Abstract
Background: Multiple sclerosis (MS), a chronic progressive, demyelinating, inflammatory disease, affects 2.5 million people worldwide. Approximately 63% of cases are classified as relapsing–remitting MS (RRMS) at the time of diagnosis. The aim of this cost-utility analysis is to evaluate alemtuzumab vs interferon beta (intramuscular [IM] interferon beta-1a, subcutaneous [SC] interferon beta-1a, SC interferon beta-1b, and SC pegylated interferon beta-1a) in previously treated, and vs SC interferon beta-1a, fingolimod, and natalizumab in untreated RRMS patients to determine the incremental cost-effectiveness ratio among the treatment alternatives as prices, the route, and the frequency of administration of considered products vary significantly.
Methods: The primary outcome was the modeled incremental cost-effectiveness ratio (ICER; €/quality-adjusted life-year [QALY] gained). Markov modeling with a 10-year time horizon was carried out. During each 3-month cycle, patients maintained the Expanded Disability Status Scale (EDSS) score or experienced progression, developed secondary progressive MS (SPMS), or showed EDSS progression in SPMS; experienced relapses; suffered from an adverse event (AE); changed treatment; or died. A published network meta-analysis (NMA) was used for indirect comparison. The possibility of a therapy switch was considered. Clinical input data and resource utilization data were derived from the literature. Costs were extracted from price lists published in Austria and were calculated from the payer’s perspective.
Results: In treatment naïve patients, alemtuzumab is associated with costs of €132,663 and 5.25 QALYs in a 10-year time horizon. Costs for SC interferon beta amount to €164,159 and generate 4.85 QALYs. Also, in the pre-treated patients, alemtuzumab dominated comparators by accumulating higher total QALYs (4.88) and lower total costs (€137.409) compared to interferon beta-1a (€200.133), fingolimod (€240.903), and natalizumab (€247.758).
Conclusion: The analysis shows that alemtuzumab is a cost-saving alternative to treat RRMS in pre-treated and therapy naïve patients. From the patient perspective, alemtuzumab improves quality-of-life.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and demyelinating disease of the central nervous system with onset in early age, usually between 20 and 40 years. The disease is characterized by inflammation, destruction of the myelin sheath, and axonal loss that lead to symptoms such as visual and sensory disturbances, dizziness and fatigue, limb weakness, gait problems, and neurogenic bladder and bowel symptomsCitation1.
MS is estimated to affect nearly 2.5 million people (30 per 100,000) at a global level and is more prevalent in areas farther from the equator, with prevalence rates (per 100,000) of 128 in Germany, 291 in Canada, 140 in North America, and 203 in the UKCitation2. In 2011 there were 12,500 MS cases in Austria, which equals ∼ 13,200 time and population adjusted cases todayCitation3–5.
The availability of disease-modifying therapies (DMTs) has improved patient management. The therapeutic goal of DMTs is to decrease the frequency of relapses and to prevent the disability that accumulates with disease progressionCitation3.
Today, there are more than 10 DMTs approved by the European Medicines Agency (EMA) for the treatment of relapsing-remitting MS (RRMS): beta-interferons, glatiramer acetate, natalizumab, ocrelizumab, as well as the oral DMTs fingolimod, dimethylfumarate, teriflunomide, alemtuzumab, and cladribine. Additional options are becoming available which further contribute to the segmentation of the market.
Alemtuzumab is a humanized monoclonal antibody that selectively targets CD52, an antigen highly expressed on T and B lymphocytes. Binding of alemtuzumab to CD52 results in depletion of circulating T and B cells, following which a distinct pattern of T- and B-cell repopulation and a shift in cytokines toward a less inflammatory pattern occur. Both mechanisms may be relevant to the durable efficacy of this drugCitation6.
The clinical efficacy of alemtuzumab compared to interferon beta-1a (Rebif 44 mcg) was documented in two randomized, single-blind studies. CARE-MS 1 included treatment-naive patients, while CARE-MS 2 included patients who had relapsed on previous therapy. Both trials demonstrated a statistically significant reduction of relapse rates compared with Rebif after two cycles of alemtuzumab. A 55% reduction in the treatment naïve population (CARE-MS 1) was achieved (annualized relapse rate 0.18 vs 0.39, p < 0.0001), and 49% (0.26 vs 0.52, p < 0.0001) in the pre-treated population (CARE-MS 2). The difference between the treatment groups in the risk of sustained accumulation of disability (SAD) was not statistically significant in CARE-MS 1 (hazard ratio = 0.70, 95% CI = 0.40–1.23); however, this endpoint was met in CARE-MS 2 with a 42% reduction in the risk of SAD (hazard ratio = 0.58, 95% CI = 0.38–0.87)Citation7,Citation8.
The objective of this health economic study was to assess the incremental cost and cost-effectiveness of alemtuzumab compared with other treatments in patients diagnosed with RRMS, either treatment naïve or pre-treated to determine the incremental cost-effectiveness ratio among the treatment alternatives.
