Abstract
Aim: To estimate the drug administration, travelling, and productivity costs associated with infusion or subcutaneous proteasome inhibitor (PI) treatments (specifically carfilzomib and bortezomib) for multiple myeloma (MM) patients in Finland.
Materials and methods: Price tariffs of Finnish hospital districts are used as the basis of invoicing sent to healthcare service payers. A review of these price tariff lists was conducted and obtained data analysed to estimate the mean unit cost of PI administration visit. Travelling costs stratified by areas with different population densities were assessed, based on the national travelling reimbursement register data maintained by the Social Insurance Institution of Finland. Productivity costs due to time spent on administration visits and travelling were estimated based on an expert interview and a spatial healthcare accessibility analysis.
Results: Nineteen (95%) of the Finnish hospital districts were included in the review. Relevant unit cost information was found for 15 (75%) of the districts. The mean PI administration cost alone was 270€ (95% CI = 189€–351€) per administration and increased to 371€ when travelling costs were included. Productivity costs added, the mean PI administration costs totalled 405€ for bortezomib and 437€ for carfilzomib.
Limitations: The costing rationale of price tariffs may vary between hospital districts. Productivity costs were estimated conservatively, due to lack of data.
Conclusions: The administration of intravenous or subcutaneous PIs to treat MM in healthcare facilities causes significant and potentially avoidable healthcare, travelling, and indirect costs, and they should be included in all health economic evaluations (HEEs). As the cost estimates utilized in this study represent most of central hospitals in the country, they provide useful information for future HEEs. A broader conclusion is that novel oral medications, such as the first oral PI, have a significant potential for reducing administration-related costs of subcutaneous or intravenous PIs.
Introduction
Multiple myeloma (MM) is a malignant disease where clonal plasma cells proliferate in the bone marrowCitation1. Despite available advanced therapies, such as autologous stem cell transplantation (ASCT) and currently less commonly used allogeneic stem cell transplantation (AlloSCT), MM remains a progressive disease which can rarely be curedCitation2,Citation3. Both ASCT and alloSCT require additional drug therapy. Yet, MM may also be treated with drugs only. Drug treatments for MM include traditional cytostatic agents, proteasome inhibitors (PI), immunomodulatory agents (IMiD), and monoclonal antibodies.
Currently, the PI bortezomib (Velcade®) is one of the most frequently used MM drugs across treatment linesCitation4. A further developed PI, carfilzomib (Kyprolis®), is a new MM treatment that has shown promising study resultsCitation5. In Finland, bortezomib is used from the first line onwards, and carfilzomib is used primarily for patients intolerant to bortezomib, and it may also be considered for patients who are refractory to bortezomibCitation4. Recently, other infusion-administered MM drugs, such as daratumumabCitation6 and elotuzumabCitation7, have been introduced. All these MM drugs are administered parenterally, specifically subcutaneously (SC) or intravenously (IV).
SC and IV administration of MM treatments is expected to cause an extra economic burden to both healthcare providers and patients, due to the need for additional administration visits. However, knowledge regarding the extent of the costs associated with the parenteral administration of MM drugs, and many others for that matter, is limited. For example, in the Finnish public healthcare setting, only the administration costs of IV biologic drugs used in the treatment of autoimmune disorders have previously been published, including also a review-based comparison between countriesCitation8. Among other things, the studyCitation8 demonstrated, e.g., that there are differences in the IV administration costs between hospitals, nations, indications and, especially, different drugs. Since the cost of transportation or travelling could be a significant cost driver in hospital-administered drugs requiring frequent administration, these direct non-healthcare costs should be considered alongside direct healthcare costsCitation9. Furthermore, visits to hospital are time-consuming for both the patients and spouses or other caregivers that may accompany them.
Information on the costs brought about by administration visit-related productivity losses, which could also be significant cost drivers in health economic evaluations (HEE), is even more limitedCitation10. This lack of information causes the true costs and burden of healthcare technologies including MM drugs to be under-estimated in HEEs. Therefore, the aim of the present study is to estimate the administration, travelling, and loss of productivity costs associated with hospital-administered MM drugs, using the administration of bortezomib and carfilzomib treatments as examples, in the public healthcare setting of Finland.
