Abstract
Background and aims: Infection is a serious and expensive complication of Cardiac Implantable Electronic Device (CIED) procedures. A retrospective based cost analysis was performed to estimate Trust level savings of using the TYRX antibacterial envelope as a primary prevention measure against infection in a tertiary referral centre in South London, UK.
Methods: A retrospective cohort of heart failure patients with reduced ejection fraction undergoing Implantable Cardioverter Defibrillator (ICD) or Cardiac Resynchronization Therapy (CRT) procedures were evaluated. Decision-analytic modelling was performed to determine economic savings of using the envelope during CIED procedure vs CIED procedure alone.
Results: Over a 12 month follow-up period following CIED procedure, the observed infection rate was 3.14% (n = 5/159). The average cost of a CIED infection inpatient admission was £41,820 and, further to economic analysis, the additional costs attributable to infection was calculated at £62,213.94. A cost saving of £624 per patient by using TYRX during CIED procedure as a primary preventative measure against infection was estimated.
Conclusions: TYRX would be a cost-saving treatment option amongst heart failure patients undergoing ICD and CRT device procedures based on analysis in the local geographical area of South London. If upscaled to the UK population, we estimate potential cost savings for the National Health Service (NHS).
Introduction
In recent decades, the number of Cardiac Implantable Electronic Device (CIED) procedures has increased substantiallyCitation1, consequent to growing indications for implantation based upon expanding evidence on improved morbidity and mortalityCitation2–7, increased survival rates from ischaemic heart disease and an ageing populationCitation8. In accordance with this, the numbers of CIED related infections have climbed, with an estimated annual prevalence of 2–4%Citation9–11, despite best practise and vigilant infection-control standard operating protocolsCitation12–15. Certain risk factors have been observed in relation to CIED infections, including a medical history of diabetes, renal failure, heart failure and prior CIED infection, and use of anticoagulant or corticosteroid therapy. Additionally, Cardiac Resynchronization Therapy (CRT) implants, revision or upgrade procedures, and procedures involving three or more pacing leads have increased infection riskCitation16–19. The risk of infection to any individual is most likely determined by the combination of risk factors that is present.
CIED infection is one of the most serious complications following the procedure, characterized by high levels of patient related morbidity and mortality. Infections are associated with prolonged hospital admissions, extended antibiotic therapy, and, if the patient is a suitable candidate, recommended complete device extraction followed by re-implantation, the former being a major procedure with non-insignificant risks of serious complications including fatalityCitation12,Citation14,Citation20,Citation21. All-cause mortality rates over a 5 year follow-up period have been reported as high as 35%Citation14,Citation19 and, in cases of CIED endocarditis without concomitant device extraction, mortality rates range from 31–66%Citation13. Consequently, CIED infections contribute considerable financial burden to healthcare systems globally. Recent estimated costs attributable to a CIED infection related hospital admission were $146,000 and £30,958 in the US and UK, respectivelyCitation12,Citation21.
Given the significant clinical and financial burden of infection complications, ongoing primary prevention at the time of device procedure is paramount. The TYRX Absorbable Antibacterial Envelope (Medtronic plc, Mounds View, Minnesota)Citation22, is a sterile, single use multifilament knitted mesh composed of a polymer made of glycolide, caprolactone, and trimethylene carbonate, that houses the CIED generator within the subcutaneous pocket which elutes two antibiotics (rifampicin and minocycline) over at least a 7 day period following device implantation and, thereby, provides a primary prophylaxis against infection. A non-absorbable version of the envelope was previously available and in several observational studies demonstrated an infection reduction rate between 69–100% with associated cost-effectivenessCitation11, Citation23,Citation24. A prospective randomized controlled trial determining the efficacy of the absorbable TYRX; the World-wide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT)Citation25 is currently underway with highly anticipated results.
