors. It is estimated that ∼48.5 million couples worldwide experience infertilityCitation3. A total of 1.9% of females aged 20–44 years were found to have difficulty in conception worldwide in 20104,Citation5. The prevalence of infertility varies considerably across countries, and there is a paucity of reliable data due to the presence of multiple factors which complicate any estimates. Among the EU member states, France recorded the highest fertility rates in 2015Citation2. However, its fertility rates fell steadily from 2.01 children per woman in 2012 to 1.93 in 2015Citation4,Citation5. The decreasing trends in fertility can be related to an overall increase in the incidence of infertility due to social, lifestyle, biological, and environmental factorsCitation6. Furthermore, it estimated that ∼20% of couples experience subfertility or infertility, while only 10% seek specialist careCitation6.
However, various treatment options are available for female fertility in France. Assisted reproductive technologies (ART) comprise one of the most commonly used treatment options for women encountering fertility issues. In ART, gonadotropins are usually administered in order to stimulate the follicular development. Exogenous gonadotropins, including follicle-stimulating hormone (FSH), are universally recognized as the key driver of controlled ovarian stimulation and maturation. Available FSH products include purified urinary-derived human menopausal gonadotropin (hMG), highly purified urinary (HP-uFSH), and recombinant human FSH (r-hFSH).
Gonal-F (follitropin alfa for injection) was the first FSH preparation of human recombinant DNA origin marketed since 1997 in several indications, including the stimulation of mutifollicular development in women undergoing superovulation for ARTCitation7. Two biosimilars, namely Ovaleap (Teva, Castleford, UK), and Bemfola (Finox AG, Burgdorf, Switzerland), are now marketed in Europe. Ovaleap was approved by the European Medicine Agency (EMA) in 2013 and recommended by the European guidelines as a biological product containing r-hFSHαCitation8. Bemfola was approved by EMA in 2015 and licensed for all indications of reference productsCitation9. The biosimilar FSH products have non-clinical pharmacological, pharmacokinetic, and toxicological profiles similar to those of the originator FSH. The clinical safety and efficacy of biosimilars is well documented in the literature among women undergoing ART in European countriesCitation8,Citation10. However, their clinical bioequivalence has been demonstrated on an intermediate criterion, that is the number of oocytes retrieved, but not on the ultimate objective of interest, which is the number of live birthsCitation11 and the secondary end-point of these randomized controlled trials (RCTs)Citation12,Citation13. Comparing live birth rates for Gonal-F vs the biosimilar FSH products for the first treatment cycle, the results are in favor of Gonal-F, but non-statistically significantly, as the RCTs were not powered for this endpoint. While there is to date no hard-clinical evidence of the superiority of Gonal-F vs the biosimilar on live birth rates, considering the results observed on existing RCTs and the need to optimize outcomes in relation to limited public resources it is of interest to compare Gonal-F and the biosimilar FSH products in a cost-effectiveness (CE) analysis.
Indeed, currently, the CE of Gonal-F in comparison to its biosimilars is yet to be demonstrated, considering the lower cost of the biosimilars. Hence, the aim of our study was to perform a CE analysis (CEA) of Gonal-F in patients undergoing a medically assisted reproduction (MAR) program compared to its biosimilars: Bemfola and Ovaleap in a French context.
Materials and method
The analysis was based on previously conducted studiesCitation12,Citation13 and did not involve any new studies with human or animal subjects performed by any of the authors. The study was performed in accordance with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Practices for Outcomes Research consensus guidanceCitation14 and relevant international and national guidelines for health economics studies. Ethics board approval and informed consent were not required because the study did not involve human or animal participants and the analysis only used publicly available anonymized data.
