?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Non-vitamin K antagonist oral anticoagulants (NOACs) have been included in international guidelines as important alternatives to vitamin K antagonists (VKAs) for the treatment of venous thromboembolism (VTE) and stroke prevention in non-valvular atrial fibrillation (NVAF). Meanwhile, in the Netherlands, NOACs are widely used next to VKAs. The objective of this study is to estimate the cost-effectiveness of treatment with rivaroxaban compared to VKAs in NVAF and VTE patients in the Netherlands, using data from international prospective observational phase IV studies.
Methods: Two models were developed to represent NVAF and VTE patients, populated with patients from the XANTUS (NCT01606995) and XALIA (NCT01619007) international prospective observational studies. The 1-year cost-effectiveness of rivaroxaban use, compared to VKAs, was explored in a population consisting of NVAF and VTE patients (base case) as well as for four scenarios with sub-populations: NVAF patients only, VTE patients only, NVAF patients with unstable international normalized ratio (INR), and NVAF patients using an INR self-measuring device.
Results: In the base case, rivaroxaban saved €72,350 and gained 21 quality-adjusted life-years (QALYs) in a simulation of 2,000 patients over the use of VKAs. Ergo, rivaroxaban was dominant over VKAs. The probabilistic sensitivity analysis showed a probability of 85% for rivaroxaban being dominant and 100% at a willingness-to-pay threshold of €20,000/QALY. Rivaroxaban appeared to be dominant in all scenarios as well, except for the NVAF-patients-only scenario where the incremental cost-effectiveness ratio (ICER) was €157/QALY.
Conclusions: In patients with NVAF or VTE, rivaroxaban treatment is likely to be cost-effective and a potentially cost-saving alternative to VKA in the Netherlands.
Introduction
Atrial fibrillation (AF) and venous thromboembolism (VTE) are diseases associated with blood clot formation, treated and prevented with anticoagulation therapy. Vitamin K antagonists (VKAs) are mainly used as standard anticoagulation therapy in the Netherlands. Non-vitamin K antagonist oral anticoagulants (NOACs) have been included in international guidelines as an important alternative to VKAs. The American College for Chest Physicians (ACCP) guidelines even suggested the use of NOACs over VKAs for the initial and secondary treatment of VTE in patients without cancerCitation1,Citation2. According to the medical report of the Federation of Dutch Thrombotic Services (FNT), a total of 465,107 patients are anticoagulated with either acenocoumarol or phenprocoumon (VKAs). Dutch reimbursement authorities presume the safety and efficacy of acenocoumarol, and phenprocoumon is comparable to warfarin, which is the most used VKA worldwideCitation3. Recent years, however, have shown a steady increase in patients who are treated with a NOAC instead of VKAsCitation4.
Non-valvular atrial fibrillation (NVAF) is a disease characterized by an irregular heart rate. The arrhythmia is caused by a “circle stimulus” which leads to uncoordinated atrial activity. This causes stagnation of the blood flow in the atria, leading to blood clot formationCitation5. As a result, patients who are diagnosed with NVAF have an increased risk of embolic events. NVAF doubles the risk of heart-related death and is associated with a 5-fold increased risk of a strokeCitation6. Furthermore, these clots are also known to block other arteries, causing systemic embolisms (SE) or myocardial infarctions (MI)Citation7.
VTE is the formation of a blood clot in the veins and can be sub-divided into deep vein thrombosis (DVT) or pulmonary embolism (PE). Long-term effects of VTE can be post-thrombotic syndrome (PTS) and chronic thromboembolic pulmonary hypertension (CTEPH)Citation8. VKAs, in combination with low-molecular-weight heparins (LMWH), have been the standard anticoagulation treatment of VTE patients for decades and have proven to be very effective in preventing thromboembolic events. However, VKAs have a very narrow therapeutic window which can be impacted by many drug and food interactions. For these reasons VKAs require frequent monitoring of the international normalized ratio (INR) value of patientsCitation4,Citation9–11. In the Netherlands, the INR measurement is managed and controlled by anticoagulation clinics.
In the Netherlands, NOACs have become available as a possible alternative to VKAs for prevention of stroke and systemic embolism in atrial fibrillation patients in 2012 and treatment and prevention of VTE in 2015Citation12,Citation13. Due to the predictable kinetics and pharmacodynamics of these drugs, routine coagulation monitoring is no longer requiredCitation14. NOACs have had a prominent place in international guidelines for several yearsCitation1,Citation15. In September 2016, the Dutch association for general practitioners issued a statement stating that anticoagulant treatment with NOACs is equally adequate as VKAs concerning the indications AF and VTECitation11. The FNT has reported a decrease in the number of patients who started a VKA for the first time in 2015. As a reason for this decrease, the FNT states that this is mainly due to the steady increase in NOAC prescriptionCitation4.
