Abstract
Background: Both public and private insurers provide drug coverage in Canada. All payers are under pressure to contain costs. It has recently been proposed that private plans leverage the public health technology assessment (HTA) evaluation process in their decision-making.
Objectives: The objectives of the current study were to examine use of public health technology assessments (HTAs) for private payer decision-making in the literature, to gather the perspectives of experts from both public and private insurers on this practice, and to summarize which value parameters of public evaluations can be used for private payer decision-making.
Methods: A targeted literature review was conducted to identify publications on the use of public HTA or cost-effectiveness data for private payer decision-making on pharmaceutical reimbursement. Concurrently, a roundtable meeting was organized with invited panelists, including private payer representatives and health economic consultants (total n = 9). The findings from both were synthesized and expressed in qualitative terms using the PICO framework.
Results: The targeted review identified 20 studies meeting the inclusion criteria, primarily originating from the US and Canada. The panelists felt that, despite some similarities, there were substantial differences between both systems. The PICO framework highlighted the issues with transferability between the two systems. Most of the value parameters were either not applicable, needed to be added, needed to be adjusted, or their applicability to private payer systems needed to be confirmed.
Conclusion: Some components of public HTA may be relevant for private payers, however there are reservations that still exist on whether the HTA process in Canada, designed for a public system, can address the informational needs of private payers. Private insurers need to use caution in assessing which value parameters from public HTAs can be used and which need to be confirmed, ignored, enhanced, or adjusted. One size HTA does not fit all applications.
Introduction
Over the past century, the life expectancy of the average Canadian has increased by roughly 20 yearsCitation1. This improvement has been attributable in large measure to advances in medicine, including a revolution in the use of therapeutic and prescription drugsCitation2,Citation3. In fact, the pace of scientific discovery in areas like genomics, molecular, and computational biology has resulted in a proliferation of innovative medicines. While these innovations offer enormous benefits to patients and to society at large, slower economic growth, coupled with demographic changes, has been projected in many developed economiesCitation4, and rising spending on new health technologies is of concern for many jurisdictions. For Canada, this economic projection raises concerns related to the funding and evaluation of innovative medicines and new health technologies, in particular for prescription drugs.
In the Canadian healthcare system, prescription drugs are funded through both public and private insurance schemesCitation5. A detailed description of the Canadian prescription insurance system is provided to contextualize the discussion on payer decision-making (Appendix A). For the majority of Canadians, prescription drug coverage is provided privately by employer sponsored benefit plans, which can include coverage for employees’ family members. While there is some variation by province, territory, or nationally, on average ∼40% of Canadians qualify to have their prescription drugs covered under public benefit plansCitation6, and the remaining 60% of the Canadian population either receive coverage through a privately funded benefit plan, or are uninsured and pay out-of-pocketCitation7,Citation8. It is estimated that ∼5% of the population is uninsuredCitation9. This is either by choice (e.g. small business owners), by necessity due to a lack of access to an employer sponsored plan, and/or not meeting eligibility criteria for public coverageCitation9, or due to an inability to afford one’s own private insurance plan. In some circumstances, depending on plan design and eligibility criteria, coordination of benefits may lead to a combination of public, private, and out-of-pocket funding.
Given that prescription drug funding in Canada is provided from individual or a mix of public and private sources, decision-making related to funding or reimbursing new health technologies raises important issues in terms of the public and private evaluation processes. Different payers may have different decision-making processes and priorities. There are also important differences between public and private plans in terms of demographics, health status, and other characteristics of beneficiaries. The majority of public drug plan funding ultimately flows to older retired individuals or those of lower socioeconomic status, whereas privately funded drug plans tend to be concentrated among employed working age individuals and their family members. For instance, private drug plans are typically more comprehensive (i.e. cover more drugs under more conditions) than public plans, which can result in varied coverage and cost sharing. Overall, the eligibility criteria, comprehensiveness, design, and features of drug plans can vary dramatically. A full overview of the organizational and structural differences between public and private health insurers in Canada is provided in Appendix A.