Model design
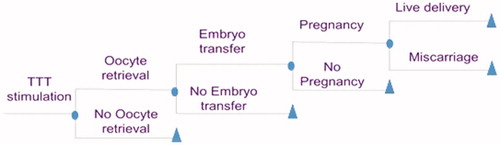
A Microsoft Excel-based pharmacoeconomic model was developed to evaluate the CE of Gonal-F vs its biosimilars in women undergoing ART, focusing on the first treatment cycle. The number of cycles used in our model was decided based on the availabilities of the clinical data used to feed the model, knowing that only data for the first cycle were available for both treatment comparisons (Gonal-F vs BemfolaCitation12 and Gonal-F vs OvaleapCitation13). The CEA was carried out from a National Health Service perspective. To delineate the cost and efficacy of Gonal-F in patients undergoing an MAR program compared to its biosimilars in a French context, a decision-tree was used. This decision-tree model depicted different relevant outcomes of fertility treatment with r-hFSHα over the first cycle. The modeling of decision-tree encompassed the following steps: (a) Treatment stimulation; (b) Oocyte retrieval; (c) Embryo transfer; (d) Pregnancy; and (e) Live delivery or miscarriage. The probability of having live births/miscarriage was taken as the final outcome of the model. At each step, the probability of succeeding or failing was calculated. Only one reimbursed IVF cycle was considered in the model.
Ovarian hyperstimulation syndrome (OHSS), the most important adverse event of gonadotropin use, was taken into account in the model.
Six treatment arms based on the following three pairwise comparisons were considered: (a) Gonal-FO vs Ovaleap using data reported by Strowitzki et al.Citation13; (b) Gonal-FB compared to Bemfola using data reported by Rettenbacher et al.Citation12; (c) Pooled Gonal-F (i.e. Gonal-FP) vs pooled biosimilars (i.e. BioS) using data from both the clinical trials. The model in epicts the main steps of ART on which clinical outcomes and costs are based, and simulates a patient’s journey from the start of IVF therapy through various treatment stages.
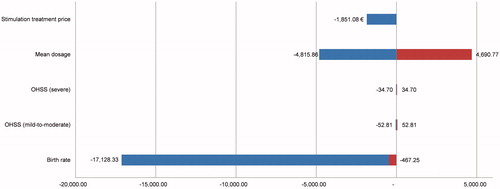
Sensitivity analyses were also performed to investigate the stability of CE for the treatment over a range of value variations. For each comparison, one-way sensitivity analysis was performed to assess the impact of different parameters on the ICER. These parameters included the percentage of live births per woman, mild/moderate OHSS and severe, r-hFSHα dose, and the price of the treatment is performed to test the robustness of the results. The variation of each parameter was determined arbitrarily [±20%], except for r-hFSHα dose, where the confidence intervals of UI dose reported in the two clinical studies have been considered. A tornado diagram was used to analyze the parameters having an impact on the ICER.
Clinical studies and evidence details
The choice of clinical outcomes in infertility trials has been debatable owing to the multistage nature of the treatment, and a recent review of outcome measures in the in vitro fertilization (IVF) clinical trials has indicated very wide diversity in the selection of these outcomesCitation12. Live births are the ultimate endpoint of fertility tests, and there is a consensus among experts in reproductive medicine to advocate the need to adopt live births as the preferred primary outcome in infertility trialsCitation13. Hence, the live birth rate was considered as the criterion of interest to assess efficacy of each treatment in our CE model. The model developed is based on the clinical evidences from head-to-head EMA registration trials of BemfolaCitation12 and OvaleapCitation13 which assessed the number of oocytes retrieved as primary end-point and the live-birth rate as secondary end-point: One of these studies was a multinational, multi-center, randomized, assessor-blind phase III study comparing the efficacy (in terms of number oocyte retrieved) and safety of Ovaleap to Gonal-F in 299 women undergoing controlled ovarian stimulation with ARTCitation13. The other study was a multi-center phase III study that compared the efficacy (in terms of number oocyte retrieved) and safety of Gonal-F to Bemfola in 372 women undergoing ovarian stimulation for IVF for ART superovulationCitation12. We mention that the clinical trials used to feed the model included women according to different criteria (such as age, which range between 20–38 years in the Gonal-F vs Bemfola clinical trialCitation12 and between 18–37 years for the Gonal-F vs Ovaleap clinical trialCitation13 or body mass index). It is, therefore, evident that, based on other populations, the CE model could lead to different results.