Data and methods
Study design
The study was designed to estimate direct and indirect healthcare costs and productivity costs (due to absenteeism, i.e. absence from paid work in this case) associated with hospital administration of bortezomib and carfilzomib in the treatment of MM. Data on treatment protocols, the number of administration visits, and cycle lengths were taken from the two drugs’ (bortezomib, carfilzomib) official Summaries of Product Characteristics (SmPCs). Based on extracted information, cycles of 3 weeks and four administrations, and 4 weeks and six administrations for bortezomib and carfilzomib, respectively, were used as a basis to estimate the total costs per treatment cycle. Based on a Finnish clinical expert interview, the average time for the administration visit at the hospital was estimated to be 60 min for bortezomib and 165 min for carfilzomib.
All cost analyses were conducted from the societal point of view in year 2016 values (drugs in year 2018 values). Value added tax was not included, and no discounting was applied, due to the short time horizon of the analysis. The study’s costs in euros can be converted to US dollars or other currencies using the 2017 European Central Bank annual bilateral exchange rates (available at sdw.ecb.europa.eu).
Administration unit costs
The average unit cost of a single administration of bortezomib and carfilzomib medication in hospital was estimated with a review of publicly available price tariffs from 20 hospital districts in Finland. The following inclusion criteria were applied to ensure the most relevant unit costs for both drugs:
The unit price mentioned infusion or drug administration specifically, OR;
The unit price was that of an inpatient visit or clinic visit that could potentially include a parenteral drug administration based on prior knowledge (parenteral drug administration is often invoiced as a clinic visit), if no specific infusion or administration unit cost was available, AND;
If multiple unit prices fulfilling these criteria were found, the most suitable was selected based on the drugs or drug categories mentioned in the description, AND;
Price information was from either hematology, internal medicine or oncology department, i.e. from departments treating MM patients and/or using bortezomib and carfilzomib for treating patients.
Confirmation requests were sent to the hospital districts to validate the extracted information. To separate the costs of the drugs from the other costs in prices including both administration and administrated drug, official Finnish wholesale prices for carfilzomib (Kyprolis® 60 mg, 1354.58€) and bortezomib (Velcade® 3.5 mg, 1188.71€) as of 1 January 2018 were used as reference prices. Tariffs with only administration costs included were used as the basis for cost calculation.
Travel costs
The average travel costs associated with an administration visit in hospital were estimated based on national reimbursement data from the Social Insurance Institution (SII) of Finland. The 2015 data covers all reimbursed travel costs in Finland exceeding the one-way deductible fee of 25€ per direction, and all travel costs regardless of the cost after a yearly total deductible fee of 300€ per person. The average travel cost estimate was calculated using weighting based on the number of trips with different vehicles (personal car, taxi, bus, train, etc.), excluding reimbursed emergency transportation. Besides reimbursed costs, deductible payments on reimbursed travels were also considered costs.
Productivity costs related to the administration visits and travelling
The average travel times to and from Finland’s central hospitals offering MM treatment were estimated using data from a spatial healthcare accessibility analysis by Lankila et al.Citation11. The analysis plotted the locations of the hospitals and the geometry of the Finnish road and street network with a 1 × 1 km population density resolutionCitation11. Furthermore, to characterize the impact of long travelling distances to and from hospitals on total administration costs, municipality-level travel times were estimated based on the distance by car between the city centre of each municipality (n = 295) and the respective central hospital. The population-weighted average results of this municipal-level analysis were validated against the results of a countrywide spatial healthcare accessibility analysisCitation11. To further explore the variability in travelling times and the associated productivity costs between municipalities, median and percentiles of travelling times were reported for the productivity losses as well.