Health technology assessment (HTA) is defined as “the systematic evaluation of the properties and effects of a health technology … aimed mainly at informing decision-making regarding health technologies”Citation10. The public HTA process in Canada is moderated by two bodies. The Canadian Agency for Drugs and Technologies in Health (CADTH) is comprised of the Common Drug Review (CDR) and the pan-Canadian Oncology Drug Review (pCODR), which undertake HTA for all provinces and territories except QuebecCitation11. The Institut National d’Excellence en Santé et en Services Sociaux (INESSS) undertakes HTA specifically for the province of QuebecCitation12. These agencies have set guidelines as to the approaches and methods preferred for new drug submissions. Key components of the submission include a clinical section and a pharmaceconomic model, typically a cost-utility analysis (CUA). It is important to note that the recommendations provided by CADTH and INESSS are not binding to the provinces. Each of the 10 provinces administer their own healthcare system; there are also some public federal prescription drug plans for specific populationsCitation12,Citation13.
Historically, private insurers’ assessments of the new drug prior to listing have been relatively simple clinical and economic reviews. Although clinical reviews of drugs may vary slightly in terms of scope and complexity, economic considerations have been more standardized, focusing primarily on the budgetary impact. Unlike public HTA submissions, there is no standardized process across private plans on the submission requirements or assessment approach for new drugs. Generally, the process has been less restrictive than public plans in choosing new drugs to reimburse.
Recently, a proposal to shift decision-making for privately covered benefit plans towards public evaluation processes for prescription drugs has been suggested, that would leverage the public HTA processCitation14 and budget impact analysis (BIA) vs a private evaluation (case-by-case clinical and economic review and assessment, and BIA). However, evidence related to the value and economic consequences of using public HTAs and evaluations in private reimbursement decision-making is limitedCitation15,Citation16.
The purpose of this study is to examine the use of public health technology assessment (HTAs) to privately funded insurance plans. To explore the benefits and potential economic consequences of changing evaluation processes, the two main objectives of the study were:
To conduct a literature review on public HTAs or pharmacoeconomic analyses that have been identified as being used for private payer drug reimbursement decision-making, and to determine what information is considered important for private insurers; and
To convene a panel of experts to determine the relevance of public HTAs or pharmacoeconomic analyses for private payer drug reimbursement decision-making.
In this work, HTA is intended to describe a report or body of work that includes the multiple dimensions typically included in a submission to a formal HTA body, which includes clinical, economic, and social implications, while pharmacoeconomic analyses are assumed to only describe comparative models, typically structured as cost-effectiveness or cost-utility analyses. Evidence from the literature and expert perspectives will be synthesized to highlight unique needs and data requirements that are important for private insurance decision-making. This summary will provide insight into what value parameters of public evaluations can be used for private payer decision-making.
Methods
The methods for each of the study objectives are presented below.
Literature review
A literature review was conducted to identify publications on the use of public HTA or cost-effectiveness data for private payer decision-making on pharmaceutical reimbursementCitation17.
Search strategy
A literature review was conducted by searching the MEDLINE database and grey literature to identify publications on the use of public HTA or cost-effectiveness data for private payer decision-making on pharmaceutical reimbursementCitation17. Medical Subject Headings (MeSH) “technology assessment biomedical”, “Canada”, “cost benefit analysis”, and other keywords “private payer”, “health technology assessment”, “cost-effectiveness”, “private insurance” were searched from inception to January 3, 2019. Combinations of terms, keywords, and pre-specified search terms were also performed in websites related to the subject matter: Benefits Canada, Blue Cross, Conference Board of Canada, C.D. Howe Institute, Canadian Centre for Policy Alternatives, Canadian Institute for Health Information (CIHI), Fraser Institute, Harvard Think Tank Search, and NHS Health Economic Evaluations Database (HEED).