One of these NOACs, rivaroxaban, has proven to be at least as effective and safe as VKAs in the ROCKET-AF (NCT00403767) and EINSTEIN (NCT00440193 and NCT00439777) clinical trialsCitation16–19. Recently, also international prospective observational studies with real-world data (RWD) have been published on the effectiveness and safety of rivaroxaban. The single-arm XANTUS (NCT01606995) study included 6,784 patients and showed low rates of stroke and major bleeding (MB) in AF patients in routine clinical practiceCitation20. The XALIA (NCT01619007) study included 5,136 patients and examined the efficacy and safety in VTE patients using rivaroxaban, compared to standard of care in the real-world setting. Results showed low MB rates in both treatment groups. Moreover, the use of rivaroxaban was associated with low recurrent VTE rates and shorter hospitalization compared to standard care in the real-world settingCitation21.
The objective of this analysis is to estimate the cost-effectiveness of treatment with rivaroxaban compared to VKA in NVAF and LMWH/VKA in VTE patients, using real-world data. Results will be compared with results from trial-based economic analyses.
Methods
For VTE and NVAF patients, two separate models were developed with a time horizon of 1 year, populated with patients from two RWD studies of rivaroxaban, XALIA (NCT01619007) and XANTUS (NCT01606995)Citation20,Citation21. The cost-effectiveness was explored in five different populations, assuming differences in costs for these groups: patients with NVAF, patients with VTE, patients with NVAF as well as patients with VTE, patients with NVAF who have unstable INR measurements and NVAF patients using INR self-measuring devices. For example, the sub-group of unstable NVAF patients is chosen separately, as these patients can be assumed to have a higher INR measurement frequencyCitation4. Unstable patients were assumed to have a time in therapeutic range (TTR) of < 60% which was consistent with 16% of the total hypothetical population, based on 3,978 patients in the Euro Heart Survey on AF with complete follow-upCitation22. The hypothetical cohort was based on previously published trials (XALIA and XANTUS) and, therefore, a formal ethics review committee approval and consent of patients was not needed.
VTE model
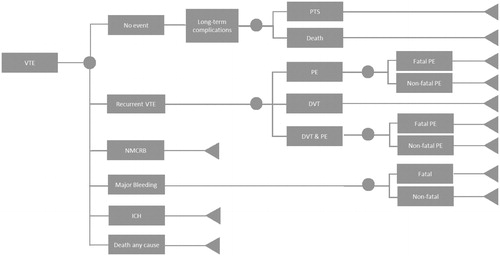
shows the model for the VTE population. A population of 2,000 patients experiencing a VTE event entered the model and moved to no event, recurrent VTE, non-major clinically relevant bleeding (NMCRB), MB, intracranial haemorrhage (ICH), and death by any cause. Recurrent VTE was sub-divided into DVT, PE, and DVT which led to PE. Because a DVT normally first leads to PE before it becomes fatal, the fatality related to DVT was not taken into account for DVT&PE patientsCitation23. Patients who did not experience a bleeding event, recurrent VTE, or death by any cause were assumed to be in the no event health state. In the no event health state it was assumed that 56% of the patients experienced a DVT in the pastCitation21. These patients were at risk of PTS. For PTS, a conservative estimation was made only accounting for severe PTS at 1% risk for “no event” patients who already experienced a DVTCitation24. Patients who experienced a PE in the past could be at risk of developing CTEPH, which was conservatively not taken into account as event rates for PE as well as CTEPH are lowCitation24, especially within the study time horizon.
Figure 1. Progression-of-disease tree for patients with VTE. The health states for both branches of rivaroxaban and VKA therapy are identical. Abbreviations. DVT, deep vein thromboembolism; ICH, intracranial haemorrhage; NMCRB, non-major clinically relevant bleeding; PE, pulmonary embolism; PTS, post-thrombotic syndrome; VTE, venous thromboembolism.
Patient data from the XALIA study were used to calculate the relative risks in the model. Death by any cause was based on the all-cause mortality from the XALIA studyCitation21. The population in the XALIA study treated with rivaroxaban was on average 59 years of age and 55% male. The population initially treated with an unfractionated heparin (UFH), LMWH, or fondaparinux followed by VKA was on average 66 years of age and 52% male. Due to this difference, a correction was made using the propensity-scored primary outcomes of the XALIA study, as shown in Citation21.
All transition probabilities, for both rivaroxaban and LMWH/VKA, included in the model are based on the treatment period of 184 days, which is the duration of the XALIA study. The transition probabilities are summarized in .
