 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims: Non-adherence is associated with poor clinical outcomes among patients with asthma. While cost-effectiveness analysis (CEA) is increasingly used to inform value assessment of the interventions, most do not take into account adherence in the analyses. This study aims to: (1) Understand the extent of studies considering adherence as part of the economic analyses, and (2) summarize the methods of incorporating adherence in the economic models.
Materials and methods: A literature search was performed from the inception to February 2018 using four databases: PubMed, EMBASE, NHS EED, and the Tufts CEA registry. Decision model-based CEA of asthma were identified. Outcomes of interest were the number of studies incorporating adherence in the economic models, and the incorporating methods. All data were extracted using a standardized data collection form.
Results: From 1,587 articles, 23 studies were decision model-based CEA of asthma, of which four CEA (17.4%) incorporated adherence in the analyses. Only the method of incorporating adherence by adjusting treatment effectiveness according to adherence levels was demonstrated in this review. Two approaches were used to derive the associations between adherence and effectiveness. The first approach was to apply a mathematical formula, developed by an expert panel, and the second was to extrapolate the associations from previous published studies. The adherence-adjusted effectiveness was then incorporated in the economic models.
Conclusions: A very low number of CEA of asthma incorporated adherence in the analyses. All the CEA adjusted treatment effectiveness according to adherence levels, applied to the economic models.
Introduction
Asthma is one of the most common chronic respiratory diseases, affecting ∼300 million people worldwideCitation1,Citation2. It is characterized by several symptoms. For example, coughing, wheezing, shortness of breath, chest tightness, and expiratory airflow limitationCitation2. Asthma symptoms may be absent for weeks or months, and may be responsively resolved to the medicationsCitation1,Citation2. The prevalence of asthma has been increasing over the last few decades. In the US, it was ∼8.2%, while in China, Malaysia, Singapore, and Thailand it was 5.1%, 9.1%, 10.6%, and 12.2%, respectivelyCitation3,Citation4.
Patient non-adherence is a common and costly problem for asthma treatmentCitation5. The evidence revealed that 50% of children and adults did not take their prescribed medications. This is associated with uncontrolled symptoms, and the increase in exacerbation rates and asthma-related deathCitation6. Healthcare utilizations for asthma was very high, being expected to reach up to 2% of the total healthcare expenditure in developed countriesCitation2. Previous studies indicated the importance of patient adherence for asthma. In brief, increasing adherence was associated with improvement in asthma control and lung function, as well as reductions in exacerbation rate and healthcare utilizationsCitation7–13.
CEA is a well-established framework, that is used to estimate the incremental costs per unit of the incremental benefits provided by an interventionCitation14. Decision model-based CEA facilitates the use of multiple data sources, including costs of treatment and long-term health outcomes, which are not captured through trial-based CEA. Results of the cost-effectiveness, referred to as an incremental cost-effectiveness ratio (ICER), is used as a supportive document, enabling healthcare professionals and policy-makers to make effective decisions pertaining to health technology assessment (HTA) of the interventionsCitation15.
Previous literature reviews investigated CEA that incorporated adherence in the analyses. A systematic review by Rosen et al.Citation16 determined the inclusion of patient adherence in the CEA. The authors found that, among 177 included studies, less than one-third (54 studies) integrated sub-optimal adherence in the economic analyses. A study by Hughes et al.Citation17 evaluated the impact of non-adherence on the cost-effectiveness of different drug therapies. The authors included 22 studies in the review, and showed that non-adherence reduced the efficacy of therapies, but its impact on health care costs was varied. Another study by Cleemput et al.Citation18 reviewed literature on the economics of therapeutic non-adherence, and identified methodology flaws. Eighteen studies were included, and assessed according to their definition, measurement of adherence, study designs, as well as identification and valuation of costs and outcomes. The results indicated that most studies lacked methodological rigor, and failed to meet qualitative standards. The most updated review, conducted in 2007 by Hughes et al.Citation19, highlighted the importance of integrating adherence in economic evaluations. Although the methods of incorporating adherence were characterized, there was a great deal of inconsistency in adherence definitions, and the integrating methods from study to study. In addition, the authors only included 10 studies in the analysis. This resulted in limited generalizability of the study findings across therapeutic areas.
Non-adherence is associated with poor clinical outcomes among patients with asthma. Although CEA is increasingly used to inform value assessment of the interventions by healthcare professionals and policy-makers, one important aspect that still lacks clarity is how to incorporate adherence in the analysis. To our knowledge, none of the studies provide an insight regarding the methods of incorporating adherence in CEA of asthma, thus the knowledge on such practices is still limited. This study aims to: (1) Understand the extent of studies considering adherence as part of the economic analyses, and (2) Summarize the methods of incorporating adherence in the economic models. Our findings will provide healthcare professionals and policy-makers with current evidence of the methods used to incorporated adherence in the CEA of asthma.
Methods
Search strategy
A literature search was performed from the inception to February 2018 using four databases: PubMed, EMBASE, NHS EED, and the Tufts CEA Registry. The search filters used for identifying economic evaluations were combined with various search terms including cost-effectiveness, cost-utility, economic evaluation, and asthmaCitation20. The bibliographies of retrieved articles were examined for the studies that were not indexed in the aforementioned databases.