Selection criteria
All full-text articles, written in English, which contained descriptions or precedents of public HTA or cost-effectiveness methodologies being used by the private insurance market were included. Other relevant studies were those that included drug-specific HTAs associated with private insurers. The selection of relevant articles was performed by two reviewers (LP and RR), who independently assessed the titles and abstracts. Papers were excluded if they were irrelevant to the selection criteria or were similar to another article. The reviewers negotiated article selection discrepancies and resolved them by consensus. Data extracted from the selected papers included publication type, year of publication, country, the approach and discussion around HTA, and the types of evidence/information that is considered important for private insurers.
Expert panel
A roundtable meeting was organized by Amgen Canada, Inc. on June 3, 2016. Invited panel participants included private payer representatives from major private insurance carriers in Canada (n = 3), along with consultants with experience reviewing public (n = 3) and private payer (n = 3) clinical and pharmacoeconomic information. In total, there were nine expert panel members. The primary objective of the panel meeting was to understand the relevance of public HTAs and the use of pharmacoeconomic analysis for private payers in their formulary decision-making.
The panel was moderated by the lead author, LP, at a face-to-face meeting held over one working day. The following key questions were discussed among panel participants:
Who assesses the products in private insurance companies and what are the criteria or information considered to be important for private insurance decision-making?
What have been the fundamental changes (if any) given the parameters that private payers use to assess value?
Do you think that private payers should be changing the way they assess value? What are the additional parameters that private payers need to be looking at in their value assessments?
Does the private payer sector look at medications as an “investment in health” in terms of disease cost/disability prevention?
What components of public HTAs can be used for private drug reimbursement decision-making?
Panellists had open discussions in a large group format followed by focused discussions in small breakaway groups. Notes from the exercise were prepared by LP. The information obtained from the expert panel during the 1-day meeting was summarized thematically in qualitative terms.
Synthesis of literature review and expert panel meeting
A summary of findings from the literature and expert opinions was prepared to highlight unique information and data requirements, which were important for private insurance decision-making in Canada. The synthesis and summary were expressed in qualitative terms organized around the traditional PICO evidence-based medicine framework (i.e. P-Patient, I-Intervention, C-Comparator, O-Outcome)Citation18, as value parameters and included public and private considerations for each value parameter. Based on a literature review and expert panel opinion, the applicability of the value parameters from public HTAs for private payer decision-making were identified (yes, no, maybe) and analyzed.
Results
Study characteristics
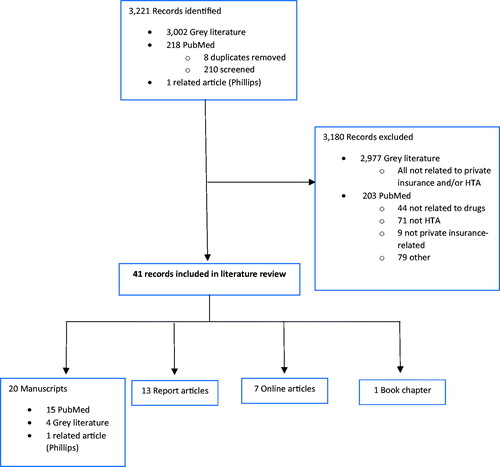
A total of 218 MEDLINE titles/abstracts and 3,002 grey literature citation titles were identified and screened, resulting in a final selection of 41 citations (). MEDLINE articles published on the topic to date (n = 20) are presented in . Publication dates ranged from 1997–2018. Of the MEDLINE articles, the most common jurisdiction of interest was the US (n = 11), followed by Canada (n = 5), not specified (n = 4), and international (n = 2) (some articles had more than one jurisdiction of interest).
Table 1. Summary of 20 identified MEDLINE manuscripts for the literature review.
Literature search findings
Based on literature review findings to date, public HTA recommendations and cost-effectiveness analysis have been used in a limited manner by private insurers. In addition, there was no mention about the use of public HTAs or their appropriateness in private reimbursement decision-making in any of the articles retrieved. Several articles made recommendations on what private insurers should consider when evaluating a drug’s value, such as ways to reduce inefficiencies. Other publications referred to the limitations of using cost-effectiveness analyses for private insurance decision-making.