This paper describes an economic model developed to demonstrate the potential financial savings of using TYRX as a preventative measure against infection within a retrospective cohort of Heart Failure patients with reduced Ejection Fraction (HFrEF)Citation26 undergoing CIED procedures inclusive of an Implantable Cardioverter Defibrillator (ICD) or CRT, to a single National Health Service (NHS) Foundation Trust within South London, UK and its local Clinical Commissioning Group (CCG).
Methodology
Retrospective audit
A retrospective audit of all ICD and CRT procedures in patients with HFrEF within two local geographical residential areas of a single tertiary referral institution and high volume device implantation centre (Guy’s and St Thomas’ NHS Foundation Trust, London, UK) was registered, approved, and conducted between 1 January 2014 and 31 September 2017. St Thomas’ Hospital is a large NHS teaching hospital in South London. It acts as a tertiary referral centre for cardiovascular disease, with services covering cardiology, cardiothoracic surgery, and congenital heart disease for both paediatric and adult populations. The estimated population of the two local geographical areas is ∼ 575,000 people. Both are socially deprived areas based on UK local authority data (40th and 44th most deprived areas, respectively). Approximately 60% of this population is Caucasian and 25–30% Black ethnic origin. Procedures were inclusive of new or de novo implant, generator change, and upgrades, and performed by appropriately skilled operators. Data collected was inclusive of those specific to the device procedure, in addition to patient demographics, co-morbidities, prescribed medications, and healthcare utilization to the Trust within a 24 month window; 12 months pre- and post-device procedure. CIED infection was defined by a hospital admission within the first 12 months following device procedure requiring a prolonged course of intravenous antibiotics with or without a device extraction procedure. Infections were identified through a combination of searching a purpose built Trust Extraction Database, which contributed to the European Lead Extraction Registry,Citation19,Citation27 and using the 10th Edition of the World Health Organization (WHO) International Classification of Disease (ICD) discharge code for the admission relating to the CIED infection containing ICD-10 code T82.7 within the Trust’s information systems.
Infection-control protocol
The Trust’s infection-control protocol when performing CIED procedures throughout the period of January 2014–September 2017 is detailed in the Supplementary Appendix and we assume 100% compliance. The TYRX envelope was not in use during ICD and CRT related device procedures within the Trust during the time period analysed.
Economic analysis
We developed a decision-analytic model seeking to assess the expected economic impact of introducing the TYRX Absorbable Antibacterial Envelope whereby the primary aim is to reduce infection rates after CIED procedures. The perspective of the model is within a single NHS Foundation Trust. The time horizon captured in the analysis was set to 1 year to be consistent with the scope of the study. The model consists of two mutually exclusive pathways; CIED procedure with TYRX vs CIED procedure alone without TYRX (see ). Mortality was not included as there were no deaths observed within the CIED infection group during the first 12 months post-discharge. Costs of healthcare utilization in the 12 months post-CIED procedure were captured subsequent to the date of discharge following the CIED procedure, therefore the procedural costs relating to this were not included in the total cost estimation. The model was probabilistic to capture the joint impact of parameter uncertainty. A Monte Carlo simulation consisting in 999 iterations was conducted in Microsoft Excel. The model parameters were cost of infection, baseline infection risk, and odds ratio of infection with TYRX relative to current practice. We used an odds ratio of 69% based on a recent meta-analysisCitation28 to derive the probability of infection within the TYRX group. A Gamma distribution was assumed for Infection cost. A log normal distribution was selected for the odds ratio of infection with TYRX relative to current practice. For both groups, the procedural costs were assumed to be equivalent, therefore were not included in the model.
Figure 1. Decision analytic model developed to analyse the expected economic impact of introducing TYRX.
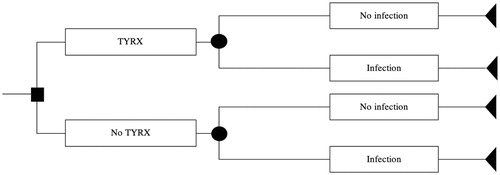
The probability of developing infection for the no-TYRX branch was defined as the proportion of procedures that resulted in an infection episode throughout the 12-month follow-up period in the 159 procedures cohort. In the absence of an experimental group, we assumed infection risk using TYRX reduced by the odds ratio obtained from a recent meta-analysisCitation28. We derived the probability of infection in the TYRX-group by combining the odds ratio for infection with TYRX and the odds of infection without TYRX observed from the retrospective audit (please refer to the Supplementary Appendix for further detail). The parameters of the model are presented in .