NVAF model
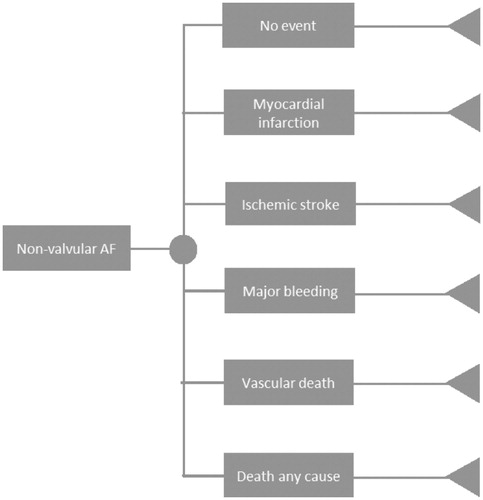
The model for NVAF is shown in . Data used for the transition probabilities of AF were corrected to reflect annual probabilities. Transition probabilities were based on a comparison of the XANTUS and ROCKET-AF studiesCitation25; therefore, we only included the health states presented in this comparative study. Patient populations of the rivaroxaban arm of both studies were matched in order to account for the differences of the real life setting and that of the clinical trialCitation25. With this correction of the groups, represented as the Matching Adjusted Indirect Comparison (MAIC ratio), the rivaroxaban transition probabilities of the ROCKET-AF reflect the XANTUS results. Application of the MAIC ratio was only possible for primary end points listed by the study of CammCitation25; i.e. ischemic stroke (IS), MB, MI, vascular death, death by any cause, and no event, as shown in .
Figure 2. Progression-of-disease tree for patients with non-valvular atrial fibrillation. The health states for both branches of rivaroxaban and VKA therapy are identical. Abbreviation. AF, atrial fibrillation.
In the ROCKET-AF study, the control group is treated with warfarin, which is a VKA that is not available in the Netherlands. Because therapy with warfarin, acenocoumarol, and phenprocoumon all depend on dose adjustments based on patient INR values, their safety and efficacy are considered the same and, therefore, the ROCKET-AF study data can be considered reflective of the Dutch situation including the comparator therapyCitation26. Treatment with rivaroxaban and VKA were continued during the 1-year time horizon of the model, which is line with the duration of the XANTUS trialCitation20.
Before adjustment, the rivaroxaban arm of the XANTUS trial comprised 60.5% of patients aged 75 years or greater and 25.5% between the age of 65 and 75. Of this same population, 50.6% had a CHADS2 (Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes Mellitus, prior Stroke/transient ischemic attack) score of 2, 27.6% had a score of 3, and 21.8% had a score of 4 or higherCitation25. The transition probabilities in the NVAF model are shown in .
Costs
In the VTE model, NOAC treatment consisted of a 21-day course of rivaroxaban, 15 mg twice a day, followed by 20 mg once dailyCitation10. Costs of the LMWH were based on the costs of a daily injection of enoxaparin for 5 days, since this was the most used low-molecular-weight heparin in the RWD studiesCitation21. NVAF patients were treated with 20 or 15 mg rivaroxaban once dailyCitation27. For both models, the costs of VKAs were based on a weighted average of the use of acenocoumarol (5 mg) and phenprocoumon (3 mg) in 2014, estimated at 77% and 23%, respectivelyCitation28. All drug costs were based on the Dutch price list (Z index) excluding 6% VATCitation29.
Based on the 2015 annual medical report of the FNT, a distinction was made between measurement at the coagulation clinic, at home, and self-measurement/self-managementCitation4. For NVAF and VTE the INR should be within the range of 2.0–3.5. For the events of PE and DVT, the costs of PE alone were used as a conservative estimate. The costs of a vascular death were assumed to be equal to the costs of one visit to an emergency roomCitation30. All costs were based on the societal perspective and corrected to the year 2016. A total overview of the event-related costs is shown in .
Table 1. Event costs used in cost-effectiveness analysis.
A complete overview of the utilization and costs associated with the treatment of VKA and rivaroxaban is shown in , as well as the frequency of INR measurements per year. To calculate the mean number of INR measurements for stable or unstable NVAF patients in 1 year, the following formula is used:
Table 2. Resource utilization and costs of NVAF and VTE.
The median number of correctly dosed (stable) NVAF patients is 80.2%Citation4.
Utilities
Specific utilities were used for the baseline health state for patients suffering from NVAF or VTE. The impact of all possible health states on the patients’ quality-of-life was taken into account (). Upon the occurrence of certain events a (dis)utility for a specific time range was used to calculate quality-adjusted life-years (QALYs).
Table 3. Utilities and disutilities used in the cost-effectiveness analysis.
Cost-effectiveness analysis
The results of the cost-effectiveness analysis are presented as the incremental cost effectiveness ratio (ICER) in costs per QALY. The ICER was calculated for each different population shown in Citation31. For the base-case scenario, the ICER was calculated using a weighted average of the VTE and NVAF population, with 18.75% and 81.25% of patients experiencing VTE and NVAF, respectivelyCitation4.
Table 4. Different populations taken into consideration for the cost-effectiveness analysis of rivaroxaban vs VKA.
Sensitivity analyses
In order to determine uncertainty around input parameters we performed a probabilistic sensitivity analysis (PSA). The distributions applied on the 95% confidence interval (CI) of the input parameters were beta for probabilities and utilities, lognormal for relative risks and differences, and gamma for costs. For the five different populations a PSA was performed using a Monte Carlo simulation with 2,000 iterations. Results were plotted in a cost-effectiveness (CE) plane with a willingness-to-pay (WTP) threshold of €20,000/QALY. Results were used to produce cost-effectiveness acceptability curves (CEACs). Additionally, a one-way sensitivity analysis was conducted to determine which parameters have the biggest influence on the ICER for the populations VTE + NVAF, VTE, and NVAF.
