Abstract
Objectives: This study aimed to analyze (1) the cost-effectiveness of olanzapine orally disintegrating tablet (ODT) compared to olanzapine standard oral tablet (SOT) and (2) the cost-effectiveness of olanzapine-SOT compared to aripiprazole-SOT for patients with schizophrenia in China.
Methods: A microsimulation model was adapted from a healthcare payers’ perspective. The model ran over a 1-year time horizon, using quarterly cycles. The costs of adverse events were acquired through a clinical expert panel. The average bidding prices in China of olanzapine-ODT, olanzapine-SOT, aripiprazole-SOT, and other switch alternatives were used. Inpatient and outpatient medical costs were sourced from the Urban Employee Basic Medical Insurance database in Tianjin. Additionally, adherence, efficacy, safety, and utility data were taken from the literature. Uncertainty of parameters were assessed through one-way and probabilistic sensitivity analyses.
Results: The total annual costs per patient in aripiprazole-SOT arm, olanzapine-SOT arm, and olanzapine-ODT arm are USD 2,296.05, USD 1,940.05, and USD 2,292.81, respectively. The average number of relapses per patient in 1 year in the aripiprazole-SOT arm, olanzapine-SOT arm, and olanzapine-ODT arm, are 0.734, 0.325, and 0.198, respectively. The quality-adjusted life years (QALYs) gained per patient in 1 year in the aripiprazole-SOT arm, olanzapine-SOT arm, and olanzapine-ODT arm are 0.714, 0.737, and 0.758, respectively. Consequently, (1) the incremental cost-effectiveness ratios (ICERs) of administrating olanzapine-ODT over olanzapine-SOT are USD 2,791.96 per relapse avoided and USD 16,798.39 per QALY gained; and (2) the ICERs of using olanzapine-SOT over aripiprazole-SOT are USD –870.39 per relapse avoided and USD –15,477.93 per QALY gained. All ICERs are under the willingness-to-pay threshold in China of USD 25,772.67. The sensitivity analyses confirmed the robustness of the results.
Conclusion: As the first-line treatment for schizophrenia in China, olanzapine-ODT is cost-effective compared to olanzapine-SOT and olanzapine-SOT is cost-effective compared to aripiprazole-SOT.
Introduction
Schizophrenia is a complex and severe mental disorder that ranked in the top 25 leading causes of disability worldwide in 2013Citation1. Although only a small group of people (0.78%) are affected by schizophrenia in their lifetime in ChinaCitation2, it can lead to heavy personal, societal, and economic burdens due to the risk of relapse, hospitalization, disability, and even premature deathCitation3,Citation4.
Antipsychotic medications play a mainstay role in treating the disorder in the acute stage as well as the long-term maintenance to prevent future relapsesCitation5–7. However, poor patient adherence has been reported to be a great challenge to treat schizophrenia in China and elsewhereCitation8,Citation9. With a large proportion of patients being non-adherent to the treatment, the risk of relapse and hospitalization is predicted to increase in the general populationCitation10–12. This, in turn, contributes the most to the economic burden on both patient families and societyCitation13.
Among many factors to affect adherence, evidence indicates that the orally disintegrating tablet (ODT) formulation may produce a better adherence to antipsychotics than the standard oral tablet (SOT) formulationCitation14,Citation15. Therefore, it can be argued that Olanzapine in ODT formulation has potential economic value, even though its price may be more expensive.
In the US, a 1-year Monte Carlo microsimulation model had been previously developed to compare the cost-effectiveness of the ODT formulation and the SOT formulation of three atypical antipsychoticsCitation16. The model provides the total annual direct costs, quality-adjusted life years (QALY) gained in 1 year, and the proportion of patients ever having a relapse in 1 year as the outputs, using the clinical and cost data in the US as the inputs. The model indicated that, in the US, olanzapine-ODT was more cost-effective than olanzapine-SOT and risperidone-SOT, and cost-saving compared to aripiprazole-SOT, risperidone-ODT, and aripiprazole-ODT.