Table 1. Parameters of economic model.
Cost analysis
An estimated cost for each patient for the 12-month period following their device procedure was calculated. This was inclusive of inpatient admissions, outpatient department (OPD) and Accident and Emergency (A&E) department visits, and Community Heart Failure Nurse visits. As HFrEF patients are frequently associated with multiple co-morbidities, we felt it was vitally important to calculate and present the “total” expense of their 12-month post-device procedure journey rather than just the cardiac related costs. In addition to the “total” 12-month cost, we were able to scrutinize our cost data in great detail, enabling us to calculate costs related specifically to cardiac and non-cardiac healthcare utilization. Unlike previous published works, which used financial data derived from reference costs, our inpatient admission, A&E, and OPD department related financial data were derived from patient level costing and represent the actual cost to the Trust. Inpatient admission costs represented the entire outlay of the hospital stay, including device, staffing, overheads, and diagnostic testing. We also recorded hospital income for patient care, based on nationally and locally agreed reimbursement tariffs, but they have not been used further in our analysis or reported results. General Practise (GP) healthcare utilization was not reported and, because their costs are fixed, they have not been included in our analysis. Community Heart Failure Nurse visit costs are separately commissioned and available to the Trust drawn from reference costings. Overall, community related costs contribute less significantly to the overall 12-month care pathway post-device procedure in contrast to inpatient admissions. Missing costs data was observed for 9% of inpatient admissions, 6% of OPD visits, and 3% of A&E attendances. For missing inpatient costs we used the predicted values from a multi-variable linear regression model which included bed-days, critical care days, type of admission, and whether a CIED procedure was performed during the admission. We added some random noise to the imputed values (with a standard deviation equal to the root-mean-square error), to give some variability around the regression line. For the missing A&E data, we used HRG-level reference costs supplied by the trust. For the majority of the missing outpatient data we again used reference costs and, where this wasn’t possible, we simply used median costs. A cost of £719 for the TYRX envelope was used for analysis purposes, which has been previously quoted and published in health economic literatureCitation22.
Estimation of costs attributable to infection
We undertook regression analysis of total costs to allow adjustment for patient case-mix in the estimation of the cost attributable to infection. We applied Generalized Linear Modelling following recommendations by Mihaylova et al.Citation29 and used the Park test to select the distribution. Given the limitations of the sample size we considered a linear and a log link (additive and multiplicative model). We assessed the robustness of estimation of infection-attributable cost through two sensitivity analyses.
We applied an ordinary least squares (OLS) model in place of the GLM model in combination with bootstrapping to quantify uncertainty.
We estimated the cost of infection through attribution of resource use deemed to have arisen from infection for the five patients with an infection.
We also conducted a scenario analysis on the TYRX cost in order to determine the threshold of positive savings to the NHS. The reported market price of the device was varied ± 50%. We report the breakeven price of TYRX. All the statistical analyses were conducted on Stata SE 15. Further details on these methods are provided in the Supplementary Appendix.
Statistical analysis
The demographics, device procedural details, comorbidities, and other associated risk factors for CIED infection were compared among the two patient groups; with and without infections and presented using descriptive statistics with measures of frequency, central tendency, and variation.