Methods
Several cost-effectiveness models of DMTs for MS have been developed for different populations and different countries. To examine the potential economic impact of alemtuzumab, we constructed a cost-effectiveness model which computed costs and health benefits associated with each treatment. The model used a Markov process to project the long-term costs and health effects. The present probabilistic Markov simulation model used a similar approach as models published previously, simulating disease progression as transfer to a higher Expanded Disability Status Scale (EDSS) state over a period of timeCitation9,Citation10. One cycle in the model comprised a 3 month time frame. No half-cycle correction was adopted due to the short cycle-length.
The present model assumed that two cohorts of patients with RRMS (untreated and pre-treated) entered the model, and were initially proportionally assigned to 10 EDSS states. The assignment is based on the baseline EDSS distribution of CARE MS I for the untreated and CARE MS II for the pre-treated population. Over time, patients move among these states according to a set of transition probabilities. The factors influencing the transition probabilities are: EDSS state, age, disease duration, treatment, being untreated or pre-treated, and the treatment duration. RRMS patients may progress to secondary progressive MS (SPMS) over their lifetime. In addition, the model considered relapses and adverse event (AE) depending on individual medication, which means that each cohort was at risk of experiencing one or more relapses or AEs during each cycle. For every 3 months cycle, a patient may have stopped treatment due to lack of efficacy, AEs, or another reason. The model did not differentiate between reasons of discontinuation, which influenced the subsequent transitions. A treatment switch to the next therapeutic alternative was considered in the model. Once death state (EDSS = 10) was reached it continued for the rest of the simulation.
For treatment comparison, a published network meta-analysis (NMA)Citation11 was used, as therapeutic alternatives were generally not studied in head-to-head trials. Relapse rates (after 12 and 24 months, which were used for the follow-up), disability worsening, and treatment discontinuation/continuation rates were derived from this NMA. Detailed information referring to the network and the studies considered is shown in the Supplementary material (Table S1).
The following health states were defined:
10 Health states (based on EDSS scores) regarding disability progression based on EDSS scores for RRMS and SPMS;
4 Health states described the treatment algorithms, including no treatment;
1 Health stage considered relapses;
The treatment discontinuation health state. In the next cycle, patients received a treatment switch or a treatment stop;
Adverse events were summarized in one cumulated health state (Supplementary Table S2); and
Death.
Health states describing the treatment algorithms consider the following treatment follow-ups to simulate the patient pathways. A more detailed description is given in the Supplementary Appendix (Figure S1). The algorithms for the untreated patient population comprise the following two arms:
Alemtuzumab followed by DMTs, followed by no treatment, compared to
Interferons (IM interferon beta-1a, SC interferon beta-1a, SC interferon beta-1b, and SC pegylated interferon beta-1a) followed by DMTs followed by fingolimod/natalizumab and vice versa and followed by no treatment.
The algorithms for the pre-treated patient population compare the following four arms:
Alemtuzumab followed by DMTs, followed by no treatment, compared to
Interferon (SC Interferon beta-1a) followed by fingolimod/natalizumab and vice versa and followed by no treatment,
Fingolimod followed by natalizumab and followed by no treatment, and
Natalizumab flowed by fingolimod and followed by no treatment.
Cladribine tablets were not considered in the algorithms, because the drug was not available at the time of model development.
Costs were calculated for each of the cycle in Euros. The model provides cumulative costs, outcomes expressed as relapse avoided and, quality adjusted life-years (QALYs) within a time horizon of 10 years, as well as the incremental cost-effectiveness ratio (ICER), which can be interpreted as additional cost QALY gained. A 10-year time horizon was chosen as appropriate to capture all important and relevant outcomes. A 5-year follow-up period was available based on CAMMS223 and estimations to the future were limited to 5 years, which represents a conservative approach. In previous published studies time horizons between 10 year and lifetime were utilizedCitation12.
To assess the robustness of our findings, we carried out deterministic and probabilistic sensitivity analyses.
The model was constructed and analyzed using Microsoft Excel 2010 ().
Figure 1. Markov model. Markov process with cycle length of 3 months and 18 defined states (10 EDSS stages; 4 treatment lines; relapse; discontinuation of treatment, followed by treatment switch or no-treatment; adverse events; and death).
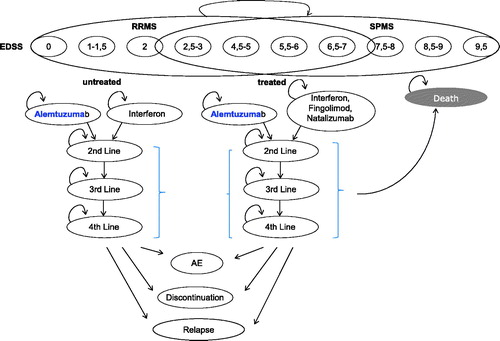
The analysis was conducted in consideration of the Modeling Good Research Practices published by the ISPOR Task ForceCitation13 and the Austrian health economic guidelinesCitation14.