In China, a national survey in 2012 suggested that a majority of patients with schizophrenia were taking atypical antipsychoticsCitation17. Among the atypical antipsychotics, olanzapine is one of the most commonly prescribed agents for patients with schizophrenia, and its ODT formulation became available in 2009; aripiprazole, another mainstream choice, is the main competitor of olanzapine in the market. There has been no comprehensive economic evaluation of olanzapine-ODT, and a limited comprehensive comparison between olanzapine and aripiprazole in China. We examined the cost-effectiveness of (1) olanzapine-ODT vs olanzapine-SOT, and (2) olanzapine-SOT vs aripiprazole-SOT from a healthcare payer’s perspective in a Chinese setting by adapting the previously published model. Due to the fact that the interchangeability of the generics had not been well evaluated until a new policy on re-evaluating the registered generics was released in early 2016Citation18, only the antipsychotics with brand names were targeted in this study.
Methods
Overview of the published model
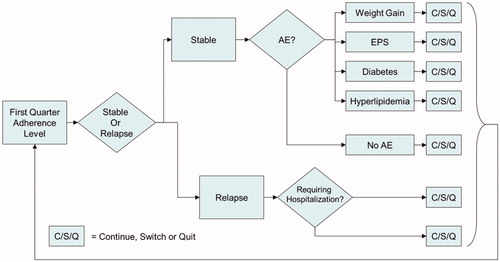
The US ODT model is a 1-year Monte Carlo microsimulation model using quarterly cycles. In the model, patients were divided into three groups according to adherence level (defined by annual medication possession ratio [MPR]), which were the fully adherent group (MPR ≥ 80%), partially adherent group (MPR ≥ 60%, <80%), and non-adherent group (MPR < 60%). For the three groups of patients, different relapse rates, hospitalization rates, suicide attempt rates, and health-related utility values were simulated. Patients may have also experienced four common treatment-emergent adverse events, which were extra-pyramidal symptoms (EPS), clinically significant weight gain (≥7%), diabetes, and hyperlipidaemia. Patients in the model may have experienced discontinuation in case of lack of efficacy, intolerability, patient decision, or other reasons. Once they discontinued, they either switched to another antipsychotic or just quit the treatment. The overview of the model is illustrated in . In a single run, 1,000,000 patients for each treatment arm were simulated to experience the events in the model, and then the results were used to generate an average direct cost, QALY, and relapse percentage for each treatment arm.
Update to Chinese inputs
The model was adapted to reflect Chinese clinical practice, resource use, and decision perspectives by updating to Chinese clinical and cost data. All updated data were sourced and in sequence of ranking order from peer-reviewed literatures, government grey literatures, unpublished data-on-file, and a clinical expert panel composed of experienced psychiatrists in China. All currency data were, however, converted from CNY to USD to enhance the comparability of the findings (exchange rate on April 12, 2018: 1 USD = 6.2834 CNY).
Probability and rate inputs
Based on the results of an observational survey conducted in China, the hospitalization rate of patients who experienced a relapse was quite different from that of the US. The survey indicated that 56.3% of the relapse cases in China required hospitalizationCitation19. The annual risk of the treatment-emergent adverse events was taken from multiple sources, which included a naturalistic trial in ChinaCitation20, a randomized controlled clinical trial in ChinaCitation21 and a network meta-analysisCitation22. We assumed that the same medication in different formulations did not have a difference in the risk of the adverse events, which was also assumed in the US ODT modelCitation16. The Chinese naturalistic trialCitation20 also reported the annual discontinuation rates, which were used in the model adaptation in place of the data in the US to represent the antipsychotic usage patterns of Chinese schizophrenia patients. Among the discontinued patients in China, 14.37% with olanzapine and 12.80% with aripiprazole switched to another treatment according to the data from the Beijing Urban Employee Basic Medical Insurance system (data-on-file). For patients who switched to another antipsychotic, multiple reasons may lead to different switch patterns. For patients who switched because of EPS, metabolic syndromes, or patient preference, the switch patterns were assumed to be consistent with the assumptions in the US ODT model, which were presented in . However, the switch patterns of patients who switched due to the lack of efficacy could be quite different from that of the US, due to the different clinical practices. Therefore, the switching patterns due to the lack of efficacy were acquired through the clinical expert panel to reflect the current Chinese clinicians’ practice. During the expert panel, three experienced psychiatrists filled in a questionnaire about the switching patterns due to the lack of efficacy, and modified their fillings after discussing on any divergence. The consensus of the clinical expert panel on this issue was presented in .
Table 1. Treatment switch patterns by reason for switching.