Study selection
Initially, the titles and abstracts were screened to identify the potential studies. Only decision model-based CEA of the pharmacological interventions for asthma, which included the results of incremental costs per unit of the incremental benefits, were included in the analysis. Decision model-based CEA of non-pharmacological interventions were out of the scope of this review. Outcomes of interest were the number of studies that incorporated adherence in the analyses, and the methods of incorporating adherence in the economic models. Only studies published in English were included. Full texts of relevant studies were assessed by BC and PD, and all disagreements between the investigators were resolved by a third reviewer (NC).
Data extraction and quality assessment
Data extraction was undertaken by BC and PD, using a standardized data collection form. The extracted data included author names, publication years, study countries, study objectives, the characteristics of participants and interventions, comparators, study outcomes, types of economic analysis, study models, perspectives, cycle lengths, time horizons, study results, and adherence data. All studies were assessed for their methodological qualities, and the quality of the reporting studies by BC and PD, using the Consensus on Health Economic Criteria-extended (CHEC-extended) and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS), respectively (Supplementary Table S1E)Citation21–23.
Data analysis
The number of CEA that incorporated adherence in the analyses were calculated as the percentage of studies considering adherence as part of the economic analyses. All the CEA were reviewed to summarize adherence data, methods of incorporating adherence, and the impact of adherence on the cost-effectiveness results.
Results
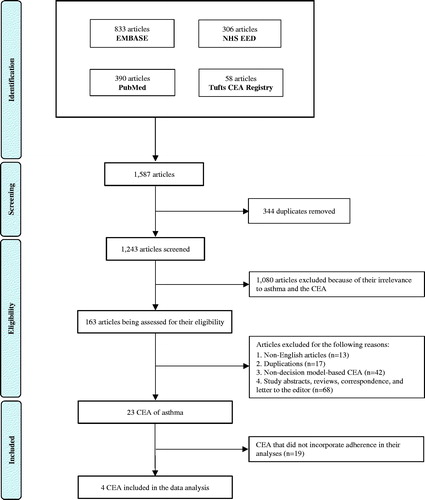
The initial search yielded 1,587 articles, of which 344 duplicates were removed. The remaining 1,243 articles were screened through the titles and abstracts. A total of 1,080 articles were excluded because of their irrelevance to asthma and the CEA. This resulted in 163 articles being assessed for their eligibility. A further 140 articles were excluded for the following reasons; non-English articles (n = 13), duplications (n = 17), non-decision model-based CEA (n = 42), as well as the abstracts, reviews, correspondence, and letters to the editor (n = 68). This yielded a total of 23 CEA of asthma, of which four incorporated adherence in the analysesCitation24–27. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is shown in . Results of the initial search are presented in Supplementary Table S2E.
Figure 1. A PRISMA flow diagram describing the study selection process. This figure demonstrates the approach of studies, included in this systematic review. Overall, 1,587 articles were identified through the initial search, of which 163 were assessed for their eligibility. In conclusion, a total of 23 CEA of asthma were identified, and four of the studies incorporated adherence in the analyses.
General characteristics
Twenty-one studies (91.3%) were conducted to carry out the cost-effectiveness of interventions in a single country; US (eight studies)Citation24,Citation27–33, Colombia (three studies)Citation25,Citation26,Citation34, UK (three studies)Citation35–37, Canada (two studies)Citation38,Citation39, Italy (two studies)Citation40,Citation41, Australia (one study)Citation42, Germany (one study)Citation43, and Sweden (one study)Citation44, whereas two studies (8.7%) were conducted in multiple countries; UK, Netherlands, and SpainCitation45, and two World Health Organization (WHO) sub-regions, countries in sub-Saharan Africa with very high adult and high child mortality, and countries in South East Asia with high adult and high child mortalityCitation46 (). The study characteristics of 23 CEA are shown in .
Figure 2. Diagrams demonstrating characteristics of the included studies: (a) Studies conducted to carry out the cost-effectiveness of interventions in a single or multiple countries. (b) Types of decision-analytic models, used in the analyses.
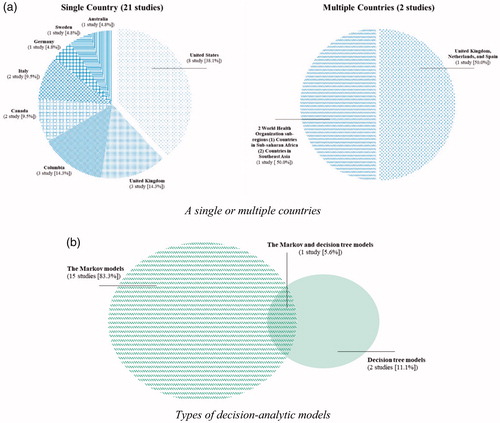
Table 1. Characteristics of the included studies.