Productivity costs due to the administration of the drugs in hospital were estimated based on travel times and time spent at the clinic, in the manner described above. A human capital approach was assumed, and absenteeism due to administration visits and associated travelling was valued at a Finnish average hourly labor cost of 33.31€ (Statistics Finland, indexed to 2016 value) for employed persons. As in a Finnish cost-utility study of actinic keratosisCitation12, the employment rate of Finnish MM patients (33.61%) was calculated based on the real-world age distribution of Finnish MM patientsCitation13, and the corresponding age-specific employment rates of most recent calendar year (2017) from Statistics Finland. Unlike in the studyCitation12, gender-specific employment rate was not applied here due to lack of age-specific gender data.
As a sub-group-based sensitivity analysis, productivity loss estimates were also calculated based on the Finnish real-world age distribution of sub-groups with different 1st line treatment choices: novel, conventional, and novel + ASCTCitation13. The respective employment rates used in the sub-group analysis were 25.83%, 9.34%, and 54.39%.
Based on the clinical expert interview, 30% of the patients were assumed to be accompanied by someone of working-age during the hospital visits. The Finnish average employment rate of working-age persons from year 2017 (69.6%, Statistics Finland) was used for the accompanying persons. For simplicity, individuals who were not employed were not included in the productivity loss estimation.
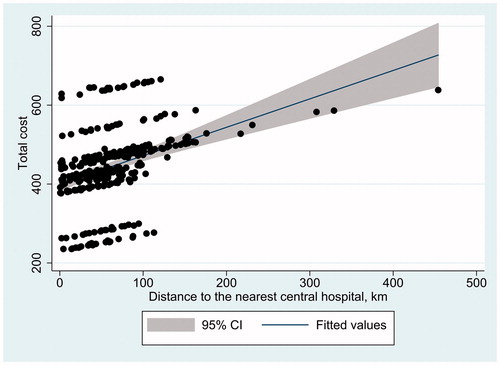
The association between the distance to the nearest central hospital and the total cost of administration—the cost of the administration visit (excluding drug cost), travelling cost from SII reimbursement data, and productivity costs due to travelling—was estimated by fitting a regression line with 95% confidence intervals on a scatter plot, with the horizontal axis representing distance and the vertical axis representing total costs. Hospital district specific administration cost data was used when available; a mean value of 269.87€ was used (imputation) for those with missing data.
Analyses were performed using Microsoft Excel 2016 (Microsoft corporation, Redmond, WA) and Stata 14 MP (StataCorp, College Station, TX).
Results
A total of 19 price tariffs for hospital administration of the drugs (95% of the possible total) and 15 price tariffs with the relevant price information for the purposes of this analysis (79% of the compiled tariffs) were found. Six hospital districts (32%) provided additional estimates after validity confirmation requests.
Administration costs
Based on this information, the mean unit cost (administration included) in Finland for using the drugs to treat MM was 1922.67€ (). After subtracting the official drug prices, the average administration cost estimates were 749.63€ for bortezomib and 533.97€ for carfilzomib.
Table 1. Costs associated with hospital administration of multiple myeloma drugs in Finland.
Unit costs for administration only (excluding drug costs) tariff values were readily available from nine tariffs (sub-information covering 47.4% of districts) and averaged 269.87€ (). This information was used for the base case analysis.
Table 2. Base case costs of administration only, from hospital district price lists (excluding drug costs).
The Finnish hospital districts reported equal administration tariff values for both bortezomib and carfilzomib. Thus, equal administration cost only estimates for both drugs were applied when estimating the base case costs of administration.
Travelling costs
The estimated weighted mean non-emergency travel cost per hospital visit based on the national reimbursement data was 100.90€ in 2016 real value (indexed), based on 2015 resources. When the administration cost only estimate was included together with the non-emergency travel cost in the base case, the average base case cost for administration increased to 370.77€ per visit when including the administration only cost. Therefore, the cost per cycle in the base case was 1483.08€ for bortezomib and 2224.62€ for carfilzomib.