Adverse events associated with the use of gonadotropins have been considered in the model. Ovarian hyperstimulation syndrome (OHSS) is one of the most important adverse events of gonadotropin and the severity, management, and costs of OHSS have been considered for all treatment options using data provided in European Public Assessment Reports of BemfolaCitation9 and OvaleapCitation8. No other publication comparing the biosimilars to the originator follitropin alfa was available at the model development date.
The transition probabilities of clinical outcomes, including OHSS rates used in the model were calculated based on the evidence submitted for biosimilars in EMA registrations considering the intent-to-treat (ITT) population data from the two clinical trials. Probability of each treatment arm derived from clinical efficacy outcomes are presented in .
Table 1. Model input data: clinical background.
Costs inputs
The resource pertaining costs associated for each step of the MAR process were retrieved from a literature review, PMSICitation15 (Programme de médicalisation des systèmes d'information) analysis (a comprehensive French hospital database), and French clinical experts opinions through a management questionnaireCitation16.
Cost assumptions taken for each step in the model for each treatment were based on a market study based on the responses from 30 gynecologists/endocrinologists and 300 patients using a questionnaire completed by French experts. The ART cost included cost of treatment induction, which was separated in three steps: blocking phase, stimulation phase, and trigger phase. Nurse tariffsCitation17 were applied for one subcutaneous injection each.
Cost analysis also considered monitoring visits, biological hormones dosagesCitation18, and IVF based on French health insurance tariffCitation19. The IVF cost is weighted between standard IVF cost (40%) and IVF Intra Cytoplasmic Spermatozoid Injection (ICSI) cost (60%)Citation16. Oocyte retrieval costs involved anesthetic visit (10% of patients based on French expert opinion), oocyte retrieval, spermatozoid retrieval (0.09%, based on French Diagnosis-Related Group (DRG) tariffCitation20), and spermatozoid preparation.
Embryo transfer involved technical examination, adding a specific fee and one beta-human chorionic gonadotropin (b-HCG) dosage. The management costs were considered similar to normal pregnancy costs from this stage onwards. Pregnancy follow-up costs were based on mensural medical visit and quarterly monitoring. The weighted mean cost of DRG for natural delivery (75%) and caesarian delivery (25%) was applied in the case of pregnancy leading to live birth, whereas DRG tariff was considered in the case of miscarriage. Costs of mild, moderate, and severe OHSS were used in the model considering the monitoring costs, unless for the severe OHSS where the cost of the associated DRG (“Autres affections de l'appareil génital féminin” – GHM: 13M041, 13M042, 13M04T, 13M043 and “Interventions sur le système utéroannexiel pour des affections non malignes” – GHM: 13C071)Citation20 was added in addition to the monitoring costs. All estimated costs and assumptions made are presented in .
Table 2. All estimated costs with assumptions.
The total cost per patient was calculated for each treatment group by multiplying the probability of having one of the clinical efficacy outcomes presented in with the related costs. The incremental CE ratio (ICER) was calculated by taking the difference in total costs divided by the difference in live birth rate of the two treatment groups in France. All resources are valued in 2017 Euros using official local tariffs sources/database.
Results
Detailed results are presented in for each set of pairwise comparisons, presenting the ICER values, cost per live birth, incremental cost, and incremental efficacy either with or without OHSS cases. In all the analyses it was observed that Gonal-F was found to be cost-effective over its biosimilars, irrespective of the consideration of OHSS.
Table 3. Results of the base case cost-effectiveness analysis.