Results
Device infection rate
One hundred and fifty-nine ICD and CRT device-related procedures occurred in 157 HFrEF patients between 1 January 2014 and 31 September 2017 within two specified catchment geographical areas for the Trust (see ). Two patients underwent two separate procedures as a result of upgrade from ICD to CRT-D, each with separate 12-month follow-up periods that did not overlap within the 45-month time period, therefore accounting for 159 procedures. Five patients developed a CIED infection within 12 months of their device procedure, requiring prolonged inpatient admission and device extraction followed by re-implantation, resulting in an infection rate of 3.14%. All five patients had confirmed microbiology evidence of CIED infection with either positive blood cultures and/or pacing lead tip cultures for typical bacterial organisms; two Staphylococcus Aureus, two Staphylococcus Epidermis, and one Enterococcus Faecalis. All five patients underwent full CIED system extraction during the CIED infection related admission followed by re-implant procedures prior to discharge. The average length of stay for the CIED infection related admission was 43.6 days, with the largest contributors to the total admission cost being inpatient bed days (inclusive of critical care bed days; 27%), devices (17%), Trust overheads (16%), and finally medical staffing costs at 12%. Patient demographics, co-morbidities, and specifics of the device procedure for both groups (those with and without a CIED infection) are shown in .
Table 2. ICD and CRT device procedures in HFrEF patients between 1 January 2014 and 31 September 2017.
Table 3. Baseline characteristics, comorbidities, and CIED risk factors in patients with and without CIED infections (n/%).
Cost of infection
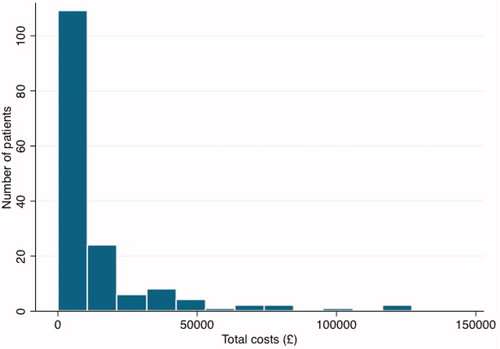
Using the raw cost data, excluding costs relating to the CIED procedural admission (as already detailed in our methodology), the average total 12-month post-device procedure healthcare cost was £13,326. The unadjusted difference between mean costs of non-infected and infected patients from the raw cost data was £59,048.82. The average cost of attributed individual resource use (n = 5) relating to a CIED infection was £41,820.40 (range = £28,377–£56,498). The distribution of total costs was strongly right-skewed (). The modified Park test indicated the Gamma distribution best fit the data. Link tests indicated a linear link (additive model) was superior to a log link. presents the results of alternative approaches to the estimation of “total” costs attributable to infection. Infection costs were highest when estimated using GLM and lowest when estimated using attribution of individual resource use. Additional costs attributable to infection costs were £62,213.94 [SE = £16,697.81] in the base case (GLM). When analysed for cardiac related costs only, the additional costs attributable to infection were £49,541.25 [SE = £10,707.48] in the base case (GLM).
Table 4. Sensitivity analysis on cost estimation method.
Economic analysis
also summarizes the results of the economic impact assessment. Utilizing a base-case device price of £719 and infection costs estimated from GLM, TYRX generated savings of £624 per procedure. In sensitivity analysis, savings were £514 and £184 per procedure using infection costs estimated from the OLS model and direct attribution of resource use, respectively. The breakeven price for the TYRX envelope was £1,361.
Discussion
We observed an infection rate of 3.14% (n = 5/159) amongst a HFrEF population undergoing ICD and CRT device-related procedures. The average cost of a CIED infection inpatient admission was £41,820 and, further to GLM analysis, the additional costs attributable to infection was calculated at £62,213.94 (“total” costs) and £46,770 (cardiac-related costs). Our economic analysis determined cost savings between £184 and £624 by using the TYRX absorbable envelope at the time of procedure as a primary preventative measure against CIED infection.
Accurate figures of the scale of CIED infection and their associated costs are not known within the UK, however predictions on continued growth in the number of CIED procedures, particularly within older and comorbid populations, mean that these figures are projected to increase. Definitions of what entails a CIED infection and during what timeframe of follow-up varies in the literature, making comparison challenging, however the observed rate of infection in our cohort within 12 months of the device procedure (3.14%) falls within the range published in current literature (2–4% annual prevalence). It is possibly not surprising, that our infection rate was nearer to the top end of this range, given that we specifically studied a “high risk” group; patients with HFrEF, of whom 80% had a CRT device, and 44% underwent a recurrent procedure (generator change or upgrade). As demonstrated, significant comorbidities were substantially prevalent within our cohort, with traditional infection risk factors of diabetes, renal failure, and COPD featuring prominently.