Study participants
Among 23 CEA, six studies investigated the CEA in all patients with asthma; moderate-to-severe (two studies [8.7%])Citation29,Citation33, severe (one study [4.3%])Citation44, and at varying levels of asthma severity (three studies [13.0%])Citation39,Citation45,Citation46. Seven studies investigated CEA in adults with asthma: moderate-to-severe (three studies [13.0%])Citation40,Citation41,Citation43, severe (two studies [8.7%])Citation31,Citation32, and at varying levels of asthma severity (two studies [8.7%])Citation24,Citation42, while four studies (17.4%)Citation25,Citation26,Citation34,Citation38 only investigated CEA in children at varying levels of asthma severity. Other groups of study participants are shown in .
Pharmacological interventions
Six studies compared the use of standard therapy plus monoclonal antibodies; omalizumab (five studies [21.7%])Citation29,Citation32,Citation36,Citation37,Citation44, and mepolizumab (one study [4.3%])Citation31, to standard therapy alone. One study (4.3%)Citation33 compared omalizumab to standard therapy. Two studies (8.7%)Citation40,Citation45 compared between the combination inhalers; beclomethasone dipropionate plus formoterol, and fluticasone propionate plus salmeterol. One study (4.3%)Citation35 compared the combined salmeterol xinafoate plus fluticasone propionate with fluticasone propionate, salmeterol xinafoate plus fluticasone propionate (separated inhalers), and budesonide plus formoterol (combination inhaler), whereas one (4.3%)Citation39 only compared the combined salmeterol xinafoate plus fluticasone propionate with fluticasone propionate. One study (4.3%)Citation27 compared the combined fluticasone propionate plus salmeterol with fluticasone propionate, non-fluticasone propionate inhaled corticosteroids, and leukotriene receptor antagonists (LTRA). One study (4.3%)Citation41 compared beclomethasone, beclomethasone-extrafine, budesonide, and fluticasone propionate with beclomethasone dipropionate or beclomethasone dipropionate-extrafine. Other pharmacological interventions are shown in .
Types of decision-analytic model, perspectives, and time horizons
Eighteen studies reported models used in the analyses. The majority of studies (15 studies [83.3%])Citation24–26,Citation28–34,Citation36,Citation37,Citation40,Citation44,Citation45 applied the Markov models, while two studies (11.1%)Citation27,Citation38 used decision tree models. One study (5.6%)Citation41 applied both Markov and decision tree models (). Twenty-two studies reported the perspectives. Most studies (16 studies [72.7%])Citation25–29,Citation31,Citation33,Citation34,Citation36,Citation37,Citation39–43,Citation45 used the healthcare perspective, five studies (22.7%)Citation24,Citation30,Citation32,Citation35,Citation44 used the societal perspective, and one (4.5%)Citation38 used the hospital perspective, which included all the costs pertaining to the emergency department and hospital admissions. The two most commonly reported time horizons were lifetime (30.4%)Citation29,Citation31,Citation36,Citation37,Citation40,Citation44,Citation46 and 1 year (30.4%)Citation25–27,Citation34,Citation35,Citation39,Citation42. Other time horizons are shown in .
Incorporating adherence in cost-effectiveness analyses
Among 23 CEA, four studies (17.4%) incorporated adherence in the analyses. A study by Shih et al.Citation27 estimated the cost-effectiveness of fluticasone propionate plus salmeterol administered in a single-inhaler, compared with fluticasone propionate, non-fluticasone propionate inhaled corticosteroids, and LTRA. Results showed that combined fluticasone propionate plus salmeterol was the most cost-effective strategy. A study by Rodriguez-Martinez et al.Citation26 compared budesonide, ciclesonide, and fluticasone propionate with beclomethasone dipropionate. The study revealed that beclomethasone dipropionate was associated with the lowest cost, while fluticasone propionate gained the highest number of QALY. Beclomethasone dipropionate was the most cost-effective therapy when the willingness-to-pay threshold was less than £21,129.22/QALY, or otherwise, it was fluticasone propionate. Another study by Rodriguez-Martinez et al.Citation25 compared once-daily budesonide with a twice-daily dose, and demonstrated that a once-daily dose was a dominant strategy. A study by Zafari et al.Citation24 investigated the cost-effectiveness of improving adherence to controller medications, comparing between the hypothetical scenario, in which all patients were fully adherent to the medications (full-adherence), and status quo scenario (the current status of patient adherence). The authors showed that full-adherence was cost-effective.
Adherence data
Adherence data, including the definitions, therapeutic levels, and data sources, were varied from study to study (). Shih et al.Citation27 used patients’ refill patterns that were adapted from observational studies and claims dataCitation47–51. Rodriguez-Martinez et al.Citation26 applied an assumption of decreasing adherence over time for a twice-daily dose of inhale corticosteroids (ICS), assessed by counting the remaining doses in the inhaler device based on a randomized controlled trial (RCT)Citation52. Subsequently, the authors applied a difference in adherence between once-daily and twice-daily administrations which was adapted from a randomized, single-blind, clinical trialCitation53. Likewise, Rodriguez-Martinez et al.Citation25 applied an assumption of decreasing adherence over time for a once-daily dose of ICS, assessed by using a device that recorded the date and time of an inhaler actuation based on an observational studyCitation54, and used the difference in adherence levels between once-daily and twice-daily administrations, adapted from a randomized, single-blind, clinical trialCitation53. Zafari et al.Citation24 calculated adherence levels using the proportion of days covered (PDC) of patients that were extrapolated from a RCTCitation55.