Base-case analysis
When Gonal-FO was compared to Ovaleap after taking OHSS into account, the use of Gonal-FO resulted in an incremental cost of €259.17 and an incremental efficacy of 0.05 over Ovaleap. This translated into an ICER of €4,804, which is the additional cost required for Gonal-FO to gain an additional live birth in comparison with Ovaleap. The costs of treatment with Gonal-FO and Ovaleap were €3,826 and €3,567, respectively. The cost per live birth was €5,799 with Gonal-FO and €5,682 with Ovaleap. Efficacy in terms of live-birth rate was 0.32 and 0.27 for Gonal-FO and Ovaleap, respectively, which indicates that there would be 32 live-born children per 100 women treated with the Gonal-F and 27 live-born children per 100 women treated with Ovaleap. Similar results were observed when Gonal-FO was compared to Ovaleap without taking OHSS into account, with an ICER of €4,878. There was no change in the incremental efficacy (0.05), while the incremental cost was relatively higher without taking OHSS into account (€263) as compared to analysis taking OHSS into account (€259).
For the second set of pairwise comparisons between Gonal-FB and Bemfola, the incremental efficacy was 0.08 in favor of Gonal-FB, irrespective of OHSS consideration. The incremental cost of Gonal-FB over Bemfola was €279 with OHSS and €299 without OHSS. This resulted in an ICER of €3,275 with OHSS and €3,505 without OHSS.
The third set of pairwise comparison between pooled Gonal-FP and BioS also showed a similar trend which favored Gonal-FP over BioS in terms of incremental efficacy (0.06), irrespective of OHSS consideration. The incremental cost of Gonal-FP over BioS was €260 with OHSS and €278 without OHSS. The observed ICER values are €4,352 with OHSS and €4,668 without OHSS.
Sensitivity analysis
The results of one-way sensitivity analysis are presented in , and the outcomes for the comparison of Gonal-FP to BioS are depicted as a tornado diagram in . This analysis indicates that uncertainty in probability of birth and dosages of r-hFSHα are the most sensitive variables and have the highest impact on ICER values. Gonal-FO vs Ovaleap (without OHSS) and Gonal-FP vs BioS (without OHSS) analyses indicated a cost-saving with higher efficacy for Gonal-F.
Table 4. Results of one-way sensitivity analyses for the comparison of Gonal-F with its biosimilars.
Nearly all sensitivity analyses support that Gonal-F is a cost-effective strategy, even cost-saving when the lower dosage limit is considered for Gonal-F compared to Ovaleap or BioS. In the case of Gonal-F where the probability of a live birth decreased by 20%, the Ovaleap and BioS are observed to be dominant.
Discussion
Cumulative potential savings to health systems in the European Union (EU) and the US, as a result of the use of biosimilars, could exceed €50 billion in aggregate by 2020 and reach as much as €100 billionCitation21. The cumulative spending in the EU5 (France, Spain, Germany, Italy, and the UK) alone is expected to reach €47 billion over the period 2016–2020 on different originator biologic medicines. In this regard, Germany and France are leading the addressable biosimilar medicines market in the EU5 with 17 billion euros and 9 billion euros of spending, respectively, for 2016–2020Citation21. France is the first European country to explicitly permit biosimilar substitution, and the drop in price of originator products ranges from 1–33% in FranceCitation22,Citation23. While biosimilars may offer a less expensive alternative for patients, it is essential to perform CEA to evaluate the cost per course of treatment of biosimilar r-hFSHα with respect to the originator on the base of equivalent therapeutic. However, to the best of our knowledge, there is a lack of published literature on any CEA of the biosimilar gonadotropin in France, and our study is the first CEA comparing biosimilars Ovaleap and Bemfola to their originator r-hFSHα Gonal-F in women undergoing ovarian stimulation for IVF in France.
Previous CEA studies conducted in Italy, Spain, Germany, and Portugal have indicated Gonal-F to be a cost-efficient treatment strategy compared to its biosimilars BemfolaCitation24,Citation25 and OvaleapCitation26,Citation27. The ICER obtained for Gonal-F vs Ovaleap was €1,517 per woman with a new-born child in PortugalCitation26 and between €415.43–€2,917.47 for the others countriesCitation27. The ICER values for Gonal-F compared to Bemfola were €3,600 in Italy and €900 in SpainCitation24. Yet another recent study in Italy also supported the fact that Gonal-F provided a lower average cost per live birth than Bemfola and an ICER of €1,210Citation25. This is despite the fact that Gonal-F had a higher acquisition cost when compared to its biosimilars Bemfola and Ovaleap.