However, when the average administration cost estimates were based on the deduction of drug prices from the costs of administration visits as an alternative sensitivity analysis scenario that covered most Finnish hospital districts, the administration and travelling costs were 850.53€ for bortezomib and 634.87€ for carfilzomib. This demonstrated the conservativity of base case estimates.
Productivity costs
An estimated mean productivity cost due to administration visit-related absenteeism per employed patient was 63.28€ per visit and 253.13€ per cycle for bortezomib, and 121.57€ per visit and 729.41€ per cycle for carfilzomib. When these estimates were adjusted to comply with the real-world age distribution of Finnish MM patients and the age-specific Finnish employment rate at the base case analysis, the mean productivity cost per patient was 21.27€ per visit and 85.08€ per cycle for bortezomib, and 40.86€ per visit and 245.15€ per cycle for carfilzomib.
When calculated based on the Finnish real-world age distribution of novel, conventional, and novel + ASCT 1st line treatment choice sub-groups of MM patients in the sensitivity analysis, per-visit productivity losses for bortezomib administration visit were 16.35€, 5.91€, and 34.42€, respectively. The respective productivity losses for carfilzomib administration visits were 31.40€, 11.35€, and 66.12€, respectively.
Per visit productivity loss for the person accompanying the patient was estimated as 13.21€ for bortezomib and 25.38€ for carfilzomib. Thus, total productivity loss came to 34.48€ per visit and 137.93€ per cycle for bortezomib, and 66.24€ per visit and 397.46€ per cycle for carfilzomib.
Total cost of administration visit
Total base case costs of one drug administration visit (including direct costs and productivity costs, but excluding drug cost) were estimated at 304.35€ per visit and 1,217.41€ per cycle for bortezomib, and 336.11€ per visit and 2,016.68€ per cycle for carfilzomib. When travel costs from reimbursement data were added to the base case, the total costs increased to 405.24€ per visit and 1,620.96€ per cycle for bortezomib, and 437.00€ per visit and 2,622.00€ per cycle for carfilzomib.
Variability of travelling costs
visualizes average travel times from municipal city centres to the nearest central hospitals in Finland. Finnish travel times and productivity costs vary greatly due to the diverse geographical distances. The population-weighted average one-way travelling time to central hospitals across the country, based on the results presented in , was found to be 27.6 min. In a separate study, Lankila et al.Citation11 reported that the average one-way travelling time to Finland’s central hospitals was 27 min. Therefore, municipality-level analysis reported here is seen to accurately represent the impact of long distances on total administration costs.
Figure 1. Travelling times in minutes from each Finnish municipality to the hospital district’s central hospital.
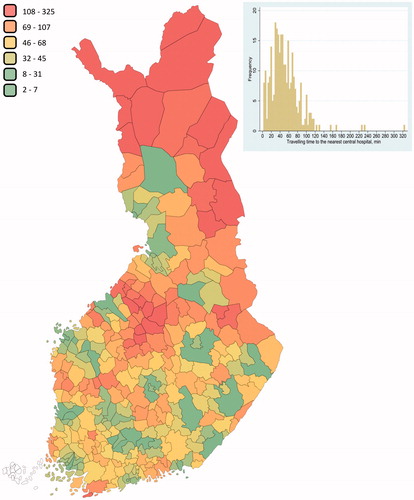
As seen in , most of the travelling times from individual municipalities are longer than the population-weighted average. To further demonstrate the impact of geographical variability on the total costs of the administration visits, the following examples were considered: The median one-way travelling time of 45 min for all municipalities increased the total productivity loss per administration visit to 47.40€ (not adjusted for employment rate), a 63% rise compared to the loss for the mean of 27.6 min. In the same way, the analysis presented the following impact on productivity loss compared to the population-weighted average: −13.98€ (−46%) for the 10th percentile, +3.23€ (+11%) for the 25th percentile, +44.31€ (+145%) for the 75th percentile, and +67.74€ (+221%) for the 90th percentile.
The impact of distance to the nearest central hospital on the total cost of travelling (including administration cost, direct travelling costs, and associated productivity costs) was observed to be significant ().