Table 2. Adherence data.
Methods of incorporating adherence
Only the method of incorporating adherence by adjusting treatment effectiveness according to adherence levels was demonstrated in this review. Two approaches were used to derive the associations between adherence and effectiveness. The first approach was to apply a mathematical formula that assumed the effectiveness slowly decreased at the first following an exponential curve, as adherence fell below 100%, and increased the rate when it was below 30% following a linear curve. This mathematical formula was derived based on the consultation with an expert panel, involved pulmonologists and allergists as demonstrated below,
The authors assumed an exponential decline with the constant rate equal to 5, and applied a modifying factor of 0.2287 to confirm the intersection between non-linear and linear functions at an adherence rate of 30%. The adherence-adjusted effectiveness was taken into account as an input parameter, and incorporated in the economic model.
This approach was developed by Shih et al.Citation27, and used in the other two studies by Rodriguez-Martinez et al.Citation25,Citation26. In the Shih et al.Citation27study, the effectiveness measures included the proportion of patients that were free of symptoms, and from the use of rescue medication, adapted from the RCT. A decision tree model was used to follow the patients at 3-month intervals, throughout the year study period, starting at the initiation of their medications. Patients were assumed to have the opportunity of switching to another therapy, or withdrawing from the study. At the end of the study, they were in one of the following health states: (1) Free of symptoms, (2) Experienced mild symptom, but had not needed rescue medication, (3) Experienced mild symptom that required the use of rescue medication, and (4) Experienced one or more exacerbations. In the Rodriguez-Martinez et al.Citation25,Citation26studies, the effectiveness was the proportion of patients at risk of exacerbation, adapted from a systematic review and meta-analysis of RCT. The Markov model, which consisted of three health states: (1) No symptoms, (2) Suboptimal control, no exacerbation, and (3) Exacerbation, was applied to the studies, using a cycle length of 1 week over a 12-month period.
The second approach of deriving the associations between adherence and effectiveness was to extrapolate the relationships from previous published studies. This approach was used in a study by Zafari et al.Citation24. First, they calculated adherence levels based on an actual dose of ICS, taken by patients in a RCTCitation55, resulting in the PDC values of 0%, 25%, and 75%. Second, an association between each of the 25% decreasing PDC and relative risk (RR) of 1.26 for the rates of exacerbation was adapted from a retrospective cohort studyCitation56. The authors combined those PDC values with this RR, and then estimated the RR of exacerbation to be ∼2 for the patients with PDC of 25%, and 1.2 for PDC of 75%. For those who did not use any medication, or having PDC of 0%, the authors applied the RR of 1.53, obtained from a systematic review and meta-analysis of RCT which compared the clinical outcomes of using ICS vs no controller medicationCitation57. Lastly, those RR associated PDC were adjusted based on follow-up periods of the studies, resulting in the RR of 1.40, 1.36, and 1.09 for patients with PDC of 0%, 25%, and 75%, respectively. The adherence-adjusted RR were then applied to the model. In the Zafari et al.Citation24 study, the authors developed the Markov model, which used a cycle length of 1 week throughout the 10-year time horizon, in which the patients transitioned between the following health states: (1) Controlled asthma, (2) Partially controlled asthma, (3) Uncontrolled asthma, (4) Exacerbation, and (5) Death.
Impact of adherence on cost-effectiveness results
Out of four CEA, two studies (50.0%) assessed the impact of adherence on cost-effectiveness results. Shih et al.Citation27 performed one-way sensitivity analysis by varying adherence levels for all the ICS to be 70%, and assumed the associations between adherence and effectiveness of the clinical outcomes; proportion of patients that were free of symptoms, and free of rescue medication use to be fully exponential or linear. Results showed that single-inhaler salmeterol and fluticasone propionate remained cost-effective. Another study by Zafari et al.Citation24 varied the RR of exacerbation associated PDC, and determined that the full-adherence scenario was cost-effective, as long as each of the 25% increases in the PDC reduced the exacerbation rates by at least 1.1-fold.