An article discussed the use of a set of principles when establishing new or assessing existing HTA activities. Several publications noted that there did not seem to be any specific best practices used by private insurers when evaluating drugsCitation14. Some of the points highlighted were:
There is a lack of best practice guidelines and a lack of a general framework for evaluationsCitation37,Citation38;
There is a need for better definitions of information to be valued and excluded in evaluationsCitation39,Citation40;
If cost-effectiveness thresholds are a socio-political decisionCitation27, it is not known how this is being used or should be applied in a private market;
One option to consider is a “value based” insurance designCitation39;
There were also some consistent themes that emerged from the literature regarding the nature of the evidence that is, or should be, important for private insurers. These themes included:
The importance of validated evidenceCitation32: i.e. health economic models must undergo comprehensive checks and decision-makers need to obtain the expertise required to fully evaluate realistic models,
Absenteeism, presentism, and disability dataCitation37,Citation39,
How a new drug is usedCitation37: e.g. administration,
Incorporation of real-world data and real world scenariosCitation27,Citation40: tailoring drug submissions to real-world scenarios and the inclusion of treatment benefits relevant to private payers, and
The use of private insurer data including measurement of outcomes and quality improvementCitation33.
Expert panel findings
Participants felt that there were some similarities between private and public plan reimbursement considerations, however, there were substantial differences between both systems in terms of coverage, funding sources, market structure (competitive vs governmental), and reimbursement decision-making criteria and processes. The panel provided the following context to highlight key private payer differences:
Private insurers cover primarily a population of employees in a corporation, and as such adopt a population approach (i.e. overall health of a group of people);
When considering health benefits, employers will have to decide how much to spend on various competing priorities (e.g. wage increases, other benefits, infrastructure investment etc.);
When assessing a new drug, private insurers look primarily at clinical value and cost of a claim;
Private payers examine the potential market size for a new drug to calculate claim exposure rates; and
Private payers do not pay for a number of healthcare costs (e.g. physician costs, hospitalizations, ambulatory care costs) and such do not view the inclusion of these costs as being relevant to them. That said, they currently review drug submissions where these costs are included.
Based on these considerations, the panel identified several “value” parameters they felt were important for reimbursement consideration from a private payer’s perspective ().
Table 2. Important private payer value assessment parameters identified from expert panel.
As with public reimbursement decision-making, effectiveness, and safety of a new drug relative to drugs currently funded by the plan is the first and most important consideration.
The panel also felt the timelines for reimbursement decision-making are different between private and public insurers. Timelines for public drug reimbursement decisions in Canada are long and increasing. However, since delayed decisions can have a direct detrimental impact on worker productivity and can affect the organization’s productivity overall, there was strong recognition that private payers tend to need their assessments quickly and probably cannot, or should not, wait for public HTA reviews.
There was consensus that some insurers have started to show some interest in public HTAs. However, even for those private payers who have shown interest, there appears to be limited understanding of some components of public HTAs including, the methods used in these assessments (e.g. probabilistic sensitivity analyses), the emphasis on clinical value within the evidence retrieved, and how to interpret the main outcome of cost-effectiveness analyses (e.g. what does a cost per QALY gained mean for private payers?).
The discussion revealed that HTAs performed by public payers may have limited applicability for private payers for the following reasons:
The comparator used in the pharmacoeconomic analysis is not the relevant standard of care for the private payer (e.g. the trial comparator is an old generic product suitable for a public drug plan, whereas the private payer covers a new branded product);
The demographic and health status profiles of public plan beneficiaries differ from those of private plan beneficiaries;
The perspective used in public payer HTAs (i.e. healthcare system) is not relevant for private payer decision-making;
Information pertaining to productivity, absenteeism, presentism is missing, and
Patient convenience and impact on family and caregivers is not captured; and
The concept and utility of QALYs is not well understood and often not directly relevant for private payer decision-making.