Simulated cohort
The patient characteristics of the simulated hypothetical cohorts align with the patients included in the CARE-MS clinical trials.
In the untreated population, RRMS patients were aged 18–50 years (median 33 years) and had MS for up to 5 years; 65% were female. During the previous 2 years, patients had two relapses and had experienced an EDSS score of 3 or lowerCitation7. The detailed EDSS score distribution used or the first Markov cycle distribution is presented in .
Table 1. Clinical input data.
In the pre-treated population, patients were aged between 18–55 years (median 35 years). Disease duration was 10 years or less. Again, 65% of the patients were female. During the previous 2 years, patients had suffered two relapses and at least one in the previous year; at least one relapse while on interferon beta or glatiramer acetate after at least 6 months of treatment. Patients exhibited an EDSS score of 5 or lowerCitation8. The detailed EDSS score distribution is presented in .
Transition probabilities
The present Markov model considered disability progression by means of 10 EDSS states. During each Markov model cycle, a patient remained in the current health state, progressed to a worse state, or improved to a better state (only possible in states 2–7). The CARE-MS trials have documented changes in EDSS score from baseline. In the untreated patient population, 11% of the interferon beta-1a-treated patients and 8% of alemtuzumab patients had SAD which did not differ between groupsCitation7, so disability accumulation did not differ in the short-term trial. In the pre-treated patient population, the rate of SAD with alemtuzumab was 13% vs 20% with interferon beta-1a. A sustainable reduction of EDSS was observed in 22% of alemtuzumab- and 9% of interferon beta-1a-treated patientsCitation8. Long-term disease progression was derived from the phase 2 CAMMS223 clinical trial which reports safety and efficacy up to 60 months from baselineCitation15. The mean disability of alemtuzumab patients at month 60 had improved compared with baseline, whereas it had worsening effects in interferon beta-1a-treated patientsCitation15. Between month 36 and 60, the change in median disability did not differ between alemtuzumab- and interferon beta-1a-treated groupsCitation15.
To compare disability worsening of alemtuzumab with other DMTs, relative risks from the Cochrane NMA were used.
The results were presented as relative effect sizes (RR) for each possible pair of treatmentsCitation11. Patients’ off-treatment experience probabilities were derived for placebo. Relative risks of disability worsening are presented in .
In addition, the model provided for progressing to SPMS. The only available source was obtained from the London, Ontario cohort study reported by the Centre for Bayesian Statistics in Health Economics of the University of Sheffield, School of Health and Related Research (ScHARR) in its final report to the National Institute for Health and Care ExcellenceCitation16. The hazard rates used for progression rates from RRMS to SPMS are shown in . Patients who progressed to SPMS discontinued present treatment and received interferons beta, recommended for SPMS, or no treatment.
Patients in EDSS 1–6, or health state 1–1.5 to 5.5–6, may experience a relapse. Patients who experienced a relapse moved to the health state “relapse”, which was associated with higher costs and quality of life decrements than the original health state. Relapse probabilities were obtained from the Cochrane NMA and were documented over 12 and 24 months, which were used for the extrapolation. Relapse probabilities for patients off-treatment were obtained from placebo results of the NMA (see ).
Rates of treatment discontinuation were obtained from the Cochrane NMA (opposite of treatment acceptability). If a patient discontinued treatment, they switched to the next treatment line or decided to stop MS treatment. Information concerning treatment stop for alemtuzumab and interferon beta-1a were extracted from the clinical trialsCitation7,Citation8. The percentage of patients receiving no further treatment at month 60 and 72 was derived from CAMMS223 for alemtuzumab and interferon beta-1a. For the other DMTs included in the model, it was assumed that there is an inverse correlation between treatment stop and relapse rate.
NMAs were not conducted for AE data. Treatment-specific AEs that occurred at a frequency greater than 5% were obtained from a health technology assessment authored by Tran et al.Citation16. The analysis includes the following AEs: influenza like symptom, fatigue, flushing, infection, depression, infusion reaction, injection side reactions, hypersensitivity, liver enzyme elevation, thyroid disorder, gastro-intestinal disorders, and cardiovascular disorders (bradycardia and atrioventricular block). Pooled data were aggregated based on 25 RCTsCitation16.
Mortality rates were required to estimate the number of patients who died within the time horizon. Expected mortality in patients with MS was significantly higher than the general populationCitation17–20. Age- and sex-specific all-cause mortality rates for the general population were obtained from Austrian public statistics. Adjusted mortality rates were derived by using the relative risk of death in an MS population compared to the general population. It was expected that the risk of MS-related mortality increased as the EDSS score rose (see ).
All probabilities were time adjusted to Markov cycle length by means of the actuarial method to make it usable for calculation.