Results
Cost-effectiveness analysis
The deterministic results of the five populations for VKA and rivaroxaban are shown in . In the Netherlands a WTP threshold of €20,000/QALY is used for preventive treatments. Rivaroxaban was not only cost-effective at this threshold but even cost-saving in the base case and three other populations. In the base-case scenario rivaroxaban leads to health gains of 24 QALYs and savings of €71,923 per 2,000 simulated patients, compared to current standard of care with VKAs. Rivaroxaban use in scenarios A, C, and D showed a dominant ICER as well. Rivaroxaban use in NVAF patients only (scenario B) was the only non-cost-saving scenario, but is still a cost-effective option with an ICER of €157/QALY.
Table 5. Deterministic costs, effects (2,000 simulations), and incremental cost effectiveness ratios per patient of the five selected populations.
Sensitivity analyses
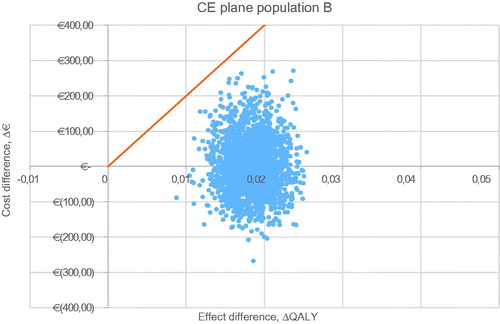
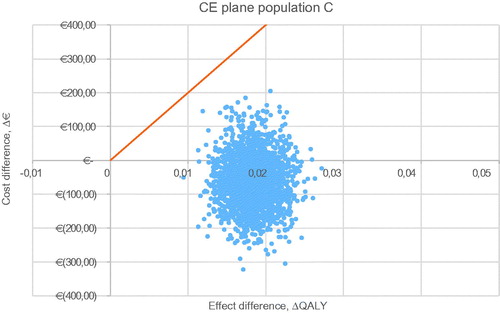
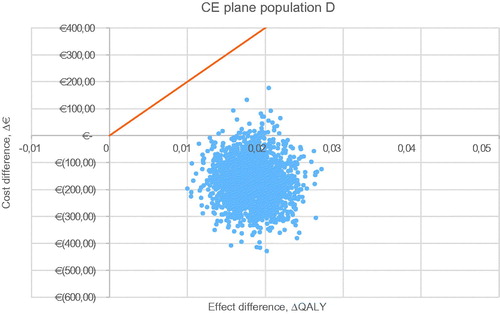
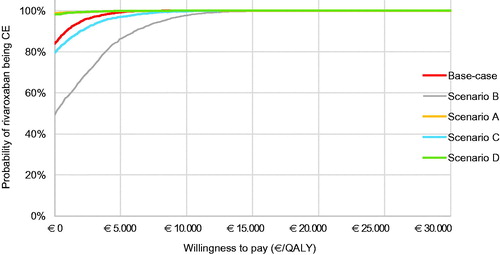
For all five populations, a probabilistic sensitivity analysis was executed with 2,000 iterations. The CE plane for the base case is shown in . The other CE planes are presented in . Cost-effectiveness acceptability curves have been established for all five populations. The probability of being cost-effective at a WTP threshold of €20,000/QALY is 100% in the base case and all other scenarios. When a WTP threshold of €0/QALY is assumed, rivaroxaban use in the base-case scenario has a probability of 84.0% of being cost-effective compared to VKA treatment. The probabilities of cost-effectiveness for scenarios A, B, C, and D were 98.7%, 49.3%, 79.6%, and 98.2% respectively, at a WTP threshold of €0/QALY. The corresponding cost-effectiveness acceptability curves (CEAC) are displayed in .
Figure 3. Probabilistic sensitivity analysis of the base-case scenario. Abbreviation. QALY, quality-adjusted life-years.
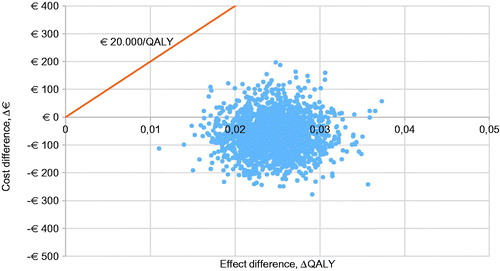
Figure 4. Cost-effectiveness acceptability curve of the base-case scenario (VTE + NVAF patients) and scenarios A, B, C, and D. Abbreviations. CE, cost-effectiveness; NVAF, non-valvular atrial fibrillation; QALY, quality-adjusted life-years; VTE, venous thromboembolism.