There was agreement that BIAs remain the key economic component in private payer decision-making. However, it was emphasized that the BIA needs to be tailored to, and done from, the perspective of the specific private payer and not from the perspective of the public payer plan. As such, the comparator drugs where a new drug would capture market shares, the market capture rates and even the relative cost of competitor drugs needs to be customized for each private payer BIA.
The expert panel expressed that there are current practices that private insurance companies do exceptionally well in assessing value. These include recognizing the benefit and leveraging patient support programs as it increases efficiency of their listing decision, and have a good understanding of what their customer values. Private insurers are complex and ultimately profit-making organizations with competing priorities. They often have a wide range of teams including pharmacists, business development, those selling insurance, account managers, etc. Each of these teams often have different goals and priorities, and how well they are communicated amongst themselves is unclear.
Synthesis of literature review and expert panel meeting
Based on the literature review and expert panel meeting, a number of criteria, factors, or value parameters were identified as important for public and private payer drug reimbursement decision-making. Some of the analytic methods and value parameters used in public payer HTAs and their applicability or transferability to private payer assessments are summarized in using the PICO (Population, Intervention, Comparator, and Outcome) framework.
Table 3. Applicability of public HTA analytic approaches and value parameters for private payer decision-making.
Some of these parameters (e.g. clinical effectiveness and safety) were identified as being important for both public and private payer decision-making. However, since these parameters are measured relative to comparators (C), whether an assessment in one sector can be used in another depends on which comparators were included or excluded from the assessment. Also, the clinical trial data in a public HTA submission may be more applicable to an elderly population (P) and the generalizability to a working age population (i.e. private payer) may be questionable. Similarly, although the intervention (I) may be similar in both public and private payer submission, the assessment of the intervention may be different.
Perhaps the biggest difference between public and private payers which limits the relevance of public HTAs to private payer assessments relates to differences in analytic methods and value parameters in cost and effect measures of outcomes (O). Public HTAs accept and use a variety of clinical outcome measures, many of which are not directly relevant for private payer decision-making. The primary outcome measure in public pharmacoeconomic assessments (i.e. QALY) has questionable relevancy for private payers. Many of the costs included in public HTAs (e.g. physician visits, hospitalizations) are not relevant for private payers. On the other hand, public HTAs exclude factors and costs which are relevant for private payer assessments such as productivity, absenteeism and presentism. Private payers tend to value attributes such as convenience and impact on caregivers that public plans typically do not consider. Even public BIAs need to be adapted and re-calculated for private payer submissions.
Overall, some value parameters from public HTAs can be used directly in private payer decision-making, but most of the value parameters were either not applicable, needed to be added, needed to be adjusted, or their applicability to private payer systems needed to be confirmed.
Discussion
The purpose of this study was to examine the use of HTAs to privately funded plans and to explore the benefits and potential economic consequences of changing evaluation processes. However, this research identified a need to further explore the application of HTAs and pharmacoeconomic models developed for public payers to private payer decision-making. As anticipated, the literature review identified a few sources describing the application of public evaluation processes to private payer decision-making. The expert panel identified numerous key differences between public and private payers in terms of perspective and priorities. A key common element that was emphasized was the importance of the BIA to both payers. A second key common element for both the public and private systems is the assessment of value offered by a new drug, with a focus on clinical and economic evidence. Overall, both sectors are interested in aspects such as efficacy, safety, and budgetary impact. However, a comprehensive assessment of what constitutes value from each perspective has not been previously conducted.
With regards to value parameters, there are obvious differences between the perspectives, needs, requirements, and expectations of public and private drug plans. Different methodological approaches are likely required for public vs private market. Based on our literature and expert panel findings, these differences need to be better understood or at least explored. The process for deciding whether to add new drugs to the public drug plan is well defined, with HTA being the primary tool used for decision-making. There is no existing formalized HTA mechanism for decision-making in the private market, although findings from our expert panel indicated that this may change in the future. Individual private market competitors may undertake evaluations of drugs in different ways. While there are private review bodies that provide assessments, these assessment procedures are not typically published or made publically available.