Quality of studies
According to the CHEC-extended, all studies clearly identified the description of the interventions, study designs, time horizons, perspectives, costs, outcomes, discounting, input parameters’ uncertainty, and study conclusions. Most studies clearly described their research questions (20 studies [87.0%])Citation24–28,Citation30–42,Citation44,Citation45, potential conflicts of interest (19 studies [82.6%])Citation25,Citation26,Citation28–33,Citation35–40,Citation42–46, study populations (18 studies [78.3%])Citation24–34,Citation36–39,Citation41,Citation43,Citation44, ethical issues (16 studies [69.6%])Citation24–28,Citation30–34,Citation36,Citation37,Citation40,Citation42,Citation45,Citation46, and generalizability of the study findings (nine studies [39.1%])Citation24–28,Citation32,Citation36,Citation38,Citation40 (). For the quality of reporting the studies, estimated by CHEERS, all studies provided the explicit statements of background and objectives, comparators, choice of health outcomes, measurement and valuation of the outcomes, estimating resources and costs, currency, price date and conversion, analytical methods, incremental costs and outcomes, study findings, limitations, generalizability, and current knowledge. Most studies reported their settings and locations (22 studies [95.7%])Citation24–30,Citation32–46, study perspective (22 studies [95.7%])Citation24–45, source of funding (22 studies [95.7%])Citation24–40,Citation42–46, study assumptions (21 studies [91.3%])Citation24–41,Citation44–46, and measurement of effectiveness (20 studies [87.0%])Citation24–39,Citation41,Citation42,Citation44,Citation45 ().
Table 3. Quality assessment of the study methodology.
Table 4. Quality assessment of the reported studies.
Discussion
Patient non-adherence is associated with asthma-related clinical outcomes and healthcare utilizations; however, one important aspect that still lacks clarity is how to incorporate adherence in the economic analysis. This study investigated the extent of studies that considered adherence as part of the economic analyses, and the methods of incorporating adherence in the CEA of asthma. Our findings show that very low numbers of the CEA incorporated adherence in the analyses. Only the method of incorporating adherence by adjusting treatment effectiveness according to adherence levels was demonstrated in this review. Two approaches were used to derive the associations between adherence and effectiveness. The first approach was to apply a mathematical formula, developed by an expert panel, and the second was to extrapolate the associations from previous published studies.
Incorporating adherence by adjusting treatment effectiveness according to adherence levels was the only method exploited in the economic analysis for asthma, while different methods were observed for other diseases. A literature review by Hiligsmann et al.Citation58 revealed that recent CEA of the interventions for osteoporosisCitation59–61 integrated the probabilities of patients that can be at risk of discontinuation over time. The patients were assumed to have a risk of stopping therapy in each cycle. In addition, offset time of the treatment that was similar to the treatment duration was also applied to the analysis. During this time, the treatment effectiveness is assumed correspondingly. This approach is based on two implicit assumptions. First, patients did not receive any medications after stopping the therapy. Second, the effectiveness of interventions throughout the offset time was estimated by the authors, i.e. the RR of fracture reduction linearly declined to zero by the end of time. Some limitations are recognized by using this method. First, many patients, in fact, can restart their medications any time after they discontinued the therapy. The evidence revealed that one-third of patients restarted their medications within 6 months after discontinuation. Second, the information of treatment effectiveness during the offset time lacked supporting data. It is, therefore, difficult to estimate the effectiveness of interventions in the long-term.
The approach recommended by Hiligsmann et al.58 is to apply real-world estimates among the patients who complied with the medications. Using this approach, patients were classified into two groups; (1) Compliant patients (medication possession ratio [MPR] ≥ 80%), and (2) Poorly compliant (MPR < 80%), were assigned the probabilities of being adhered or not based on the real-world adherence data. The associations between adherence levels and the RR of fracture reduction were also assumed accordingly. This approach is in line with what we found in a study by Zafari et al.Citation24. Both studies classified adherence levels into various groups. However, the difference is that, in the Zafari et al.Citation24 study, patients who did not use any medication were applied the RR, obtained from a systematic review and meta-analysis of RCT, while in the Hiligsmann et al.Citation62,Citation63 studies, the authors used this information that was derived from a real-world database. Even though a systematic review and meta-analysis of RCT demonstrates the highest quality of evidence compared to other types of study designCitation64, using adherence data extrapolated from this will not be able to provide real-world data, since the pooled estimates were calculated based on the RCT. Therefore, using adherence data adapted from a real-world database would provide more accurate findings, but the quality of evidence is needed to be confirmed whether it is sufficiently high to synthesize the information that meets healthcare requirements.
Use of the Markov model captures the entire cohort of patient adherence in the economic analysis, but not that of individuals. It is noteworthy to highlight the method of incorporating adherence that is exploited in a study by Slejko et al.Citation65, where the authors applied a microsimulation modeling technique to determine the real-world adherence scenario of patients with statin therapy for the primary prevention of cardiovascular (CV) disease. A Markov model was modified to simulate individual adherence to statins, by integrating three additional health states in the existing Markov structure. These health states represented the different levels of adherence that were measured as the PDC; (1) PDC < 20%, (2) 20% ≤ PDC < 80%, and (3) PDC ≥ 80%. They assigned transitional probabilities between the PDC levels, and applied the associations between changes in adherence to statins and the risk of CV events, according to pharmacy claims data particularly reflecting patient adherence history. The microsimulation technique identifies individual patients by tracking their characteristics and disease backgrounds, and then used this record information to adjust the transition probabilities, effectiveness, utility values, and costs, to reflect the patient history over the study period. The use of microsimulation models has the potential to provide more accurate data than the cohort-based models. However, the drawbacks of this are the difficulty in obtaining relevant input parameters, and more detail required for the data set in the modeling approach. In addition, there is greater variance in the results because of the random variation in individual outcomesCitation66. With respect to some advantages of the technique, a microsimulation modeling might be considered one of the methods used to integrate adherence for the future economic analyses of asthma.