The US ODT model conservatively assumed that the relapse rate with each antipsychotic in ODT formulation was equal to the rate with it in SOT formulation if patients had the same adherence. However, this disagrees with a more recent clinical studyCitation23. This clinical study, through a multivariate logistic regression, indicated that, in non-adherent patients, the odds ratio of relapse rate with ODT vs SOT was 0.368 (95% CI = 0.183–0.739). We updated the model with this new evidence. The parameters regarding probability and rate are summarized in .
Table 2. Chinese input values applied in the updated model (probability and rate).
Cost and resource utilization inputs
The clinical expert panel also discussed and summarized the common clinical practice for patients in stable stage of schizophrenia, and for managing EPS, clinically significant weight gain, and treatment-emergent diabetes and hyperlipidaemia, respectively. According to the panel’s indications on the common clinical practice, the resource utilizations in different scenarios were generated to calculate the quarterly costs of the stable state and the management of the treatment-emergent adverse events. The average unit costs of the medical resources were acquired from the governmental publications in four major cities in China. The costs of hospitalization, and outpatient visits were from local cost studies conducted in TianjinCitation24 and Beijing. The unit costs of all antipsychotics were the most recent average bidding prices in all available provinces in China. A summary of all updated cost data was presented in .
Table 3. Chinese input values applied in the updated model (cost and resource utilization).
Model outputs
Clinical, economic, and cost-effectiveness outcomes were projected in the model. The proportions of patients in the model who have ever experienced outpatient relapse, inpatient relapse, or never experienced a relapse were estimated as clinical outcomes. The direct costs by the medication groups were calculated as economic outcomes. In terms of the cost-effectiveness outcomes, incremental cost per QALY gained and incremental cost per relapse avoided were estimated.
Sensitivity analysis
A one-way sensitivity analysis was conducted on the inputs that were confirmed important in the US ODT model studyCitation16 based on the sequential bifurcation methodCitation25, which included adherence, annual discontinuation, and differential rates of relapse between olanzapine-ODT and olanzapine-SOT. Additionally, a multivariate probabilistic sensitivity analysis (PSA) was conducted to assess the uncertainty and the overall robustness of the model results. In the PSA, the adherence rates, relapse rates, and treatment discontinuation rates were varied following the beta distribution, and the costs of medical resource utilizations and management of adverse events varied following the log-normal distribution.
Results
Deterministic analysis
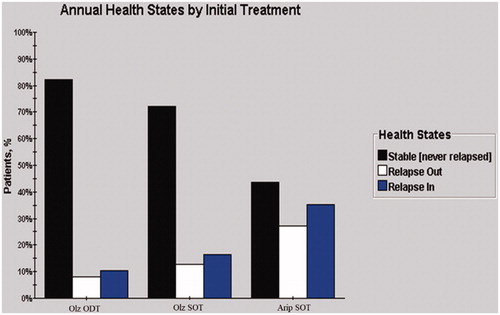
The proportions of patients experiencing outpatient relapse, inpatient relapse, and stable state of the three medication groups are presented in . Olanzapine-SOT is associated with a lower relapse rate compared with aripiprazole-SOT, while the ODT formulation of olanzapine further decreases the proportion of patients who experience relapse.
The cost, QALY, average number of relapses per patient, incremental cost per QALY gained, and incremental cost per relapse avoided are presented in . The total annual costs per patient in the aripiprazole-SOT arm, olanzapine-SOT arm, and olanzapine-ODT arm are USD 2,296.05, USD 1,940.05, and USD 2,292.81, respectively. The average number of relapses per patient in 1 year in the aripiprazole-SOT arm, olanzapine-SOT arm, and olanzapine-ODT arm are 0.734, 0.325, and 0.198, respectively. The QALY gained per patient in 1 year in the aripiprazole-SOT arm, olanzapine-SOT arm, and olanzapine-ODT arm are 0.714, 0.737, and 0.758, respectively. Consequently, the incremental cost-effectiveness ratios (ICERs) of administrating olanzapine-ODT over olanzapine-SOT are USD 2,791.96 per relapse avoided and USD 16,798.39 per QALY gained; the ICERs of using olanzapine-SOT over aripiprazole-SOT are USD –870.39 per relapse avoided and USD –15,477.93 per QALY gained (olanzapine-SOT costs less and gains more effectiveness than aripiprazole-SOT). All ICERs are under 3-time-GDP threshold in China in 2016, which is USD 25,772.67.