Discussion
The study results demonstrated that the hospital administration of MM drugs causes significant and potentially avoidable costs. The average base case total costs for bortezomib were 405.24€ per administration visit, and 1620.96€ per cycle, when administration-, travelling-, and productivity loss-related costs were included. The respective costs for carfilzomib were 437.00€ per visit and 2622.00€ per cycle.
The base case estimates were conservative. The administration costs obtained by subtracting the official drug prices from the tariff values which included also drug costs were significantly higher in comparison to the base case estimates. In addition, costs associated with travelling to the hospital for administration (productivity loss and cost of transportation) form a significant proportion of total costs, particularly in sparsely populated areas. In the present study, productivity loss and transportation formed 33% of the total costs of bortezomib administration visit and 38% of those of carfilzomib.
The changes in employment rate between different 1st line treatment patterns had significant impacts on the total productivity cost. Patients receiving novel agents and ASCT experience more productivity loss compared to the average MM patient as they are generally younger. On the contrary, patients receiving conventional treatments experience less productivity loss due to older age.
Municipality-level analysis shows that travel times to central hospitals are extensive for residents of most Finnish municipalities. However, the average population-weighted travel time remains moderate, as most of the population is concentrated in urban areas that contain central hospitals.
As expected, longer distances to hospitals were strongly associated with higher total costs. The linear trend of association was evident, even though the differences in administration costs among hospital districts were also responsible for some variation in total cost. Furthermore, travel times to central hospitals in the most highly populated cities were not always the shortest, even if the central hospital was in the city. This was likely due to slower trips due to lower speed limits and congestion.
Based on previously published international literature, the administration of intravenous or subcutaneous MM treatment causes significant healthcare costsCitation14–18. These studies report single session administration costs ranging from 145€ to 516€ (converted to euros at 2016 rates). The average cost estimate based on this previous data is 271€, which falls very close to our price tariff review result. We are not aware of any previous studies of Finnish administration and travelling costs for MM treatments.
For example, two Finnish HEEs have included assumed administration costs for MM treatments in their estimates. The Finnish Medicines Agency, Fimea, presented an administration cost of 3.95€ for bortezomib and 23.80€ for carfilzomib in its first February 2016 assessment of relapsed and refractory MMCitation19. The results of our study and the previous literature described above prove these estimates to be very low. A second Fimea MM assessment (elotuzumab) from December of that same year stated an administration cost of 85.00€ for bortezomib and 255.00€ for carfilzomibCitation20. In this case, the latter estimate matches our conservative base case result of 269.87€ quite closely.
A recent study of newly-diagnosed MM patients from the US described a favorable effect of oral chemotherapy on productivity loss compared to injectable chemotherapy. Patients receiving only oral chemotherapy missed on average 22 work days less during the first year after MM diagnosis than those receiving injectable therapy, and their monetary productivity loss was US$3862 smallerCitation21. Productivity loss based on absenteeism alone is a significant cost driver. However, the heterogeneity of parenteral and non-parenteral chemotherapy user populations in the study may have biased the results.
Bortezomib- and carfilzomib-based treatments were used as examples in this study because they are currently among the most common hospital-administered treatments available for MM patients in Finland. One limitation of our study is that hospitals use drug price categories for determining administration pricing, making the estimation of actual administration costs based on combined drug and administration unit prices and drug wholesale prices difficult. This naturally increases the imprecision of our unit cost estimate, although high administration costs do seem evident nonetheless.
Our study also revealed an inconsistency: hospital prices that included the cost of the drugs yielded significantly higher administration cost estimates when the drug costs were subtracted than prices that did not include drug costs. This portends inadequate cost estimation for tariffs that do not include the price of the drug, as the “hidden” costs of drug ordering, storage, preparation, logistics, and/or disposal can be inadvertently omitted. This oversight could lead to significant under-estimates of average administration-related healthcare costs. Despite this potential imprecision, the conservative base case administration cost results provided in this study can be useful estimates for future HEEs.