Results of the one-way sensitivity analysis in the base-case scenario are shown in . Tables for scenarios A and B are displayed in . In the base-case scenario, utilities for the use of VKAs and the unit cost of rivaroxaban were the parameters with the biggest influence on the ICER.
Table 6. Results of the one-way sensitivity analysis for the base-case scenario.
Discussion
In the base-case analysis, rivaroxaban treatment was associated with a gain of additional 21 QALYs and saved €72,350 for 2,000 patients over a period of 1 year compared to VKA treatment. These results suggest that, in the base case, rivaroxaban is dominant over the standard treatment with a VKA for a combined population of patients with NVAF or VTE. Also in the scenarios including unstable NVAF patients and NVAF self-measurers/self-managers, the use of rivaroxaban provided cost savings and health gains compared to VKA treatment. Even though the drug costs of rivaroxaban are higher than those of VKAs, the total treatment costs are lower, due to the additional costs for INR monitoring and the relatively higher bleeding risks associated with VKA treatment. In the total NVAF population, the intervention was more costly than current treatment, at €2,894, however rivaroxaban use is still considered cost-effective with an ICER of €157/QALY.
To our knowledge this is the first Dutch economic evaluation of rivaroxaban vs VKA based on RWD studies, whereas most cost-effectiveness studies are based on clinical trial data. Our model is unique in the fact that it includes VTE and different NVAF populations in one analysis. The input parameters for the INR measurement were obtained from real-life data of the coagulation clinics, as documented in the FNT reportCitation4. Still, there are some uncertainties and limitations to be discussed.
The incidence proportions reported by Ageno et al.Citation21 are not propensity-score-adjusted, like the hazard ratios. However, we were unable to use these adjusted values, since they were only available for MB, recurrent VTE, all-cause mortality, major adverse cardiovascular events, and other thromboembolic events. The use of relative risks might have led to an over-estimation of the effect of rivaroxaban, and therefore an under-estimation of the cost-effectiveness.
The therapeutic and target range for INR in the Netherlands was not similar to the international range (2.5–3.5 vs 2.0–3.0). The FNT has recently decided to adjust the therapeutic INR range to reflect the international valuesCitation32. Given this, we assumed that it is not necessary to extrapolate the INR values from the XANTUS, XALIA, and ROCKET-AF studies to the Dutch situation. The XANTUS study has Dutch data available, however it was not suitable for this analysis because of the absence of a control group. We compared results from the total XANTUS populationCitation20 to the Dutch XANTUS sub-populationCitation33, and found the number of major bleedings and thromboembolic events to be comparable (1.9% vs 2.1% and 1.6% vs 1.4%). The number of non-major bleedings was slightly lower in the total XANTUS population compared to the Dutch XANTUS sub-population: 12.9% vs 15.8%Citation25,Citation33. More non-major bleedings costs are higher, which makes the calculated ICER a conservative outcome.
The XANTUS study did not include a control groupCitation20. To overcome this problem, data from the analysis of Camm et al.Citation20 were used to make a comparison between the XANTUS and ROCKET-AF studiesCitation16,Citation25. The MAIC ratio was used to convert transition probabilities of the rivaroxaban arm from the ROCKET-AF study to resemble the results in the real world. This leads to an indirect comparison which is a limitation, but, because there was no control group included in the XANTUS study, it was the only way to make a reasonable assumptionCitation20. Also, because the study of CammCitation25 only included five primary outcomes as stated in , the comparison was limited to these outcomes and it was not possible to include, for example, the severity of a stroke or MB (ICH vs non-ICH). These factors might have contributed to either an over- or under-estimation of the ICER in the NVAF arm of the results. On another note, it can be discussed that the 1-year time horizon might not be ideal for modelling a population suffering from NVAF, since this is a chronic disease requiring lifelong treatment and associated with significant cardiovascular complications. Therefore, a model which includes the entire lifespan of the patients suffering from NVAF might give more robust results.
The XALIA study consisted of 184 treatment days (equal to 6 months). It should be stated that 6 months of VKA treatment differs from the Dutch guidelines in which 3 months of treatment is recommended for a first episode of recurrent VTECitation10. The Dutch guideline for treatment duration of NOACs is not specific. Therefore, we remained consistent with the 6-month treatment in the XALIA study, although it might over-estimate the ICER in the base case and scenario B, mainly due to increasing incremental costs, driven by the large difference in drug costs. Since there were no specified costs available for DVT&PE, a conservative estimation was made to only account for the costs of a PE, since this was the more expensive outcome of the two outcomes. This may have resulted in an under-estimation of the costs of this outcome and, therefore, an under-estimation of the ICER. Unfortunately the XANTUS study did not have a sub-group analysis of Dutch patients, therefore a similar comparison of outcomes of the NVAF model was not possible.
The results for the VTE population in this analysis are comparable with a VTE study done in the UK. In this study rivaroxaban also proved to be dominant over the standard treatment in three different treatment durations of 3, 6, and 12 monthsCitation34. Previously, rivaroxaban was already calculated to be dominant over LMWH/VKA treatment in Dutch VTE patients, using data from the EINSTEIN clinical trialsCitation17–19.