Table 4. The cost, effectiveness, and incremental cost-effectiveness ratios (ICERs).
One-way sensitivity analysis
The ICERs of olanzapine-ODT over olanzapine-SOT in different scenarios are presented in .
Table 5. The results of one-way sensitivity analysis.
Among these scenarios evaluated, only when the ODT formulation has no effect to enhance the adherence, the ICER of olanzapine-ODT is above the 3-time-GDP threshold in China.
Probabilistic sensitivity analysis
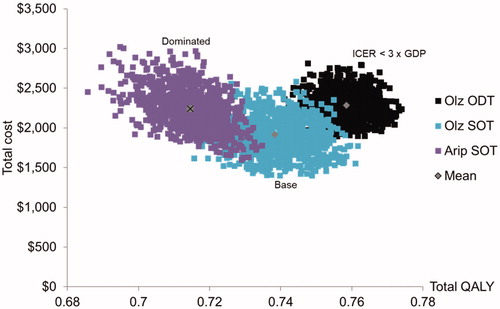
The costs and QALYs of three arms are plotted in a cost-effectiveness plane, as shown in .
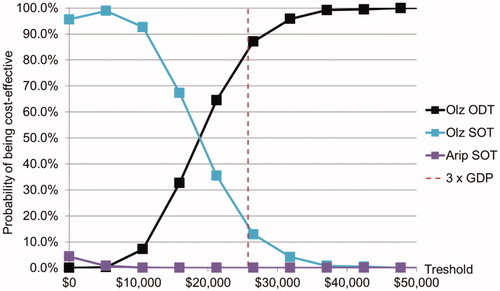
Multiple thresholds were used to check the probabilistic cost-effectiveness of olanzapine-ODT, olanzapine-SOT, and aripiprazole-SOT, which are illustrated as the cost-effectiveness acceptability curves in .
When the 3-time-GDP (USD 25,772.67) threshold was used, the probabilities of olanzapine-ODT, olanzapine-SOT, and aripiprazole-SOT being cost-effective were 84.4%, 15.6%, and 0.0%, respectively.
Discussion
Our study is the first cost-effectiveness evaluation of olanzapine ODT vs its respective SOT formulation as the first line treatment of schizophrenia in China; we further compared the cost-effectiveness of olanzapine-SOT with aripiprazole-SOT, another commonly prescribed second generation atypical antipsychotic. Our model indicates that olanzapine-ODT is cost-effective compared to olanzapine-SOT, and olanzapine-SOT is a dominant cost-effective choice vs aripiprazole-SOT because it is associated with greater effectiveness at lower total healthcare costs. In testing the stability of the model using the one-way sensitivity analysis and PSA for the model’s core assumptions, the model displays the robustness of the base case. Note that only targeting the originator drugs over any generics can limit the implications of the findings. That is, we only use the effectiveness and cost data of the brand-names olanzapine-SOT, olanzapine-ODT, and aripiprazole-SOT. Therefore, the findings would only inform the cost-effectiveness of the brand-name drugs. Caution should be exercised when extrapolating the findings to the cost-effectiveness of generics.
Since the first olanzapine was introduced into China in 1999, this atypical antipsychotic agent has been used for almost 20 years in China. More recently, its ODT formulation has become available at a higher price. However, no studies have evaluated the use of the new formulation in China. Health economic evaluations on olanzapine SOT vs other antipsychotics were also limited. One study published in 2009 concluded that the long-acting risperidone has a lower cost per successfully treated patient than olanzapine in SOT formulation, but without reporting the incremental cost-effectivenessCitation26. Moreover, several studies in Chinese language evaluated the cost-effectiveness of olanzapine-SOTCitation27–29. The main limitations of these studies included acute treatment only (successfully treated/relapse rate), medication cost only, or an overly simplified model structure. Therefore, we believe the findings from these studies are not comprehensive or sufficiently robust to support the practical decision-making for payers regarding reimbursement and resource allocation. This study informs the decision-makers with the first economic evaluation of olanzapine in both ODT and SOT formulations.