Another limitation of our study is that we assumed only one administration cost for bortezomib, even though bortezomib can be administered both intravenously and subcutaneously. IV bortezomib is given as a bolus injection, so it is justified to assume that the cost of an SC administration would not significantly deviate from this, as both require a visit to the hospital in Finland.
Home administration is used for subcutaneous bortezomib in some countries, and it seems to reduce administration-related costs compared to hospital administrationCitation18,Citation22. However, home administration of bortezomib is not a part of regular treatment practice in Finland, and, therefore, it was excluded from this analysis.
In addition, as the unit cost estimate of travelling was based on national register data on travel reimbursements, it did not include trips that fall under the deductible fee. The Finnish State covers a portion of transport or travel costs to heathcare facilities for reasons of illness, pregnancy, or childbirth. There is a deductible payment of 25€ per one-way travel and 300€ per year for all travel, regardless of whether the cost exceeded the one-way deductible fee. Thus, all transportation costs that fell under the per person limits were excluded from the analysis. Furthermore, reimbursement for travel costs needs to be personally applied for from the SII. Therefore, all patients eligible to a travel reimbursement are probably not included in the data.
This analysis utilized publicly available official wholesale prices for bortezomib and carfilzomib. However, in Finland hospitals can negotiate (tender) the drug prices privately, which can lower the actual price paid by the hospital. Thus, the proportion of administration cost in tariffs including both administration and drug costs may be higher than the estimates suggest. This, together with the potentially under-estimated list tariff costs of MM treatment administration mentioned above, further supports the conclusion that this study’s base case estimates are very conservative and by no means over-estimate the costs of PI administrations at Finnish hospitals.
There is no unambiguous definition of what kind of hospital administration visit from the price tariffs is required for bortezomib and carfilzomib administration, and, for this reason, the information can vary. Only two hospital districts (10% of the total) listed an explicit unit cost for a single administration visit with no included drug costs. These costs were 97€ and 215€ (mean 156€). The price lists lead us to believe that this can be considered a minimum price, although it also probably excludes such things as the drugs’ ordering, storage, logistics, preparation, and disposal costs.
The present analysis considered average travelling time and time spent at the hospital during the administration visit as the basis for the cost estimation of productivity losses. However, these estimates were based on optimal circumstances, assuming no waiting, delays, or other time-consuming interruptions. This is probably unrealistic, leading to under-estimated time use and costs in these areas. Actual productivity loss could, therefore, be higher than our estimates suggest. The inclusion of waiting, delays, or other time-consuming events would be subject to high amount of uncertainty, and were excluded due to lack of data and for simplicity.
Finland is a sparsely populated country, and direct non-healthcare costs and productivity costs due to travelling have a significant impact on the expected total costs of hospital-administered treatments. Our results indicate that administration costs vary substantially according to where the MM patients live. This poses a risk to equal treatment access, especially in cases where the total costs of treatment administration could present a significant burden for both the care providers and the patients. This highlights the importance of considering the costs to society (societal perspective) in HEEs, as well as the potential impact medicines and health technologies can have outside of the healthcare sectorCitation23.
In addition to the significant societal costs arising from the hospital administration of MM drugs, treatment administration can also cause potential quality-of-life losses for patients. It is, therefore, no surprise that patients tend to prefer drugs that can be orally administeredCitation24–27. Despite the HEE focus of this study, we fully acknowledge that economics and financial aspects are just one perspective to consider when selecting a treatment for MM patient. The components of HEE considering both clinical and economic viewpoints have been summarized into the PICOSTEPS principle, which has been utilized in e.g. the Finnish Current Care CriteriaCitation28, as well as real-world evidence-basedCitation29 and modelledCitation30 HEEs. PICOSTEPS reports the content of HEE in its order of importanceCitation30, starting from the patient and ending with sensitivity analysis. This study covered the essential components of PICOSTEPS.