Out of four CEA that were included in the current systematic review, two performed a one-way sensitivity analysis to assess the impact of varying adherence levels on the cost-effectiveness results. One study showed the findings were robust across all change of adherence, while another demonstrated that the results were cost-effective as long as each of the 25% increases in adherence reduced the exacerbation rates by at least 1.1-fold. This reveals that adherence alters the results when it potentially affects treatment effectiveness of the interventions. To date, knowledge on when adherence should be considered in the economic model is still unclear, since the majority of studies do not take into account adherence in the analyses. Further research is warranted to understand more on the effects of adherence on cost-effectiveness findings.
Another aspect worth mentioning is the application of adherence in coverage and reimbursement decision-making. It is important to consider patient adherence as part of the economic analysis, since varying adherence levels can alter the cost-effectiveness results. Among the CEA included in the current review, various levels of adherence were used across the studies. This is because the adherence data were varied from study to study. In addition, some CEA assumed the constant rates of adherence throughout the study periods, while some modified the rates over time. The findings of this may not be sufficient to draw a valid conclusion on which adherence level should be used as a point of reference to incorporate in the analysis. Therefore, incorporating which level of patient adherence in the CEA depends on the medications and therapeutic areas under specified context of the study. In real-world practice, it may be difficult to confirm that the patients adhere with their prescribed medications at a certain level. An effort in improving adherence should be considered as one of the routine components in diagnostic and management of asthma care. Healthcare professionals and policy-makers may consider to provide additional interventions to improve adherence to ensure such patients achieve their adherence targets satisfactorily. There is a call for future research pertaining to adherence strategies that are feasible for implementation in the clinical settings.
Among the included CEA which incorporated adherence in the models, RCT was used as a source of adherence data in two studies, while observational studies were applied in the other two studies. Although RCT minimizes the potential biases and confounders that may arise from study methodology and the clinical heterogeneities, it restricts the characteristics of participants, types of intervention, and the outcomes of interest. This raises concerns on generalizability of the study findings that may be limited by restrictions. Adherence data would ideally be derived from the observational studies or patient claims data. Many factors, i.e. age, comorbidities, and the numbers of medications, are associated with patient adherenceCitation67, and, thus, having an impact on economic consequences. However, it is vital to ensure the quality of observational studies to obtain accurate estimates based on real world evidence under specified contexts.
The aim of this work was to conduct a systematic review that complied with a PRISMA guideline, while some limitations are acknowledged in this review. First, the majority of included studies fail to report the structural assumptions and validation methods of their economic models, as well as the values, ranges, and probability distributions among input parameters. Caution should be exercised when interpreting the study findings, because biases arising from these could affect their reported outcomes, and thus limit the generalizability of the results. This suggests that further research with rigorous methodology pertaining to this area is warranted to prevent the potential for bias and imprecise results. Second, some of the non-English articles were identified through our search results. However, only the studies published in English were included in this review, because of the lack of experts in other languages involved in our study. This may be one of the reasons why a limited number of studies incorporating adherence have been identified. In addition, although a number of non-decision model based CEA were identified from the search results, our primary objective was to summarize the methods of incorporating adherence in the economic models, by only using the model-based CEA. We believe that the current review provides the most updated evidence relating to the methods of incorporating adherence in the CEA of asthma, based on justified assumptions and study methodology.
Conclusions
This systematic review gathered all relevant evidence pertaining to the CEA of asthma, and summarized the methods of incorporating adherence in the economic models. A very low number of CEA of asthma incorporated adherence in the analyses. All the CEA adjusted treatment effectiveness according to adherence levels, applied to the models. Our findings will provide healthcare professionals and policy-makers with current evidence of the methods used to incorporate adherence in the CEA of asthma.
Transparency
Declaration of funding
The authors received no specific funding for this work.
Declaration of financial/other interests
BC, NC, and PD declare no potential conflicts of interest with respect to the research, organization, and publication of this work. BC received a scholarship from Naresuan University Graduate School for postgraduate study. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
This research work was selected as the best podium research presentation at the International Society for Pharmacoeconomics and Outcomes Research 8th Asia-Pacific conference, held in Tokyo, Japan, on September 8–11, 2018.
Supplemental Material Incorporating Adherence
Download MS Word (23.6 KB)Acknowledgements
The authors wish to thank Peter Barton from the writing clinic at Naresuan University Language Center for editing this work.
References
- World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva: WHO Press; 2007.
- Global Initiative for Asthma. Global strategy for asthma management and prevention; Jul 18, 2018. Available from: https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/.
- The International Union Against Tuberculosis and Lung Disease. The global asthma report; Jul 18, 2018. Available from: https://globalasthmareport.org.
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;32:1–14.
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44.
- Lindsay JT, Heaney LG. Nonadherence in difficult asthma – facts, myths, and a time to act. Patient Prefer Adherence. 2013;7:329–336.