Cost assessment
The cost assessment was based on the assignment of costs to the health states. The costs of each health state were determined by the resource utilization associated with a health state. Resource use (e.g. the type and frequency of medical goods and services rendered to the patient) and monetary value (prices, tariffs, and/or opportunity costs) for each unit of medical goods and services were used to calculate the total direct costs in the Austrian setting. In order to estimate the costs of RRMS in Austria, only direct medical costs were included in the analysis. These comprised MS medication costs, monitoring costs, relapse costs, adverse event costs, and costs associated with the EDSS level. Direct medical costs were collected from the payer’s perspective for the year 2017.
Alemtuzumab is administered on 5 consecutive days as the initial treatment course (60 mg total dose) and on 3 consecutive days (36 mg total dose) as the second treatment course 12 months later in the inpatient setting. Based on CARE MS data, the number of patients who received retreatment was reported. According to CARE MS I data, 68.5% of patients needed no retreatment over 60 months beyond the initial two courses, 22.1% of patients received one additional course, 8% two, and 1.4% three courses. In pretreated patients of CARE MS II, 59.8% needed no retreatment, 28.8% received one additional course, 9.9% two, and 1.5% threeCitation6,Citation21. For the following cycles, analogous retreatment rates were adopted. Medication costs of alemtuzumab was valued by the means of ex manufacturing prices due to an inpatient application; comparator costs represented reimbursement prices and were extracted from the official Austrian classified index of goods (“Warenverzeichnis”). Administration costs were considered in the model. For administering alemtuzumab a hospital stay was necessary.
The annual cost of monitoring whilst on treatment was considered separately for the first and the subsequent years. Treatment specific resource use data were obtained from relevant product information and are shown in detail in the Supplementary material for the first and subsequent years of treatment. For alemtuzumab, monitoring is mandatory for 48 months after the last infusion. The outpatient monitoring costs were derived from the tariff catalogs of the nine Austrian regional health insurance funds and represent weighted average values.
Relapse costs comprise treatment of MS exacerbations in the inpatient and outpatient setting. An Austrian survey among MS patients showed that 27% of all cases were treated in the hospital settingCitation4. We assessed in-patient costs using the Austrian DRG-System called LKF. We used the 2017 version of the LKF System for this analysis, which incorporated the procedure coding based on ICD-10. In the case of an outpatient treated MS relapse, visits at general practitioners were more frequent (7-times per quarter), and methylprednisolone (Urbason 1000 mg iv) over 5 days was administered.
The cost of treating serious AE was considered in the model. The cost of treating each AE was calculated from the expected resource use of treating a patient with a specific AE and multiplied by appropriate unit costs. In some instances, published data were insufficient, for which clinical experience by the authors provided resource use estimates. The final cost for each AE was calculated using the proportion of AEs that are serious, multiplied by costs of a serious AE.
Costs associated with the different EDSS levels were taken from Kobelt et al.Citation22. The costs presented in Kobelt et al.Citation22 include direct costs (inpatient care, ambulatory care, tests, drugs costs (DMTs and other), investments, services, and informal care), as well as indirect costs (short-term absence, long-term sick leave, and early retirement)Citation22. Resource utilization data of this survey were used to calculate 2017 costs, based on current reimbursement tariffs. Only direct costs were included in the calculation. Costs for DMTs were excluded.
All data represented costs from 2017 and are shown in . A discount rate of 5% per year was applied, due to the 10 year time horizonCitation14.
Table 2. Model inputs: costs.
Health state utilities
Utilities are a measure of preference between health states, where preference can be equated with value or desirability. Utilities for health states included in the model were obtained from international literature, and, if necessary, re-expressed on a utility scale from 0 to 1 (where 0 represents death and 1 represents full health) by using weighting factors. The QALY (Quality-adjusted life year) concept allows combining the effects of health interventions on quantity and quality of the remaining life years into a single index. QALYs were calculated by multiplying the length of time spent in a certain health state by the utility score associated with itCitation23.
Utility weights for EDSS states were derived from Berger et al.Citation3, estimated with the EQ-5D. Utility scores declined with increasing disability (EDSS). Mean utility was 0.602 (SD = 0.371), and 0.778, 0.579, and 0.244 in the mild, moderate, and severe groups, respectivelyCitation3. For RRMS and SPMS, the identical utilities were used.
To calculate the quality-of-life impact per AE, the disutility per event was multiplied by the event duration. The treatment-specific incidence of serious adverse events was then used to calculate the disutility of each treatment. Data on the disutility due to AEs were derived from different literature sources. Our analysis was based on the assumption that these utilities can be applied to the Austrian population. All utilities are shown in .
Table 3. Model inputs: utilities.
Sensitivity analysis
A deterministic one-way sensitivity analysis was done to assess how variations of individual input parameter values affect the model outputs, specifically the resulting incremental cost-effectiveness ratio (ICER), and thus to judge the robustness of our findings. Input ranges for sensitivity analysis were obtained from 95% confidence intervals (CI) when available. Otherwise (e.g. for costs), input ranges were derived by adding or subtracting percentage values to or from the baseline estimates. In addition, a probabilistic sensitivity analysis was carried out. This global probabilistic sensitivity analysis allows the contribution of each parameter to model outcomes to be investigated, while also taking into account the uncertainty of other model parameters. For this purpose, we incorporated a probability distribution of the input variables by means of a second order Monte Carlo simulation. Each simulation was based on a different value drawn randomly from the distribution of each variable. Second order Monte Carlo simulations of 500 hypothetical patients were carried out based on the distributions of all input variables; gamma distribution for costs and beta distribution for probabilities and utilities.