The integrity of future customer-oriented health services in Finland hinges partly on patient wellbeing and knowledge management based on digitized customer-responsive health and social care servicesCitation31,Citation32 and data lakesCitation33. Drug treatments that require multiple visits to hospital for administration may prove a poor fit with new developments like eHealth, self-monitoring, and digital customer interfaces, and evolution extensively supported by numerous Finnish government actors.
Novel oral MM medications such as the first oral PI ixazomib, and IMiDs lenalidomide and pomalidomide, have a significant potential to reduce the administration, travelling, and productivity costs for MM treatment compared to hospital-administered drugs. Oral solutions also support the continued digitalization and customer-orientated development of Finnish healthcare services, to the benefit of MM patients throughout the country.
Transparency
Declaration of Funding
This study was funded by Takeda Oy, Helsinki, Finland.
Declaration of financial/other interests
P.M. is an employee of ESiOR Oy, Kuopio, Finland. J.M. and E.S. are employees, partners, and directors of ESiOR. ESiOR carries out studies, statistical analysis, consultancy, education, reporting, and health economic evaluations for several pharmaceutical, food industry, diagnostics and device companies, hospitals, consultancies, and academic institutions. Neither P.M., J.M., or E.S. received any direct financial support as individuals. P.M., J.M., and E.S. declare no personal conflict of interest. V.V. and S.T. are employees of Takeda Oy, Helsinki, Finland. V.V. and S.T. declare no personal conflict of interest. J.M.E. peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Abstract and congress poster: Value Health 2017;20:A558; Value Health 2017;20:A233.
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385:2197–2208.
- Danylesko I, Shimoni A, Nagler A. Allogeneic stem cell transplantation and targeted immunotherapy for multiple myeloma. Clin Lymph Myelom Leuk. 2013;13:S330–S348.
- van Rhee F, Giralt S, Barlogie B. The future of autologous stem cell transplantation in myeloma. Blood. 2014;124:328–333.
- Suomen myeloomaryhmän (FMG) hoitosuositus 11/2017 [Treatment recommendation of the Finnish Myeloma Group 11/2017]. Finnish Hematology Association. Updated 2017 Nov 1 [cited 2018 Dec 11]. Available from: https://www.hematology.fi/sites/default/files/uploads/fmg_suositus_2017_1.pdf
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. New Engl J Med. 2015;372:142–152.
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. New Engl J Med. 2016;375:1319–1331.
- Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. New Engl J Med. 2015;373:621–631.
- Soini E, Leussu M, Hallinen T. Administration costs of intravenous biologic drugs for rheumatoid arthritis. SpringerPlus. 2013;2:531.
- Olofsson S, Norrlid H, Karlsson E, et al. Societal cost of subcutaneous and intravenous trastuzumab for HER2-positive breast cancer – An observational study prospectively recording resource utilization in a Swedish healthcare setting. The Breast. 2016;29:140–146.
- Krol M, Papenburg J, Koopmanschap M, et al. Do productivity costs matter? The impact of including productivity costs on the incremental costs of interventions targeted at depressive disorders. Pharmacoeconomics. 2011;29:601–619.
- Lankila T, Kotavaara O, Antikainen H, et al. Sosiaali- ja terveyspalveluverkon kehityskuva 2025 – Paikkatieto- ja saavutettavuusperusteinen tarkastelu. [Vision of the development of social and health care network in Finland 2025 – Focus on location and availability] Helsinki: Oulun yliopisto, Maantieteen tutkimusyksikkö; 2016.
- Soini E, Hallinen T, Sokka AL, et al. Cost-utility of first-line actinic keratosis treatments in Finland. Adv Ther. 2015;32:455–476.
- Remes K, Anttila P, Silvennoinen R, et al. Real-world treatment outcomes in multiple myeloma: multicenter registry results from Finland 2009–2013. PLoS One. 2018;13:e0208507.
- Hornberger J, Rickert J, Dhawan R, et al. The cost-effectiveness of bortezomib in relapsed/refractory multiple myeloma: Swedish perspective. Eur J Haematol. 2010;85:484–491.