In context of extending these CEA results to other countries, our study was able to show that Gonal-F could remain the cost-effective strategy compared to its biosimilars, owing to its incremental efficacy in terms of the number of live births.
The CE model was fed with data reported in clinical trials and cannot be substituted for the direct real-life comparisons. Also, it should be noted that the methods used in our study were slightly different than other CE models in Portugal, Spain, and Italy, owing to differences in the number of stimulation cycles, stimulation steps, and/or costs considered. All patients included in the second cycle of treatment in the Ovaleap trial were treated with Ovaleap, irrespective of whether they received Gonal-F or Ovaleap during the first cycle of treatmentCitation13. Furthermore, the objective of the second treatment cycle in the Bemfola trial was to assess the immunogenicity and safety of Bemfola. Hence, the CE model was based on only one stimulation cycle, since the introduction of second cycle efficacy data could have raised bias not only due to the trial design of the study, but also for the small number of patients who underwent the second treatment cycleCitation12.
The outcomes used to feed the model derive from clinical trials that were designed to compare the number of oocytes retrieved between the biosimilars and Gonal-F. In our analysis we used the second end-point, which is the live birth rate, to calculate the ICER. Even though the first end-point is the number of oocytes retrieved, the number of live births represents the most meaningful and relevant clinical outcome for these types of treatments as shown in multiple studies from the literature. In the French Technology Assessment labelCitation11 for Gonal-F, multiple sources are presented, such as: the meta-analyses made by Gerli et al.Citation28 and Al-Inany et al.Citation29 which involved studies having the live birth rate as the first end-point, or other studies, such as Gholami et al.Citation30 or Sagnella et al.Citation31, where the first end-point is the pregnancy rate. The recent cost-effectiveness modeling evaluations, such as Gizzo et al.Citation24,Citation27, the effectiveness outcome used was also the live birth. Moreover, in order to prove the significance of the used outcome, we analyzed the level where the difference between the two groups was significant for the clinical efficacy outcomes used to feed the model. We observed that the difference becomes significant at a p-value >25%, going up to 50% for some outcomes. However, in our CE model the outcome is the recalculated live birth rate, presented differently than in the original paper. Using the same statistical proportion test (Z-test) as above, this difference is significant between the groups at a p-value equal to 13%. By accepting a higher value for the risk of being wrong, we can make the hypothesis that our live birth rate difference is significantly different from 0. As mentioned above, the studies used for these analyses were not designed to compare the live birth rates, and the clinical trials showed that the second end-point was not significantly different between treatment groups. Such results represent an uncertainty for the conclusion. If sufficient data were available with a study design powered to demonstrate the live-birth rate, it might be interesting to analyze the success rate of all the cycles needed to obtain a child and also to confirm our results.
Another potential limitation is that the biosimilar Bemfola is available only as single-use, fixed-dose, prefilled pens, in contrast to Gonal-F, which is available as multi-dose vials and prefilled pens. As doses need to be individually tailored to response, it is not possible to determine the impact of potential dose wastage on costs arising from the use of the different presentations. Furthermore, dissimilarities in dose reduction were observed between the biosimilar and originator groups, which also could have resulted in a higher incidence of OHSS for the biosimilarCitation32. Clinical outcomes data stratified by age groups and types of ART would have access to model sub-populations avoiding biases on treatment-related benefits and potential harms due to different population characteristics.
In our study, treating 100 women with Gonal-F resulted in nine, six, and five additional live births compared to Bemfola, pooled biosimilars, and Ovaleap, respectively, irrespective of OHSS occurrence. The ICER values were €4,804 per live birth for Ovaleap, €3,275 per live birth for Bemfola, and €4,353 per live birth when considering OHSS, and the respective ICERs were relatively higher when OHSS was taken into account.