Results
Base case results of untreated group
From the perspective of the Austrian healthcare system, total costs of MS treatment with alemtuzumab led to discounted costs of €132,663 within a 10 year time horizon. Per-patient costs for interferon-beta (IM interferon beta-1a, SC interferon beta-1a, SC interferon beta-1b, and SC pegylated interferon beta-1a) 1st line treatment were estimated at €164,159. Alemtuzumab therapy resulted in a quality-adjusted life expectancy of 5.25 years, and interferons were associated with the 4.85 quality-adjusted life years (QALY). With alemtuzumab 0.4 QALYs or 4.8 month in perfect health were gained. Compared to interferon-beta, alemtuzumab resulted in a cost saving of €31,496; alemtuzumab dominated interferons (negative ICER –€79,901). In a disaggregated view, costs savings appeared in all cost components; –€14,360 in the case of MS medication and –€11,159 for managing AEs. In addition, alemtuzumab patients had a QALY gain of 0.39 or 4.7 months in perfect health. Within the framework of the observed time period, the median EDSS score increased from EDSS 2 to EDSS 2.6 in alemtuzumab-treated patients and to EDSS 3.8 in the comparator group. EDSS distribution over time is shown in the Supplementary material (Figure S2A).
Base case results of pre-treated group
Within a 10 year time horizon, use of alemtuzumab was associated with total discounted direct costs of €137,409 and resulted in 4.88 QALYs. Treating RRMS patients with interferon beta (SC interferon beta-1a) including the follow-up algorithm accounted for €200,133 and 4.38 QALYs within 10 years. Costs associated with the fingolimod treatment amounted to €240,903 and achieved 4.64 QALYs. Costs of using natalizumab amounted to €247,758 with 4.40 QALYs.
Summarizing the analysis highlights the following points: Alemtuzumab dominated all comparator treatment strategies, because it is associated with savings and QALY-gains. All pairwise comparisons yielded a negative ICER for the comparator. Applying alemtuzumab in pre-treated RRMS patients resulted in cost savings between €62,725 and €110,349 per patient vs comparator DMTs for the Austrian healthcare system within 10 years. Markov model-predicted results showed lower MS medication costs with alemtuzumab compared to comparator DMTs (–€44,423 to –€91,251). Also other cost components were lower in the case of alemtuzumab, e.g. costs managing AEs (–€7,549 to –€18,111). For QALYs, gains ranged between 0.25 and 0.50, equaling 3–6 months in perfect health.
Within the observed time horizon, the median EDSS score increased from 2.7 to 3.3 for alemtuzumab pre-treated patients. Using an interferon beta or fingolimod, EDSS scores increased to 4.6, and with natalizumab to 4.5. EDSS distribution over time is shown in the Supplementary material (Figure S2B).
The results of costs, QALYs, and ICERs of the untreated and pre-treated group are shown in .
Table 4. Cost-effectiveness results.
Deterministic sensitivity analysis
A one-way deterministic sensitivity analysis was carried out to assess the impact of variations in single parameter assumptions on the ICER of alemtuzumab treatment vs comparator DMTs in the untreated and pre-treated patient groups. Results were expressed as incremental costs per QALY gained. Tornado diagrams () where chosen to display the results.
Figure 2. Deterministic sensitivity analysis was used to identify the critical variables. Results are displayed as Tornado diagrams, where each bar represents a one-way sensitivity analysis, and width of bars represents impact on model results. The ICER per patient is plotted on the x-axis.
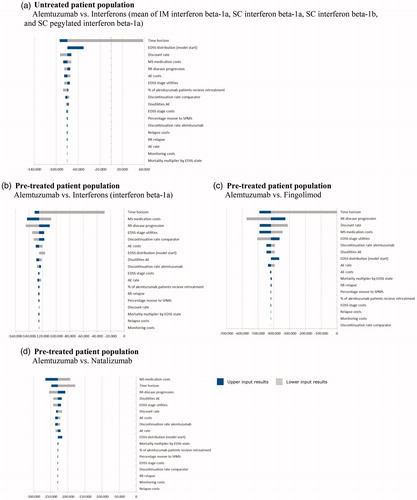
As the base case results, all input variations yielded negative ICER in the untreated and pre-treated patient group. One exception was the lower limit result of the variation of the time horizon. A 5-year time horizon yielded a positive ICER value. Results of the sensitivity analyses showed that the model was sensitive to a number of inputs, including time horizon, MS medication costs, EDSS stage utilities, disease progression, and the discount rate. For example, results were more favorable if outcomes were projected over a longer time frame rather than a shorter time period, because benefits accrue gradually over time due to delayed MS progression. Results of the deterministic sensitivity analysis demonstrated a limited impact on the results.