To really understand the financial implications of CIED infections, the first key measures are to define a dedicated geographical area and utilize accurate and validated cost data. This project addresses these specifically; we created a purpose-built database able to capture both community and secondary care health utilization for patients with HFrEF and implanted ICD or CRT devices within a pre-defined local residential geographical location. Our cost data has been obtained directly from the Trust Finance department, and provides the actual cost incurred by the Trust at an extremely accurate and detailed level for any individual patient and any individual healthcare transaction. We have been able to provide a robust “total” cost estimate for any patient within our cohort, which more accurately represents the entire cost of their healthcare journey within 12 months of their device procedure, but are also able to interrogate our cost data by type of healthcare utilization (inpatient, A&E, etc.) or whether this is cardiac or non-cardiac related. Having reviewed the literature, we believe this is uniquely different to any previously published work in this area.
Previous estimates of the cost of CIED infection to healthcare systems globally within the last decade vary from $146,000 (US), £30,958 (UK), and €20,211–23,237 (France)Citation12,Citation21,Citation30. Methodologies to determine cost estimates differed across these studies, all encompassed an estimated cost for a hospital admission relating to the CIED infection; however, the acquisition of cost data varied from a cost-to-charge ratio, diagnosis-related group (DRG) coding (similar to income reimbursements paid to NHS service providers) to the use of reference costings. Another important difference to highlight between our work and these studies is that they were all inclusive of single and dual chamber pacemakers for treatment of bradyarrhythmia, which incur a lower device tariff/cost compared to ICD or CRT-D for the replacement device procedure and one study included CIED infections that were medically managed without system extraction. All these factors in synergy may account for the higher comorbidity-adjusted estimate of cost attributable to CIED infection-related hospital admission in our cohort (£61,585). The fact that we also observed an inflated cost when adjusted to include only the cardiac related costs specifically (£49,541.25) reinforces our results and the strengths of our costing methodology. Discrepancies could also be explained by un-observed cofounders or due to a lack of earmarking of additional resource costs within the infection group to that of infection costs.
One of the main limitations of this report is the small sample size. In total, five patients from our limited cohort experienced a CIED infection within 12 months of their device procedure, therefore deriving definitive conclusions from this analysis should not be done without due care and attention. Alongside the small sample size, which had implications on the robustness and accuracy of our estimates, the fact that this was retrospective data with the lack of a control or placebo group necessitated making strong assumptions on similarities between groups to perform economic analysis to estimate cost savings from the intervention of using the TYRX envelope. Our first assumption was the probability of infection within the TYRX group, in the absence of results from the awaited randomized controlled WRAP-ITCitation25 trial on CIED infection rate reduction. We used a reduction of relative risk of 69% previously published in the literatureCitation28 to derive the probability of infection within the TYRX group. This calculation was made under the assumption of enough similarity amongst studies’ population that enables reverse engineering combining primary and secondary data. Despite this, the structural sensitivity analysis on cost estimation demonstrated cost savings, although there is a marginal difference among approaches. Whilst our economic model analysis calculated a cost saving of ∼ £600 per patient using TRYX as a preventative measure against infection, this was observed in a “high risk” studied group and, therefore, it cannot be assumed that, when applied to patients without heart failure or those undergoing bradyarrhythmia pacemaker procedures, the same savings effect would be observed.