Our results in NVAF patients are more favourable for rivaroxaban compared to results from other studies performed in Belgium and GermanyCitation35,Citation36. This might be caused by the fact that the other studies are both only based on the clinical trial data from the ROCKET-AF study and that RWD shows a more positive outcome for rivaroxaban. Another explanation could be that the drug costs of rivaroxaban have decreased substantially since these studies were published. This is a major contributor to the cost of the treatment and, for our study, we have used a cost of €2.16 per 20 mg rivaroxaban, whereas the Belgian and German studies used €2.70 and €3.26, respectivelyCitation29,Citation35,Citation36. The use of €2.16 in this study is based on data from the Dutch Healthcare institute, which shows the price before a discount is agreed upon by the government and the manufacturerCitation29. This makes it a conservative assumption; the price could in fact be lower, leading to an over-estimation of the ICERCitation13. However, price negotiations regarding the costs associated with the coagulation clinics occur as well, which could have a negative effect on the ICER. Moreover, differences between INR values, risk factors of patients, clinical outcomes, and differences in social health costs may explain differences of cost-effectiveness results between countries.
Limitations
As with all cost-effectiveness analyses, this study has its limitations. In the real-world the initial therapy with LMWH in VTE treated patients might not be necessary, or needs to be prolonged, which is not taken into account. Moreover, treatment interruption, treatment switch, or permanent discontinuation was not taken into account. Furthermore, the health states in the model were based on the measured outcomes included in the XANTUS and XALIA trialsCitation21,Citation25, which were much less explicit than the outcomes included in the trialsCitation16–18. In the NVAF model we included the health state “vascular death”, while not taking into account whether this death was caused by MI or IS. Since costs related to the patient’s death might differ by cause of vascular death, this is a limitation of our model. Another point of discussion is the difference in definition of MB between the NVAF and VTE model. The XANTUS study used International Society of Thrombosis and Haemostasis (ISTH) criteria as definition for MBCitation25, while the XALIA study defined MB as
overt bleeding associated with a fall in haemoglobin of 20 g/L or more; a transfusion of two or more units of packed red blood cells or whole blood; critical site bleeding (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, and retroperitoneal); or fatal bleeding [e14]Citation21.
In the model we applied the same costs for both definitions, which might have led to over- or under-estimation of the ICER. Second, MB may differ between the VTE and NVAF models, since patient characteristics were based on the populations included in the XALIA and XANTUS trials, wherefore risk factors for bleeding events may differ between these populations. Last, we did not make a distinction between different sorts of MB. In NVAF patients, rivaroxaban is associated with decreased risk of ICH, but an increased risk of non-ICH. ICH is associated with very high (long-term) costs. Since rivaroxaban would prevent higher costs, this is a conservative assumption. Nevertheless, varying risks of MB in the one-way sensitivity analysis still showed the ICER to be cost-effective.
Conclusions
In conclusion, treatment with rivaroxaban was cost-effective or even cost-saving and provided health gains compared to the standard treatment with a VKA in Dutch NVAF and VTE patients, as well as the other examined scenarios including only VTE patients (A), only NVAF patients (B), unstable NVAF patients (C), and NVAF self-measurers (D). In the first year of treatment, rivaroxaban showed higher benefit for VTE than NVAF patients. In sensitivity analysis, the model has shown to be robust. At a WTP threshold of €20,000/QALY, rivaroxaban appeared to be 100% cost-effective in all scenarios.
Transparency
Declaration of funding
This study was funded by Bayer Pharmaceuticals.
Declaration of financial/other interests
MP has received research grants from various pharmaceutical companies, including, but not limited to, Bayer, Pfizer, Bristol-Myers Squibb, GSK, Roche, and Novartis. MB is an employee at Bayer and was involved with the start of the research, but did not influence the results and discussion. JG, LJ, and MK have no conflict of interest with relation to the subject. One peer reviewer declares their role as a co-principal investigator of the Rocket AF trial, the pivotal trial for rivaroxaban. The remaining peer reviewers for this manuscript have no conflicts of interest to disclose.
Previous presentations
Poster presentation at the 19th ISPOR European Congress 2016, Vienna, Austria: Cost-effectiveness of rivaroxaban for atrial fibrillation and venous thromboembolism in the Netherlands.
Acknowledgements
The models used in this study were provided to the journal’s peer reviewers for their reference when reviewing the manuscript.
References
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962.
- Kearon C, Akl E, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352.
- College voor Zorgverzekeringen. Rivaroxaban (Xarelto) bij behandeling van pulmonale embolie. CvZ; 2014 [cited 2017 Sept]. The Hague, the Netherlands. Available from: https://www.zorginstituutnederland.nl/publicaties/rapport/2014/02/18/rivaroxaban-xarelto-bij-behandeling-van-pulmonale-embolie.