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis was carried out using a Monte Carlo simulation of 500 hypothetical patients. The incremental costs and health effects due to alemtuzumab were plotted against alternative DMTs. The location of the majority of data points in the lower right quadrant indicates that these simulations yield cost savings and improved health outcomes. The resulting scatter plots () revealed that alemtuzumab dominates all comparator DMTs in the untreated patient population; 94% of simulations are in the lower right quadrant.
Figure 3. Probabilistic sensitivity analysis: the scatterplot shows results of the Monte Carlo probabilistic sensitivity analysis for 500 patients. Incremental costs are plotted on the y-axis, incremental effectiveness is plotted on the x-axis. The location of majority of points in the lower right quadrant shows that the simulations yields cost savings and improved health outcomes. Alemtuzumab dominates comparators. The base case results attest to the model’s low level of uncertainty. (a) Untreated patient population; (b) pre-treated patient population.
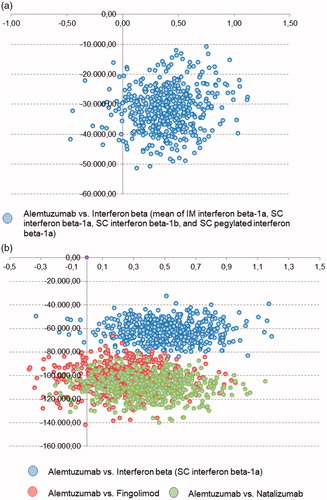
In the pre-treated patient populations the analyses show a similar pattern. Compared to interferon beta (SC interferon beta-1a) results showed that 97% of simulations are in the lower right quadrant, vs fingolimod 90% and vs natalizumab 95%. Hence, results were consistent with the base case results and the deterministic sensitivity analysis.
Discussion
The objective of this modeling study was to analyze the cost-effectiveness of alemtuzumab compared with other DMTs in patients diagnosed with RRMS, either untreated or pre-treated over a 10 year time horizon. The results suggested that alemtuzumab saved costs in comparison with other DMTs. Compared to other DMTs, alemtuzumab was dominant, which means it is associated with lower cost and higher health benefits. Cost savings were higher in pre-treated patients than in the untreated population. The reason is that first or second line medications are lower priced than medications after several treatment failure (e.g. Natalizumab); e.g. interferon-beta showed total direct costs of €164,159 for untreated patients and €200,133 for pre-treated patients. When interpreting the results, it is important to consider that discounting treatment costs had a larger impact on comparator algorithms as costs accrued gradually over time; alemtuzumab costs are accrued in the first 2 years.
Published health economic evaluations for alemtuzumab are scarce. Genzyme’s analysis of cost-effectiveness, based on 2013 costs, showed that alemtuzumab was more effective at lower costs compared with interferon beta-1b, fingolimod, and natalizumab. The probabilistic ICER for alemtuzumab compared with glatiramer acetate was £7,017 (€7,822, exchange calculation: September 24, 2018) per QALY gained. Also, in patients with highly active RRMS despite interferon beta-1b treatment or rapidly developing severe-RRMS, alemtuzumab was more effective at a lower cost than fingolimod or natalizumabCitation24.
In an independent analysis by the Institute for Clinical and Economic Review of 15 DMTs for the treatment of relapsing-remitting and primary-progressive MS, alemtuzumab was shown to be the most cost-effective. Results revealed an ICER of $34,659 (€29,439, exchange calculation: September 24, 2018) per QALY compared with best supportive care, based on 2016 costsCitation25.
In an extensive analysis, Hamidi et al.Citation26 analyzed the cost-effectiveness of alemtuzumab compared to 11 DMTs over a 20 year time-horizon, based on 2015 costs. The economic analysis indicated that alemtuzumab was more effective in terms of QALYs (8.05) and less costly (€547,068) than the other treatment alternatives. Following alemtuzumab, natalizumab was the second most effective treatment regarding QALYs (7.63); associated with costs of €779,977. Cost advantages range from €126,622 vs interferon beta-1b and €239,396 vs fingolimodCitation26.
A discrete event simulation compared fingolimod with alemtuzumab. The model considered alemtuzumab re-treatment. Consideration of treatment effect alone found that alemtuzumab generated 0.2 additional QALYs/patient; the inclusion of AEs up to duration of 1 year reduced this advantage to 0.14 QALYs/patient. Findings also show that alemtuzumab was the lowest cost option when fingolimod was at list price; a 5% discount would reverse this resultCitation27.
A recent cost-effectiveness analysis compared cladribine tablets with alemtuzumab and natalizumab from the perspective of the National Health Service (NHS) in the UK. Results showed that cladribine tablets were dominant vs both comparators. Findings also demonstrated that alemtuzumab dominated natalizumab; life-time costs savings amounts to £108,833 (€121,320) and 0.743 QALYs gained, calculated based on 2015/16 costsCitation28.