- Lasmar L, Camargos P, Champs NS, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy. 2009;64:784–789.
- Stern L, Berman J, Lumry W, et al. Medication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care data. Ann Allergy Asthma Immunol. 2006;97:402–408.
- Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128:1185–1191.
- Marceau C, Lemiere C, Berbiche D, et al. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J Allergy Clin Immunol. 2006;118:574–581.
- Small M, Anderson P, Vickers A, et al. Importance of inhaler-device satisfaction in asthma treatment: real-world observations of physician-observed compliance and clinical/patient-reported outcomes. Adv Ther. 2011;28:202–212.
- Gamble J, Stevenson M, Heaney LG. A study of a multi-level intervention to improve non-adherence in difficult to control asthma. Respir Med. 2011;105:1308–1315.
- Delea TE, Stanford RH, Hagiwara M, et al. Association between adherence with fixed dose combination fluticasone propionate/salmeterol on asthma outcomes and costs. Curr Med Res Opin. 2008;24:3435–3442.
- Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
- Towse A, Pritchard C, Devlin N. Cost-effectiveness thresholds: economic and ethical issues. London: BSC Print Ltd. King's Fund and Office of Health Economics; 2002.
- Rosen AB, Spaulding AB, Greenberg D, et al. Patient adherence: a blind spot in cost-effectiveness analyses? Am J Manag Care. 2009;15:626–632.
- Hughes DA, Bagust A, Haycox A, et al. The impact of non-compliance on the cost-effectiveness of pharmaceuticals: a review of the literature. Health Econ. 2001;10:601–615.
- Cleemput I, Kesteloot K, DeGeest S. A review of the literature on the economics of noncompliance. Room for methodological improvement. Health Policy. 2002;59:65–94.
- Hughes D, Cowell W, Koncz T, et al. Methods for integrating medication compliance and persistence in pharmacoeconomic evaluations. Value Health. 2007;10:498–509.
- Glanville J, Fleetwood K, Yellowlees A, et al. Development and testing of search filters to identify economic evaluations in MEDLINE and EMBASE. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009.
- Evers S, Goossens M, de Vet H, et al. Criteria list for assessment of methodological quality of economic evaluations: consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21:240–245.
- Odnoletkova I, Goderis G, Pil L, et al. Cost-effectiveness of therapeutic education to prevent the development and progression of type 2 diabetes: systematic review. J Diabetes Metab. 2014;5:438.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049
- Zafari Z, Lynd LD, Fitzgerald JM, et al. Economic and health effect of full adherence to controller therapy in adults with uncontrolled asthma: a simulation study. J Allergy Clin Immunol. 2014;134:908.
- Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Cost-utility analysis of once-daily versus twice-daily inhaled corticosteroid dosing for maintenance treatment of asthma in pediatric patients. J Asthma. 2016;53:538–545.
- Rodriguez-Martinez CE, Sossa-Briceno MP , Castro-Rodriguez JA. Cost-utility analysis of the inhaled steroids available in a developing country for the management of pediatric patients with persistent asthma. J Asthma. 2013;50:410–418.
- Shih Y-CT, Mauskopf J, Borker R. A cost-effectiveness analysis of first-line controller therapies for persistent asthma. PharmacoEconomics. 2007;25:577–590.
- Altawalbeh SM, Thorpe JM, Thorpe CT, et al. Cost-utility analysis of long-acting beta agonists versus leukotriene receptor antagonists in older adults with persistent asthma receiving concomitant inhaled corticosteroid therapy. Value Health. 2016;19:537–543.
- Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65:1141–1148.
- Fuhlbrigge AL, Bae SJ, Weiss ST, et al. Cost-effectiveness of inhaled steroids in asthma: impact of effect on bone mineral density. J Allergy Clin Immunol. 2006;117:359–366.
- Whittington MD, McQueen RB, Ollendorf DA, et al. Assessing the value of mepolizumab for severe eosinophilic asthma: a cost-effectiveness analysis. Ann Allergy Asthma Immunol. 2017;118:220–225.
- Wu AC, Paltiel AD, Kuntz KM, et al. Cost-effectiveness of omalizumab in adults with severe asthma: results from the Asthma Policy Model. J Allergy Clin Immunol. 2007;120:1146–1152.
- Zafari Z, Sadatsafavi M, Marra CA, et al. Cost-effectiveness of bronchial thermoplasty, omalizumab, and standard therapy for moderate-to-severe allergic asthma. PLoS One. 2016;11:e0146003.
- Rodriguez-Martinez CE, Nino G, Castro-Rodriguez JA. Cost-utility analysis of daily versus intermittent inhaled corticosteroids in mild-persistent asthma. Pediatr Pulmonol. 2015;50:735–746.
- Doull I, Price D, Thomas M, et al. Cost-effectiveness of salmeterol xinafoate/fluticasone propionate combination inhaler in chronic asthma. Curr Med Res Opin. 2007;23:1147–1159.