The strength of our study lies in its adaptation of the model previously published in the US setting, which largely aimed for the same objectivesCitation16,Citation30. Our model, however, has one major deviation from the earlier model by incorporating the latest finding, which showed that, in non-adherent patients, patients taking olanzapine-ODT had significantly lower risk for relapse or hospitalizationCitation21. This referenced study is an observational study, which should be preferable in evaluating treatment adherence and its impact because of the absence of protocol driven follow-ups and interventions as in controlled clinical trials. Nevertheless, clinical observational studies are generally more likely subject to channelling bias (a form of selection bias), which can confound the study results. However, the study findings were not likely biased in favour of olanzapine-ODT, as patients taking ODT had more severe illness at baseline (p < 0.001) as assessed with the Clinical Global Impression. Earlier research consistently showed that patients taking ODT formulation had a better adherence to antipsychotics than those taking SOT formulationCitation14,Citation15. It is plausible and expected that ODT is more beneficial for patients with higher risk of treatment non-adherent.
It is worth noting that the risk of lower disease relapse associated with olanzapine-ODT may be attributable to factors beyond convenience. In a randomized, double-blind controlled clinical trial of outpatients treated with olanzapine of different formulations to evaluate body weight gain, patients taking ODT experienced significantly better adherence to treatment compared to those taking SOT (92.9% vs 78.5%, p = 0.015), which cannot be explained by convenience and patient awareness of different formulations as the trial had a double dummy feature for the study medicationsCitation31. It was hypothesized that ODT may have some favourable effect, such as less appetite increase, influencing patients to want to take the medication more regularlyCitation31.
Our study has a number of limitations. As our work was adapted from the previously published model, naturally the limitations inherent in the original model remain, such as lack of published medical literature for some model input parameters (e.g., QALYs by health states), the model with only 1-year time horizon, although schizophrenia is a life-long illness, and only direct medical cost is considered. Finally, although we would prefer to use local data for model inputs where applicable, we employed US data in a number of places where local data were not available, e.g. adherence data (proportions of full/partial/non-adherence) from the California Medicaid and US-SCAP were applied instead. In order to validate whether the data from the US are appropriate, more research on adherence to atypical antipsychotics in China is needed.
Conclusion
As the first-line treatment for schizophrenia in China, the brand-name olanzapine-ODT is cost-effective compared to the brand-name olanzapine-SOT, and the brand-name olanzapine-SOT is cost-effective compared to the brand-name aripiprazole-SOT.
Transparency
Declaration of funding
This study was funded by Eli Lilly and Company.
Declaration of financial/other interests
No potential conflict of interest was reported by the authors. YZ and YC are full-time employees of Eli Lilly and Company. The authors have no other relevant financial or other relationships to disclose. One peer reviewer on this manuscript discloses receiving manuscript or speaker’s fees from Astellas, Dainippon Sumitomo Pharma, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Meiji Seika Pharma, Novartis, Otsuka Pharmaceutical, Wiley Japan, and Yoshitomi Yakuhin and research grants from Eisai, Mochida Pharmaceutical, and Meiji Seika Pharma. Another peer reviewer discloses employment at the pharmaceutical company Lundbeck, LLC, which markets a long-acting injectable formulation of aripiprazole, one of the antipsychotic drugs described in the manuscript. The remaining peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Acknowledgements
The authors thank Lee J. Smolen and Timothy M. Klein from Medical Decision Modeling Inc. (MDM) for the technique help for the probabilistic sensitivity analysis. The authors also thank Henry Hu for helping with the English language and proofreading the article.
References
- Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602.
- Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: an epidemiological survey. Lancet. 2009;373(9680):2041–2053.
- Montgomery W, Liu L, Stensland MD, et al. The personal, societal, and economic burden of schizophrenia in the People’s Republic of China: implications for antipsychotic therapy. Clin Outcomes Res. 2013;5:407–418.
- Liu T, Song X, Chen G, et al. Prevalence of schizophrenia disability and associated mortality among Chinese men and women. Psychiatry Res. 2014;220:181–187.
- Falkai P, Wobrock T, Lieberman J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 1: acute treatment of schizophrenia. World J Biol Psychiatry. 2005;6(3):132–191.
- Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1–56.
- Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36:71–93.
- Lin J, Chen G, Guan B, et al. The compliance of schizophrenic patients and factors that affect it. Chin J Nerv Ment Dis. 2000;26:152–155.
- Ascher-Svanum H, Zhu B, Faries D, et al. Medication adherence levels and differential use of mental-health services in the treatment of schizophrenia. BMC Res Notes. 2009;2:1–6.