The results cited were not strictly comparable to the present analysis as the models applied did not allow for treatment switch. Moreover, medication costs for follow-up treatments were not considered, and patients received best supportive care after treatment stop. Overall, the vast majority of health economic studies were in line with our results showing the superiority of alemtuzumab vs most of the comparators.
The key strength of the present model is the fact that treatment algorithms were compared. Thus, after a treatment stop of a DMT the next treatment lines were taken into account of cost and outcome calculation. This reflects clinical practice and increases the comparability of alemtuzumab with other treatment alternatives. This approach is reliable as clinical trials for second-line treatments recruited pre-treated patients. Costs and outcomes were documented in a more realistic way, when considering patient pathways. Furthermore, the model was developed based on an academic-developed NMA published by Cochrane in the absence of head-to-head RCTs. Finally, resource utilization data depicted Austrian clinical practice and reimbursement rules as cost inputs represented prices and tariffs for 2017, so no estimations were necessary.
Among the limitations of this analysis, one has to mention the fact that the effect on disability progression for alemtuzumab was derived from the CAMMS223 clinical trial covering only 5 years of follow-up. Relative risks vs comparators were extracted from the NMA and represent data over 24 months. As with previous analysis, extrapolating the efficacy results over 10 years implies an unknown degree of certainty. Therefore, disease progression was exposed to sensitivity analyses, which did not change the outcomes. When interpreting the results, it has to be taken into account that RCTs recruit selected patient groups and drugs are often less restrictively applied in a real world situation. Treatment discontinuation rates were extracted from the NMA out of RCTs. In routine clinical practice, a patient is more likely to withdraw from treatment, if treatment was judged to be ineffective or intolerable. Discontinuation rates may substantially influence the treatment effect and costs. This study did not consider indirect costs. MS is associated with significant economic burden, not only in terms of direct costs, but also indirect costs including loss of income, reduction of productivity, burden on caregivers and family members, as well as a reduction in patient’s quality-of-life (QoL). The disease has a marked effect on employment with less than 20% of patients of working age in employment at higher disability levels (Expanded Disability Status Scale [EDSS] 6.0–8.0)Citation29. As indirect cost data were scarce, incorporating such costs is difficult.
Results described from the present modeling study highlighted the cost effectiveness and cost saving of alemtuzumab compared to comparative DMTs. Alemtuzumab dominated all other commonly-used treatments used for previously treated and untreated RRMS patients.
Transparency
Declaration of funding
This study was funded by a grant from Sanofi-Aventis GmbH, Österreich, Austria.
Declaration of financial/other relationships
EW, FD, BB-K, and TB have previously received lecture fees from Sanofi-Aventis GmbH, Austria. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (249 KB)Acknowledgments
None reported.
References
- Hauser S. Multiple sclerosis and other demyelinating diseases. In: Isselbacher K, Braunwald E, Wilson J, et al., editors. Harrison’s principles of internal medicine. New York: McGraw-Hill Inc.; 1994. p. 2281-2294.
- Kanavos P, Tinelli M, Efthymiadou O, et al. London School of Economics. Towards Better Outcomes in Multiple Sclerosis by Addressing Policy Change: The International MultiPLE Sclerosis Study (IMPrESS). March 2018. Available from: http://eprints.lse.ac.uk/66219/1/Kanavos_IMPRESS-Report-March-2016.pdf
- Berger T, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: Results for Austria. Mult Scler. 2017;23(2_suppl):17-28. DOI:10.1177/1352458517708099
- Baumhackl U. Multiple Sklerose, Prävalenz und Therapie im 12-Jahres Vergleich in Österreich. Wien: Facultas wuv Universitäts; 2014.
- Salhofer-Polanyi S, Cetin H, Leutmezer F, et al. Epidemiology of multiple sclerosis in Austria. Neuroepidemiology. 2017;49(1–2):40-44. DOI:10.1159/000479696 Epub 2017 Aug 19.
- Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy. Neurology. 2017;89(11):1107-1116. DOI:10.1212/WNL.0000000000004313 Epub 2017 Aug 23.
- Cohen JA1, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819-1828. DOI:10.1016/S0140-6736(12)61769-3 Epub 2012 Nov 1.
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Mult Scler. 2012;380(9856):1829-1839. DOI:10.1016/S0140-6736(12)61768-1 Epub 2012 Nov 1.
- Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26(7):617-627.
- Soini E, Joutseno J, Sumelahti ML. Cost-utility of first-line disease-modifying treatments for relapsing-remitting multiple sclerosis. Clin Ther. 2017;39(3):537-557.e10. DOI:10.1016/j.clinthera.2017.01.028 Epub 2017 Feb 14.
- Tramacere I1, Del Giovane C, Salanti G, et al. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2015;(9):CD011381. DOI:10.1002/14651858.CD011381.pub2
- Yamamoto D1, Campbell JD. Cost-effectiveness of multiple sclerosis disease-modifying therapies: a systematic review of the literature. Autoimmune Dis. 2012;2012:784364.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices—overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 15(6):796-803.