Sensitivity analyses confirmed the robustness of base case model, and the probability of birth was the most sensitive variable followed by Gonal-F dosage. When the lower dosage limit of FSH was taken into account, Gonal-F was found to be cost-saving and the dominant strategy. The biosimilars are considered dominant only when Gonal-F probability of birth is decreased by 20%. Similar results were also observed in two CEA studies conducted in Portuguese women, wherein the probability of birth and lower dosage limits in the sensitivity analyses indicated originator FSH to be the dominant strategyCitation10,Citation26.
Our study was conducted with a National Health Service (NHS) perspective and, eventually, the preferred strategy depends on the NHS willingness-to-pay threshold. However, no national or international thresholds have been defined regarding ICER per live-birth, and, hence, no clear implication of our findings on the willingness-to-pay per live-birth can be derived at the moment. Theoretically, the biosimilar r-hFSHα will be preferred if the NHS is willing to pay less than the value of the ICER for one extra live-born child, while the originator Gonal-F will be preferred if it is willing to pay the value of the ICER or more per extra live-born child.
In conclusion, the results of this CEA indicate that the originator r-hFSHα Gonal-F could be a cost-efficient treatment strategy from the perspective of French health services in the treatment of infertility, as compared to the biosimilars. Given the limitations of the model, reliability of CEA can be greatly improved over time as evidence continues to grow and long-term data, especially in real-life scenario, are available in the individual patient populations of interest for different geographies.
Transparency
Declaration of funding
This study has been funded by Merck Santé S.A.S, which is developing products related to research described in this publication.
Declaration of financial/other interests
The authors declare no financial/other interests. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
The subject of this article was presented during the 2017 ISPOR conference.
Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 2007;22:1506–12
- Macaluso M, Wright-Schnapp TJ, Chandra A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril 2010;93:16.e1–10
- Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37
- Datta J, Palmer MJ, Tanton C, et al. Prevalence of infertility and help seeking among 15 000 women and men. Hum Reprod 2016;31:2108–2118
- Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356
- Magali M, Magali B. Breton D, et al. Recent demographic developments in France: a decline in fertility, an increase in mortality. Population-F 2016;71(3):395–454
- Mazuy M, Barbieri M, d’Albis H. Recent demographic trends in France: the number of marriages continues to decrease. Population-E 2014;69(3):273–322
- EMA. Ovaleap: EPAR Public Assessment Report. United Kingdom; 2013
- EMA. Gonal-F: Summary of Product Characteristics. United Kingdom; 2010
- EMA. Bemfola: EPAR Public Assessment Report. United Kingdom; 2014
- Haute Autorité de Santé. Avis de la commision de transparence- GONAL-F. 2014. Available at: https://www.has-sante.fr/portail/jcms/c_1773269/fr/gonal-f [Last accessed August 16, 2018]
- Rettenbacher M, Andersen AN, Garcia-Velasco JA, et al. A multi-centre phase 3 study comparing efficacy and safety of Bemfola(®) versus Gonal-f(®) in women undergoing ovarian stimulation for IVF. Reprod Biomed Online 2015;30:504–13
- Strowitzki T, Kuczynski W, Mueller A, et al. Randomized, active-controlled, comparative phase 3 efficacy and safety equivalence trial of Ovaleap® (recombinant human follicle-stimulating hormone) in infertile women using assisted reproduction technology (ART). Reprod Biol Endocrinol 2016;14:1
- ISPOR. Good Practices for Outcomes Research. USA; 2003. Available at: https://ispor.org/heor-resources/good-practices-for-outcomes-research [Last accessed August 16, 2018]
- ATIH. Présentation | Publication ATIH PMSI MCO. France; 2017. Available at: https://www.atih.sante.fr/mco/presentation [Last accessed August 16, 2018]
- IQVIA. Questionnaire de prise en charge des patientes ayant recours à une aide médicale à la procréation. France; 2017.