- Faria R, McKenna C, Palmer S. Optimizing the position and use of omalizumab for severe persistent allergic asthma using cost-effectiveness analysis. Value Health. 2014;17:772–782.
- Norman G, Faria R, Paton F, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess. 2013;17:1–342.
- Doan Q, Shefrin A, Johnson D. Cost-effectiveness of metered-dose inhalers for asthma exacerbations in the pediatric emergency department. Pediatrics. 2011;127:e1105–1111.
- Ismaila AS, Risebrough N, Li C, et al. Cost-effectiveness of salmeterol/fluticasone propionate combination (Advair(®)) in uncontrolled asthma in Canada. Respir Med. 2014;108:1292–1302.
- Gerzeli S, Rognoni C, Quaglini S, et al. Cost-effectiveness and cost-utility of beclomethasone/formoterol versus fluticasone propionate/salmeterol in patients with moderate to severe asthma. Clin Drug Investig. 2012;32:253–265.
- Marchetti M, Cavallo M, Annoni E, et al. Cost-utility of inhaled corticosteroids in patients with moderate-to-severe asthma. Expert Rev Pharmacoecon Outcomes Res. 2004;4:549–564.
- Simonella L, Marks G, Sanderson K, et al. Cost-effectiveness of current and optimal treatment for adult asthma. Intern Med J. 2006;36:244–250.
- Bruggenjurgen B, Ezzat N, Kardos P, et al. Economic evaluation of BDP/formoterol fixed vs two single inhalers in asthma treatment. Allergy. 2010;65:1108–1115.
- Dewilde S, Turk F, Tambour M, et al. The economic value of anti-IgE in severe persistent, IgE-mediated (allergic) asthma patients: adaptation of INNOVATE to Sweden. Curr Med Res Opin. 2006;22:1765–1776.
- Paggiaro P, Patel S, Nicolini G, et al. Stepping down from high dose fluticasone/salmeterol to extrafine BDP/F in asthma is cost-effective. Respir Med. 2013;107:1531–1537.
- Stanciole AE, Ortegón M, Chisholm D, et al. Cost effectiveness of strategies to combat chronic obstructive pulmonary disease and asthma in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ. 2012;344:e608
- Stoloff SW, Stempel DA, Meyer J, et al. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113:245–251.
- Stempel DA, Meyer JW, Stanford RH, et al. One-year claims analysis comparing inhaled fluticasone propionate with zafirlukast for the treatment of asthma. J Allergy Clin Immunol. 2001;107:94–98.
- Bukstein DA, Henk HJ, Luskin AT. A comparison of asthma-related expenditures for patients started on montelukast versus fluticasone propionate as monotherapy. Clin Ther. 2001;23:1589–1600.
- Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy. 2002;22:166–174.
- Stempel DA, Mauskopf J, McLaughlin T, et al. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med. 2001;95:227–234.
- Jonasson G, Carlsen K, Mowinckel P. Asthma drug adherence in a long term clinical trial. Arch Dis Child. 2000;83:330–333.
- Mallol J, Aguirre V. Once versus twice daily budesonide metered-dose inhaler in children with mild to moderate asthma: effect on symptoms and bronchial responsiveness. Allergol Immunopathol. 2007;35:25–31.
- Jentzsch NS, Camargos PA, Colosimo EA, et al. Monitoring adherence to beclomethasone in asthmatic children and adolescents through four different methods. Allergy. 2009;64:1458–1462.
- Bateman ED, Bousquet J, Busse WW, et al. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy 2008;63:932–938.
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114:1288–1293.
- Sin DD, Man J, Sharpe H, et al. Pharmacological management to reduce exacerbations in adults with asthma: a systematic review and meta-analysis. JAMA. 2004;292:367–376.
- Hiligsmann M, Boonen A, Rabenda V, et al. The importance of integrating medication adherence into pharmacoeconomic analyses: the example of osteoporosis. Expert Rev Pharmacoecon Outcomes Res. 2012;12:159–166.
- Strom O, Borgstrom F, Kanis JA, et al. Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int. 2009;20:23–34.
- Jonsson B, Strom O, Eisman JA, et al. Cost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22:967–982.
- Hiligsmann M, Reginster JY. Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics. 2011;29:895–911.
- Hiligsmann M, Rabenda V, Bruyere O, et al. The clinical and economic burden of non-adherence with oral bisphosphonates in osteoporotic patients. Health Policy. 2010;96:170–177.
- Hiligsmann M, Gathon HJ, Bruyere O, et al. Cost-effectiveness of osteoporosis screening followed by treatment: the impact of medication adherence. Value Health. 2010;13:394–401.
- Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334–336.
- Slejko JF, Sullivan PW, Anderson HD, et al. Dynamic medication adherence modeling in primary prevention of cardiovascular disease: a Markov microsimulation methods application. Value Health. 2014;17:725–731.
- Hiligsmann M, Ethgen O, Bruyere O, et al. Development and validation of a Markov microsimulation model for the economic evaluation of treatments in osteoporosis. Value Health. 2009;12:687–696.
- Papaioannou A, Kennedy CC, Dolovich L, et al. Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging. 2007;24:37–55.