- Walter E. Österreichische Guidelines zur gesundheitsökonomischen Evaluation. PharmacoEconomics German Research Articles. 2006;4(2):55-63.
- Coles AJ1, Fox E, Vladic A, et al. Alemtuzumab more effective than interferon β-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology. 2012;78(14):1069-1078. DOI:10.1212/WNL.0b013e31824e8ee7 Epub 2012 Mar 21. 2012a
- Tran K, Milev S, Jabr MF, et al. Canadian Agency for Drugs and Technologies in Health. CADTH therapeutic review. Comparative clinical and cost-effectiveness of drug therapies for relapsing-remitting multiple sclerosis [Internet]. Ottawa: The Agency; 2013. (CADTH Therapeutic Review vol.1, no. 2b). Available from: http://www.cadth.ca/media/pdf/TR0004_RRMS_ScienceReport_e.pdf
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med. 1997;29(2):101-106.
- Sumelahti ML, Tienari PJ, Wikstrom J, et al. Survival of multiple sclerosis in Finland between 1964 and 1993. Mult Scler. 2002;8(4):350-355.
- Sadovnick AD, Ebers GC, Wilson RW, et al. Life expectancy in patients attending multiple sclerosis clinics. Neurology. 1992;42(5):991-994.
- Bronnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain. 2004;127(Pt 4):844-850.
- Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology. 2017;89(11):1117-1126. DOI:10.1212/WNL.0000000000004354 Epub 2017 Aug 23.
- Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life of multiple sclerosis in Austria. Eur J Health Econ. 2006;7(suppl. 2):S14-S23.
- Gold MR, Siegel JE, Russell LB, Weinstein MC (eds). Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996, 176–209.
- Diaz RA, Doss S, Burke MJ, et al. Alemtuzumab for relapsing-remitting multiple sclerosis. Lancet Neurol. 2014;13(9):869-870.
- Institute for Clinical and Economic Review. Disease-modifying therapies for relapsing-remitting and primary-progressive multiple sclerosis: effectiveness and value. 2016. Available from: https://icer-review.org/wp-content/uploads/2016/08/CTAF_MS_Final_Report_030617.pdf Accessed April. 2017.
- Hamidi V, Couto E, Ringerike T, et al. A multiple treatment comparison of eleven disease-modifying drugs used for multiple sclerosis. J Clin Med Res. 2018;10(2):88-105.
- Montgomery SM, Kusel J, Nicholas R, et al. Costs and effectiveness of fingolimod versus alemtuzumab in the treatment of highly active relapsing-remitting multiple sclerosis in the UK: Re-treatment, discount, and disutility. J Med Econ. 2017;20(9):962-973.
- Hettle R, Harty G, Wong SL. Cost-effectiveness of cladribine tablets, alemtuzumab, and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England. J Med Econ. 2018;21(7):676–686
- Brandes DW, Rieckmann P. The manifold economic impact of multiple sclerosis – indirect and direct costs of managing patients. Eur Neurol Rev. 2013;Suppl 1:17-23.
- Bundesministerium für Gesundheit und Frauen (BMGF). Austrian DRG System (LKF); 2017. Available from: https://www.sozialministerium.at/site/Gesundheit/Gesundheitssystem/Krankenanstalten/LKF_Modell_2017/ Accessed March 2017.
- Lilja U, Berg J, Plesnilla C, Lindgren P, Kobelt G, Jönsson B. Costs and quality of life in Multiple Sclerosis: A cross-sectional study in Austria. Stockholm, October 2005.
- Matza LS, Sapra SJ, Dillon JF, et al. Health state utilities associated with attributes of treatments for hepatitis C. Eur J Health Econ. 2015;16(9):1005-1018. DOI:10.1007/s10198-014-0649-6 Epub 2014 Dec 7.
- Vera-Llonch M, Brandenburg NA, Oster G. Cost-effectiveness of addon therapy with pregabalin in patients with refractory partial epilepsy. Epilepsia. 2008;49(3):431-437.
- Jakubowiak AJ, Campioni M, Benedict Á, et al Cost-effectiveness of adding carfilzomib to lenalidomide and dexamethasone in relapsed multiple myeloma from a US perspective. J Med Econ. 2016;19(11):1061-1074. Epub 2016 Jun 16.
- Mauskopf J, Fay M, Iyer R, et al. Cost-effectiveness of delayed-release dimethyl fumarate for the treatment of relapsing forms of multiple sclerosis in the United States. J Med Econ. 2016;19(4):432-442. DOI:10.3111/13696998.2015.1135805 Epub 2016 Jan 20.
- Bergenheim K, Williams SA, Bergeson JG, et al. US cost-effectiveness of saxagliptin in type 2 diabetes mellitus. Am J Pharm Benef 2012;4(1):20-28.
- NICE. Natalizumab for the treatment of adults with highly active relapsing-remitting multiple sclerosis. 2007. Available from: http://www.nice.org.uk/TA127. Accessed March 2014.