However, using our methods of a fixed geography, unique costings; which provide full and accurate costs at a Trust level, and using our calculated cost saving of £624 per procedure, suggests that using the TYRX envelope would result in reductions in costs to the NHS. Based on national figures in 2015–2016Citation31, whereby 295 new or de novo ICD and CRT device implants per million population were performed In England, potential savings to the NHS could be estimated at over £9,700,000. This figure is under-estimated, as it is not inclusive of generator change and upgrade procedures or those from the Welsh, Scottish and Northern Ireland populations. Additionally, the use of the TYRX would have resulted in an estimated reduction in the infection rate to 1.02%, meaning three out of the five patients with CIED infection observed in our cohort would not have experienced an infection with a consequent reduction in the associated morbidity and mortality.
Conclusion
Overall, our study findings suggest that the TYRX envelope would be a cost-saving treatment option amongst HFrEF patients undergoing ICD and CRT device procedures within the local geographical area of the Trust.
Transparency
Declaration of funding
This work was funded by Medtronic.
Declaration of financial/other relationships
AR and RR have received research funding from Medtronic. MP has received consulting advisory fees from Merck. GCW has received research funding from Medtronic and Abbott. The remaining authors on this manuscript have no financial or other relationships to disclose. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplemental data for this article is available online at https://doi.org/10.1080/13696998.2019.1581621.
Download MS Word (23.4 KB)Acknowledgements
The authors would like to thank Medtronic for funding the article. Additional thanks are extended to Bart Gerritse for his statistical advice and supervision during the economic analysis.
References
- Uslan DZ, Tleyjeh IM, Baddour LM, et al. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J. 2008;155:896–903.
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237.
- Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338.
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883.
- Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158.
- Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150.
- Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549.
- Kurtz SM, Ochoa JA, Lau E, et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993-2006. Pacing Clin Electrophysiol. 2010;33:705–711.
- Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355.
- Uslan DZ, Baddour LM. Cardiac device infections: getting to the heart of the matter. Curr Opin Infect Dis. 2006;19:345–348.
- Kolek MJ, Dresen WF, Wells QS, et al. Use of an antibacterial envelope is associated with reduced cardiac implantable electronic device infections in high-risk patients. Pacing Clin Electrophysiol. 2013;36:354–361.
- Ahsan SY, Saberwal B, Lambiase PD, et al. A simple infection-control protocol to reduce serious cardiac device infections. Europace. 2014;16:1482–1489.
- Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998;97:1796–1801.
- Sandoe JA, Barlow G, Chambers JB, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70:325–359.
- de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–34.
- Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95:715–720.
- Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol. 2006;29:142–145.
- Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17:767–777.
- Gould J, Klis M, Porter B, et al. Predictors of mortality and outcomes in transvenous lead extraction for systemic and local infection cohorts. Pacing Clin Electrophysiol. 2019;42:73–84.
- Sohail MR, Henrikson CA, Braid-Forbes MJ, et al. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011;171:1821–1828.
- Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–1006.
- Kay G, Eby EL, Brown B, et al. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ. 2018;21:294–300.
- Bloom HL, Constantin L, Dan D, et al. Implantation success and infection in cardiovascular implantable electronic device procedures utilizing an antibacterial envelope. Pacing Clin Electrophysiol. 2011;34:133–142.
- Shariff N, Eby E, Adelstein E, et al. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol. 2015;26:783–789.
- ClinicalTrials.gov. World-wide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02277990.
- Escobar C, Divison JA. [New recommendations of 2016 European Guidelines for the diagnosis and treatment of heart failure]. Semergen. 2017;43:328–329.
- Bongiorni MG, Kennergren C, Butter C, et al. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur Heart J. 2017;38:2995–3005.
- Koerber SM, Turagam MK, Winterfield J, et al. Use of antibiotic envelopes to prevent cardiac implantable electronic device infections: a meta-analysis. J Cardiovasc Electrophysiol. 2018;29:609–615.
- Mihaylova B, Briggs A, O’Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20:897–916.
- Clementy N. Cardiovascular implantable electronic device infection: time to discuss reimbursement-Authors’ reply. Europace. 2018;20:2046.
- NICOR. National Audit of Cardiac Rhythm Management Devices 2015-2016. London (UK): National Institute of Cardiovascular Outcomes Research; 2016.