- Ascher-Svanum H, Zhu B, Faries D, et al. A comparison of olanzapine and risperidone on the risk of psychiatric hospitalization in the naturalistic treatment of patients with schizophrenia. Ann Gen Hosp Psychiatry. 2004;3(1):11.
- Law MR, Soumerai SB, Ross-Degnan D, et al. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008;69(1):47–53.
- Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med. Care. 2002;40(8):630–639.
- Marcus SC, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr Bull. 2008;34(1):173–180.
- Wertheimer AI, Santella TM, Finestone AJ, et al. Drug delivery systems improve pharmaceutical profile and facilitate medication adherence. Adv Ther. 2005;22(6):559–577.
- Zhao J, Ou J, Xue H, et al. Clinical utility of orally disintegrating olanzapine in Chinese patients with schizophrenia: a review of effectiveness, patient preference, adherence, and other properties. Neuropsychiatr Dis Treat. 2014;10:355–359.
- Ascher-Svanum H, Furiak NM, Lawson AH, et al. Cost-effectiveness of several atypical antipsychotics in orally disintegrating tablets compared with standard oral tablets in the treatment of schizophrenia in the United States. J Med Econ. 2012;15:531–547.
- Li Q, Xiang Y-T, Su Y-A, et al. Antipsychotic polypharmacy in schizophrenia patients in China and its association with treatment satisfaction and quality of life: findings of the third national survey on use of psychotropic medications in China. Aust New Zeal J Psychiatry. 2015;49:129–136.
- Huang B, Barber SL, Xu M, et al. Make up a missed lesson-new policy to ensure the interchangeability of generic drugs in China. Pharmacol Res Perspect. 2017;5:e00318.
- Xiao J, Mi W, Li L, et al. High relapse rate and poor medication adherence in the Chinese population with schizophrenia: results from an observational survey in the People’s Republic of China. Neuropsychiatr Dis Treat. 2015;11:1161–1167.
- Guo X, Fang M, Zhai J, et al. Effectiveness of maintenance treatments with atypical and typical antipsychotics in stable schizophrenia with early stage: 1-year naturalistic study. Psychopharmacology (Berl). 2011;216:475–484.
- Guan D, Zhuo D. Comparative study on adverse reactions of the second generation of antipsychotics. Hainan Med J. 2011;22:46–48.
- Lin L, Zhao YJ, Zhou HJ, et al. Comparative cost-effectiveness of 11 oral antipsychotics for relapse prevention in schizophrenia within Singapore using effectiveness estimates from a network meta-analysis. Int Clin Psychopharmacol. 2016;31:84–92.
- Novick D, Montgomery W, Treuer T, et al. Comparison of clinical outcomes with orodispersible versus standard oral olanzapine tablets in nonadherent patients with schizophrenia or bipolar disorder. Patient Prefer Adher. 2017;11:1019–1025.
- Wu J, He X, Liu L, et al. Health care resource use and direct medical costs for patients with schizophrenia in Tianjin, People’s Republic of China. Neuropsychiatr Dis Treat. 2015;11:983–990.
- Bettonvil B, Kleijnen JPC. Searching for important factors in simulation models with many factors: sequential bifurcation. Eur J Oper Res. 1997;96:180–194.
- Yang L, Li M, Tao L, et al. Cost-effectiveness of long-acting risperidone injection versus alternative atypical antipsychotic agents in patients with schizophrenia in China. Value Heal. 2009;12:S66–S69.
- Hu S, Zhang Y, Yang L, et al. Evaluation of the drugs for serious mental illness in National Essential Medicine List of 2012 edition using pharmacoeconomics method. China Licens Pharm. 2013;10:3–10.
- Yang L, Tao L. Evidence-based evaluation on cost-effectiveness of second generation antipsychotics. China Pharm. 2007;18:1288–1290.
- Guan X, Liu Q, Tang W, et al. Cost-effectiveness analysis of five clinical commonly used atypical antipsychotics as the first-line treatment of first-episode schizophrenia in China based on a decision tree model. Chinese J New Drugs. 2017;26:2101–2106.
- Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness model comparing olanzapine and other oral atypical antipsychotics in the treatment of schizophrenia in the United States. Cost Eff Resour Alloc. 2009;7(1):4.
- Karagianis J, Grossman L, Landry J, et al. A randomized controlled trial of the effect of sublingual orally disintegrating olanzapine versus oral olanzapine on body mass index: the PLATYPUS Study. Schizophr Res. 2009;113:41–48.