- Armoiry X, Fagnani F, Benboubker L, et al. Management of relapsed or refractory multiple myeloma in French hospitals and estimation of associated direct costs: a multi-centre retrospective cohort study. J Clin Pharm Ther. 2011;36:19–36.
- Picot J, Cooper K, Bryant J, et al. The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1–204.
- Roy A, Kish JK, Bloudek L, et al. Estimating the costs of therapy in patients with relapsed and/or refractory multiple myeloma: a model framework. Am Health Drug Benefits. 2015;8:204–215.
- Touati M, Lamarsalle L, Moreau S, et al. Cost savings of home bortezomib injection in patients with multiple myeloma treated by a combination care in outpatient hospital and hospital care at home. Support Care Cancer. 2016;24:5007–5014.
- Rannanheimo P, Härkönen U, Kiviniemi V, et al. Carfilzomib in the treatment of relapsed multiple myeloma. Finnish Medicines Agency Fimea. Serial publication “Fimea develops, assesses and informs” 2016;2/2016:1–27.
- Rannanheimo P, Härkönen U, Kiviniemi V, et al. Elotuzumab in the treatment of relapsed multiple myeloma. Finnish Medicines Agency Fimea. Serial publication “Fimea develops, assesses and informs” 2016;12/2016:1–38.
- Merola D, Yong C, Noga S, et al. Costs associated with productivity loss among U.S. patients newly diagnosed with multiple myeloma receiving oral versus injectable chemotherapy. Blood. 2017;130:3423.
- Lassalle A, Thomaré P, Fronteau C, et al. Home administration of bortezomib in multiple myeloma is cost-effective and is preferred by patients compared with hospital administration: results of a prospective single-center study. Ann Oncol. 2016;27:314–318.
- Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. 2009;10:357–359.
- Muhlbacker AC, Lincke H, Nubling M. Evaluating patients’ preferences for multiple myeloma therapy, a discrete-choice-experiment. GMS Psycho-Social Med. 2008;5:Doc10.
- Jin J, Zhu L, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adher. 2015;9:923–942.
- Leleu X, Mateos M, Delforge M, et al. Assessment of multiple myeloma patient preferences in treatment choices: an international discrete choice study. Blood. 2015;126:2086.
- Eek D, Krohe M, Mazar I, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adher. 2016;10:1609–1621.
- Soini E. Biologisten lääkkeiden kustannusvaikuttavuus nivelpsoriaasin hoidossa [Cost-effectiveness of biologic drugs in the treatment of psoriatic arthritis]. Suomalaisen Lääkäriseuran Duodecimin ja Suomen Ihotautilääkäriyhdistyksen asettama työryhmä [Working group of Finnish Medical Society Duodecim and Finnish Dermatologist Society]. Helsinki: Suomalainen Lääkäriseura Duodecim. Updated 2017 Mar 1 [cited 2018 Dec 15]. Available from: http://www.kaypahoito.fi/web/kh/suositukset/suositus?id=nix02465&suositusid=hoi50062
- Soini E, Joutseno J, Sumelahti ML. Cost-utility of first-line disease-modifying treatments for relapsing-remitting multiple sclerosis. Clin Ther. 2017;39:537–557.e10.
- Soini E, Riekkinen O, Kröger H, et al. Cost-effectiveness of pulse-echo ultrasonometry in osteoporosis management. ClinicoEcon Outcomes Res. 2018;10:279–292.
- Prime Minister's Office. Finland, a land of solutions. Mid-term review. Government Action Plan 2017–2019. Finland: Government Publications; 2017.
- Soini E, Väätäinen S, Arvonen S. Predicted cost-benefit of Virtual Hospital 2.0 in terms of health care capacity freed: Towards potential economic efficiency with digitalization and customer-responsive secondary care services. Belfast, Northern Ireland: WHO International Healthy Cities Conference; 2018.
- Soini E, Hallinen T, Kekoni A, et al. Efficient secondary use of representative social and health care data in Finland: Isaacus data lake, analytics and knowledge management pre-production project. Value Heal. 2017;20:A777.