The literature review and round table discussion have highlighted the substantial differences between both the public and private systems in terms of coverage, funding sources, market structure, and reimbursement decision-making criteria and processes. HTA is designed from a public perspective to assist with public policy-making, taking into account society’s view on paying for a new drug. For example, a public HTA dossier is structured to demonstrate the cost-effectiveness of a new drug compared to other drugs that the public system is currently paying for, and these drugs may not be the same drugs covered in private drug plans. Also, the analytical perspective of a public HTA focuses on costs to the healthcare system and does not consider important attributes such as worker productivity like absenteeism and disability. Private payers, in contrast, take a broader view of value and consider a full range of the product benefits that are important to those purchasing insurance as well as those receiving the benefits of private health coverage. Ultimately, if a product is desirable to a customer (or purchaser of insurance) they will elect to pay for it—i.e. the pot of money is not finite, as is the case for public budgets.
The results of this study would not be sufficient to provide clear guidance to payers, but is useful for raising several key questions to be addressed by stakeholders. It is clear that information from the public sector may not be directly relevant for the private sector, and so cannot and should not be transposed. There is a big question around whether cost-effectiveness is the correct tool for private insurers based on their goals. On a more methodological note, and one which certainly needs further exploration, is if private insurers do use cost-effectiveness analyses, what willingness-to-pay thresholds will they be using? Public thresholds have been tailored specifically for a public market and so are not relevant with respect to the private market where different preferences and willingness-to-pay thresholds come into effect. What is the outcome they should be considering when listing new products, and/or what do private customers value? Private health insurance is usually provided as a “perk” for employees. On the other hand, private insurers are providing a service to employers and are profit-making, so what their customer wants is key. Therefore, a private insurance perspective when looking at each new drug needs to include all the purchasers’ needs, while also weighing their own internal priorities such as maintaining competitive insurance premiums, retaining customers, satisfying their shareholders, and their profits and losses.
As previously noted, private insurers are complex and ultimately profit-making organizations with competing priorities. They often have a wide range of teams including pharmacists, business development, those selling insurance, account managers, etc. Each of these teams often have different goals and priorities, and how well are they communicated amongst themselves is unclear. Such a complex and specialized ecosystem would surely require its own customized approach to assessing value outside of any other system that exists—using assessment tools tailored to specific needs—only this will optimize their decision-making and lead to the best decisions for plan sponsors and best outcomes for members alike.
In an example of how these differences in perspectives have played out in practice and resulted in different decisions is the case of Concerta (methylphenidate) for the treatment for Attention Deficit Hyperactivity disorder (ADHD)Citation41. Public payers did not seem to recognize the value of the convenience, adherence, and lessened family caregiving burden impact of twice daily dosing with Concerta (methylphenidate) and chose not to pay for itCitation14,Citation41. In contrast, private payers acknowledged these attributes as being valuable to patients, employees, and employers, and so list and pay for it. Another example is the case of Invega Sustenna (paliperidone palmitate) for the treatment of schizophreniaCitation42. This product allowed a shift from an injection regimen of every 2 weeks to monthly injections, and resulted in a positive impact for patients and caregivers in terms of administration-related travel time and time away from work, which was not valued by public payers, and was by private payersCitation14.
Overall, it was not surprising that there were few publications that implicitly address the use of public HTA in the private market. This is partly due to the fact that this information may not always be published and/or made available to the public. Given the lack of visibility into the actual assessments and decision-makers conducted by payers, our findings should be interpreted with caution. However, the paucity of literature also speaks to the fact there is no defined best practice for private insurers using HTA or cost-effectiveness and the methodology appears to be used in a limited manner by private insurers in Canada and internationally.
Conclusions
This review of decision-making by both private and public payers in Canada suggests that there are important differences between the two processes. Some components of public HTA may be relevant for private payer assessment to some extent, but there remains significant doubt if the HTA process in Canada, which is designed to address concerns from public payers, will address the informational needs of the private payers. To date these topics have not been well explored in the literature. Fundamentally, private insurers need to exercise caution in assessing which value parameters from public HTAs can be used and which need to be confirmed, ignored, enhanced, or adjusted. One size HTA does not fit all applications.