- AMELI. Actes à domicile. France; 2017. Available at: https://www.ameli.fr/infirmier/exercice-liberal/facturation-remuneration/tarifs-conventionnels/actes-domicile [Last accessed August 16, 2018]
- AMELI. Le codage des actes biologiques - NABM. France; 2017. Available at: https://www.ameli.fr/medecin/exercice-liberal/facturation-remuneration/nomenclatures-codage/codage-actes-biologiques-nabm [Last accessed August 16, 2018]
- AMELI. CCAM en ligne - CCAM. Available at: https://www.ameli.fr/accueil-de-la-ccam/index.php [Last accessed August 16, 2018]
- ATIH. Tarifs MCO et HAD | Publication ATIH. France; 2017. Available at: https://www.atih.sante.fr/tarifs-mco-et-had [Last accessed August 16, 2018]
- Aitken M. Delivering on the potential of biosimilar medicines: The role of functioning competitive markets introduction. IMS Institute for Health Care Informatics 2016;3–36
- GABI. France to allow biosimilars substitution. Generics and Biosimilars Initiative 2014. Available at: http://gabionline.net/Policies-Legislation/France-to-allow-biosimilars-substitution
- Freiberg M, Schwarz R, Khoury C. Biosimilars: market access and market penetration – a comparison of France and Germany. Value Health 206;19:A442. Available at: https://www.valueinhealthjournal.com/article/S1098-3015(16)31919-2/abstract [Last accessed August 16, 2018]
- Gizzo S, Garcia-Velasco JA, Heiman F, et al. A cost-effectiveness evaluation comparing originator follitropin alfa to the biosimilar for the treatment of infertility. Int J Womens Health 2016;8:683–9
- Ripellino C, Visentin E, Gizzo S, et al. A cost-effectiveness evaluation comparing biosimilar Bemfola to Gonal-F for the treatment of infertility in an Italian contest. Value Health 2015;18:A735
- Silverio N, Botica F, Batista AR. Cost-effectiveness of recombinant human follicle stimulating hormone biosimilars in Portugal. Value Health 2016;19:A402–A403
- Gizzo S, Ferrando M, Lispi M, et al. A cost-effectiveness modeling evaluation comparing a biosimilar follitropin alfa preparation with its reference product for live birth outcome in Germany, Italy and Spain. J Med Econ 2018;1–6 [doi:10.1080/13696998.2018.1511567]
- Gerli S, Bini V, Favilli A, et al. Clinical efficacy and cost-effectiveness of HP-human FSH (Fostimon®) versus rFSH (Gonal-F®) in IVF-ICSI cycles: a meta-analysis. Gynecol Endocrinol 2013;29:520–9
- Al-Inany HG, Abou-Setta AM, Aboulghar MA, et al. Highly purified hMG achieves better pregnancy rates in IVF cycles but not ICSI cycles compared with recombinant FSH: a meta-analysis. Gynecol Endocrinol 2009;25:372–8
- Gholami H, Vicari E, Molis M, et al. Pregnancy outcome following in vitro fertilization-embryo transfer (IVF-ET) in women aged <37, undergoing ovulation induction with human FSH compared with recombinant FSH: a randomised controlled study. Eur Rev Med Pharmacol Sci 2010;14:97–102
- Sagnella F, Moro F, Lanzone A, et al. A prospective randomized noninferiority study comparing recombinant FSH and highly purified menotropin in intrauterine insemination cycles in couples with unexplained infertility and/or mild-moderate male factor. Fertil Steril 2011;95:689–94
- AMELI. Présentation BdM_IT. France; 2017. Available at: http://www.codage.ext.cnamts.fr/codif/bdm_it/index_presentation.php?p_site=AMELI [Last accessed August 16, 2018]
- Financement. Modalités de FINANCEMENT 2016 des activités d’AMP et de CPDPN. Agence de la biomédecine, France; 2016