- Federation Dutch Thombotic Services (FNT). Samenvatting medische jaarverslagen. FNT; 2015 [cited 2016 Nov]. Leiden, the Netherlands. Available from: https://s3.eu-central-1.amazonaws.com/storage.topsite.nl/fnt.nl/uploads/docs/jaarverslagen/Medisch_Jaarverslag_2015.pdf.
- Mainardi L, Sörnmo L, Cerutti S. Understanding atrial fibrillation: the signal processing contribution, part II. Synth Lect Biomed Eng. 2008;3(1):1–139.
- Kirchhof P, Auricchio A, Bax J, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J. 2007;28(22):2803–2817.
- Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174(1):107–114.
- Ozaki A, Bartholomew J, Cleveland Clinic Foundation. Venous thromboembolism. CCF; 2012 [cited 2016 Nov]. Available from: http:/www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/venous-thromboembolism/.
- NHG werkgroep atriumfibrilleren. NHG standaard atriumfibrilleren. Huisarts Wet. 2013;56(8):392–401.
- Oudega R, Van Weert H, Stoffers H, et al. NHG-Standaard Diepe veneuze trombose en longembolie. Huisarts Wet. 2008;51(1):24–37.
- Van den Donk M, de Jong J, Geersing G, et al. Cumarinederivaten en DOAC’s voortaan gelijkwaardig. Huisarts Wet. 2016;59(9):406–409.
- Letter from the Dutch Ministry of Health to parliament: Herbeoordeling uitbreiding nadere voorwaarden rivaroxaban (Xarelto®) bij VTE. 2015 [cited 2017 Sept]. Dutch Ministry of Health Location: The Hague, the Netherlands. Available from: https://www.zorginstituutnederland.nl/publicaties/rapport/2015/07/06/rivaroxaban-xarelto-bij-diepveneuze-trombose-pulmonale-embolie-en-preventie-van-recidief-dvt-en-pe.
- Letter from the Dutch Ministry of Health to parliament: Kamerstuk Tweede Kamer der Staten-Generaal 29477 Geneesmiddelenbeleid nr. 207. 2012 [cited 2017 May]. Dutch Ministry of Health Location: The Hague, the Netherlands. Available from: https://zoek.officielebekendmakingen.nl/kst-29477-207.pdf.
- The National Thrombotic Service. Informatie over NOACs. NTS [cited 2017 May]. Ministry of Health: The Hague, the Netherlands. Available from: http://www.denationaletrombosedienst.nl/informatie-over/noacs.
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069, 3069a–3069k.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- EINSTEIN Investigators, Bauersachs R, Berkowitz S, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510.
- EINSTEIN–PE Investigators, Büller H, Prins M, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297.
- Prins M, Lensing A, Bauersachs R, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11(1):21.
- Camm AJ, Amarenco P, Haas S, et al. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37(14):1145–1153.
- Ageno W, Mantovani LG, Haas S, et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study. Lancet Haematol. 2016;3(1):e12–21.
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100.
- Goldhaber SZ, Morrison RB. Cardiology patient pages. Pulmonary embolism and deep vein thrombosis. Circulation. 2002;106(12):1436–1438.
- Kahn SR, Ginsberg JS. The post-thrombotic syndrome: current knowledge, controversies, and directions for future research. Blood Rev. 2002;16(3):155–165.
- Camm A. Real-world versus randomized trial outcomes in similar populations of rivaroxaban treated patients with non-valvular atrial fibrillation in ROCKET-AF and XANTUS: Abstract 084 presented at the American College of Cardiology (ACC) 66th annual scientific session and expo, Washington DC, USA, 17–19 March 2017.
- Zorginstituut Nederland (ZiN). Recommendation to the Minister of Health: reassessment dabigatran. ZiN [cited 2017 May]. Diemen, the Netherlands. Available from: https://www.zorginstituutnederland.nl/binaries/zinl/documenten/rapport/2012/06/06/dabigatran-pradaxa-bij-preventie-van-cerebrovasculair-accident/Dabigatran+%28Pradaxa%29+bij+preventie+van+cerebrovasculair+accident.pdf.
- Dutch Healthcare Institute. Farmacotherapeutisch kompas, preparaattekst rivaroxaban. DHI [cited 2017 May]. Diemen, the Netherlands. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/r/rivaroxaban#dosering.
- Federation Dutch Thombotic Services (FNT). Samenvatting medische jaarverslagen. FNT; 2014 [cited 2016 Nov]. Leiden, the Netherlands. Available from: https://s3.eu-central-1.amazonaws.com/storage.topsite.nl/fnt.nl/uploads/docs/jaarverslagen/FNT_Samenvatting_Medisch_JV_2014.pdf.
- Zorginstituut Nederland (ZiN). Dutch Drug reimbursement [cited 2016 Nov]. The Hague, the Netherlands. Available from: https://www.medicijnkosten.nl/.
- Nederlandse Zorgautoriteit (NZa). Table of Dutch healthcare product tariffs (2016). [cited 2017 Jan]. Utrecht, the Netherlands. Available from: https://www.nza.nl/regelgeving/tarieven-en-prestaties/20150710_Tarieventabel_DBC_zorgproducten_en_overige_producten_per_1_januari_2016.
- Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, et al. Kostenhandleiding. Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. In opdracht van Zorginstituut Nederland. Geactualiseerde versie 2015.
- Federation of Dutch Thrombosis Services (FNT). Adjustment of INR cut-off points. Leiden, the Netherlands. 2015; Available from: https://www.fnt.nl/nieuws/therapeutische-waarden-aangepast-5.
- Pisters R, van Vugt SPG, Brouwer MA, et al. Real-life use of rivaroxaban in the Netherlands: data from the Xarelto for Prevention of Stroke in Patients with Atrial Fibrillation (XANTUS) registry. Neth Heart J. 2017 Oct;25(10):551–558.
- Bamber L, Muston D, McLeod E, et al. Cost-effectiveness analysis of treatment of venous thromboembolism with rivaroxaban compared with combined low molecular weight heparin/vitamin K antagonist. Thromb J. 2015;13:20-015–0051-3. eCollection 1–2, 2015.
- Kleintjens J, Li X, Simoens S, et al. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in atrial fibrillation in the Belgian healthcare setting. Pharmacoeconomics. 2013;31(10):909–918.
- Mensch A, Stock S, Stollenwerk B, et al. Cost effectiveness of rivaroxaban for stroke prevention in German patients with atrial fibrillation. Pharmacoeconomics. 2015;33(3):271–283.
- Baeten S, van Exel N, Dirks M, et al. Lifetime health effects and medical costs of integrated stroke services - a non-randomized controlled cluster-trial based life table approach. Cost Eff Resour Alloc. 2010;8(21):7547–7548.
- Stevanovic J, de Jong LA, Kappelhoff BS, et al. Dabigatran for the treatment and secondary prevention of venous thromboembolism; a cost-effectiveness analysis for the Netherlands. PLoS One. 2016;11(10):e0163550.
- van Leent MW, Stevanovic J, Jansman FG, et al. Cost-effectiveness of dabigatran compared to vitamin-K antagonists for the treatment of deep venous thrombosis in the Netherlands using real-world data. PLoS One. 2015;10(8):e0135054.
- Stevanovic J, Pompen M, Le HH, et al. Economic evaluation of apixaban for the prevention of stroke in non-valvular atrial fibrillation in the Netherlands. PLoS One. 2014;9(8):e103974.
- College voor Zorgverzekeringen (Zorginstituut Nederland, ZiN). Recommendation to the minister: assessment rivaroxaban. CvZ; 2012 [cited 2017 Jan]. The Hague, Netherlands. Available from: https://www.zorginstituutnederland.nl/binaries/zinl/documenten/rapport/2012/10/26/rivaroxaban-xarelto-bij-preventies-bij-diep-veneuze-trombose-dvt/Rivaroxaban+%28Xarelto%29+bij+preventies+bij+Diep+veneuze+trombose+%28DVT%29.pdf.
- van Bellen B, Bamber L, Correa de Carvalho F, et al. Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin. 2014;30(5):829–837.
- Laliberte F, Cloutier M, Crivera C, et al. Effects of rivaroxaban versus warfarin on hospitalization days and other health care resource utilization in patients with nonvalvular atrial fibrillation: an observational study from a cohort of matched users. Clin Ther. 2015;37(3):554–562.
- Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc. 1997;4(1):49–56.
- Ahmad Y, Lip GY. Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: NICE guidance. Heart. 2012;98(19):1404–1406.
- Robinson A, Thomson R, Parkin D, et al. How patients with atrial fibrillation value different health outcomes: a standard gamble study. J Health Serv Res Policy. 2001;6(2):92–98.
Appendix
Table A1. XALIA treatment-emergent clinical outcome results with propensity score adjustment.
Table A2. Transition probabilities used in the VTE model.
TABLE A3. Transition probabilities used in the NVAF model.
Table A4. Results of the one-way sensitivity analysis for scenario A.
Table A5. Results of the one-way sensitivity analysis for scenario B.
Figure A1. Probabilistic sensitivity analysis of population A, venous thromboembolism. Abbreviations. CE, cost-effectiveness; QALY, quality adjusted life-year.
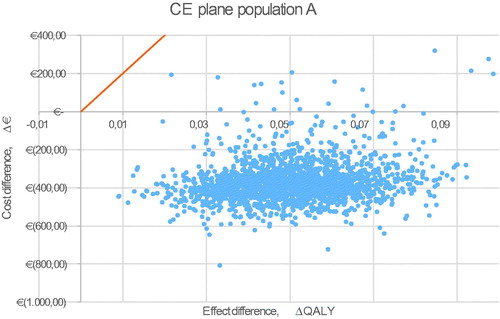
Figure A2. Probabilistic sensitivity analysis of scenario B, non-valvular atrial fibrillation. Abbreviations. CE, cost-effectiveness; QALY, quality adjusted life-year.