Transparency
Declaration of funding
This study was funded by Amgen, Inc.
Declaration of financial/other interests
LP and MA are employees of Amgen Canada and may own Amgen Inc. stock and/or stock options. RG declares no personal conflict of interest. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
The authors would like to acknowledge Judith Glennie and Raina Rogoza. Rebecca Liu and Rebecca Hancock-Howard provided writing assistance with this manuscript.
References
- Decady Y, Greenberg L. Ninety years of change in life expectancy, H.S. Division, Editor. Statistics Canada. 2014;1–10. https://www150.statcan.gc.ca/n1/en/pub/82-624-x/2014001/article/14009-eng.pdf?st=2oJBS-HR
- Lichtenberg FR. The impact of biomedical innovation on longevity and health. Nordic J Health Eco. 2015;5:45–57.
- Kontis V, Bennett JE, Mathers CD, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335.
- ISPOR Council. ISPOR 2018 Top 10 HEOR Trends. 2018 [cited 2018 Sept 18]; Available from: https://www.ispor.org/docs/default-source/publications/top10trends.pdf?sfvrsn=e6052ae7_2.
- Government of Canada. Canada’s health care system. 2016 [cited 2018 Sept 18]; Available from: https://www.canada.ca/en/health-canada/services/canada-health-care-system.html.
- Canadian Life and Health Insurance Association, Canadian life and health insurance facts. Canadian Life and Health Insurance Association (CLHIA); Canada (Toronto, Montreal, Ottawa); 2017. https://www.clhia.ca/web/clhia_lp4w_lnd_webstation.nsf/resources/Factbook_2/$file/2018+FB+EN.pdf
- Picard A. National pharmacare is possible – but it won’t come easy. The Globe and Mail. The Globe and Mail Inc: Canada: Toronto; 2018.
- Fréchette J-D. Federal cost of a national pharmacare program. Ottawa; Office of the Parliamentary Budget Officer; 2017. http://www.pbo-dpb.gc.ca/web/default/files/Documents/Reports/2017/Pharmacare/Pharmacare_EN_2017_11_07.pdf
- Sutherland G, Dinh T. Understanding the gap: A Pan-Canadian analysis of prescription drug insurance coverage. The Conference Board of Canada; Canada; 2017.
- The International Network of Agencies for Health Technology Assessment. The International Network of Agencies for Health Technology Assessment. 2019 [cited 2019 Jan 14]; Available from: http://www.inahta.org/
- Battista RN, Côté B, Hodge MJ, et al. Health technology assessment in Canada. Int J Technol Assess Health Care. 2009;25:53–60.
- Institut National d'Excellence en Santé et Services Sociaux. Evaluation Process and Criteria. 2018 [cited 2018 Oct 04]; Available from: https://www.inesss.qc.ca/en/activities/drug-products/evaluation-process-and-criteria.html.
- Government of Canada. Federal Public Drug Benefit Programs. 2018 [cited 2018 Sept 18]; Available from: https://www.canada.ca/en/health-canada/services/health-care-system/pharmaceuticals/access-insurance-coverage-prescription-medicines/federal-public-drug-benefit-programs.html.
- Glennie J. Assessing Medication Value – One Size Does Not Fit All! 2016 [cited 2018 Sep 18]; Available from: http://bppgcreative.ca/pdfs/r/HTA-private-payer-article-submission-to-Benefits-Canada.pdf.
- Sáaid HB, Stewart D, England I, et al. The Impact of Health Technology Assessment on Decision-Making Processes in Public Versus Not-for-Profit Private Hospitals. American Medical Journal. 2011;2:72–78.
- Sullivan SD, Watkins J, Sweet B, et al. Health Technology Assessment in Health-Care Decisions in the United States. 2009; 12:S39–S44.
- Pericleous L, Rogoza R, Goeree R. Use of health technology assessment for drugs in the private payer environment in canada: a literature review. Canadian Association of Population Therapeutics. Toronto, Ontario: Amgen Canada Inc; 2016.
- Santos CMdC, Pimenta CAdM, Nobre MRC. The PICO strategy for the research question construction and evidence search. Rev Latino-Am Enfermagem. 2007;15:508–511.
- Fernandez AM, Schrogie JJ, Wilson WW, et al. Technology assessment in healthcare: a review and description of a “best practice” technology assessment process. Best Practices and Benchmarking in Healthcare. 1997;2:240–253.
- Perry S, Thamer M. Health technology assessment: decentralized and fragmented in the US compared to other countries. Health Policy. 1997;40:177–198.
- Davis JB. Cost containment mechanisms in Canada. Croat Med J. 1999;2:287–293.
- Philips Z, Ginnelly L, Sculpher M, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;36:iii–iv, ix–xi, 1–158.
- Drummond MF, Schwartz JS, Jönsson B, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care. 2008;24:244–258. discussion 362-8.
- Bresnahan BW, Neumann PJ, Carlson R, et al. Findings related to assessing value for private us health plans. Am J Pharm Benefits. 2010;2:219–228.
- Bryan S, Sofaer S, Siegelberg T, et al. Has the time come for cost-effectiveness analysis in US health care? Health Econ Policy Law. 2009;4:425–443.
- Goeree R, Levin L, Chandra K, et al. Health technology assessment and primary data collection for reducing uncertainty in decision making. J Am Coll Radiol. 2009;6:332–342.
- Weintraub WS, Cohen DJ. The limits of cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2009;2:55–58.
- Mohr PE, Tunis SR. Access with evidence development: the US experience. Pharmacoeconomics. 2010;28:153–162.
- Marra CA, Bansback N, Anis AH, et al. Introduction to economic modeling for clinical rheumatologists: application to biologic agents in rheumatoid arthritis. Clin Rheumatol. 2011;30(Suppl 1):S9–S18.
- Trosman JR, Van Bebber SL, Philiips KA. Health technology assessment and private payers’ coverage of personalized medicine. Am J Manag Care. 2011;17:SP53–SP60.
- Kratzer J, McGrail K, Strumpf E, et al. Cost-control mechanisms in canadian private drug plans. Healthc Policy. 2013;9:35–43.
- Caro JJ, Möller J. Decision-analytic models: current methodological challenges. Pharmacoeconomics. 2014;32:943–950.
- Klein I, Kolodziej M. Private payers and cancer care: land of opportunity. J Oncol Pract. 2014;10:15–19.
- Moloney R, Mohr P, Hawe E, et al. Payer perspectives on future acceptability of comparative effectiveness and relative effectiveness research. Int J Technol Assess Health Care. 2015;31:90–98.
- Trosman JR, Weldon CB, Douglas MP, et al. Decision making on medical innovations in a changing health care environment: insights from accountable care organizations and payers on personalized medicine and other technologies. Value Health. 2017;20:40–46.
- Garrison LP, Pauly MV, Willke RJ, Jr, et al. An overview of value, perspective, and decision context-A health economics approach: An ISPOR special task force report [2]. Value Health. 2018;21:124–130.
- Garel E. Rewriting the prescription: creative ways to control drug costs. 2011 [cited 2018 Sep 19]; Available from: https://www.benefitscanada.com/benefits/health-benefits/rewriting-the-prescription-creative-ways-to-control-drug-costs-14201.
- Scott-Clarke A. Moving toward a healthier drug plan. 2013 [cited 2018 Sep 19]; Available from: https://www.benefitscanada.com/uncategorized/moving-toward-a-healthier-drug-plan-36176.
- Bonnett C. Making U.S. benefits plan design work in Canada. 2011 [cited 2018 Sep 19]; Available from: https://www.benefitscanada.com/benefits/health-wellness/making-u-s-benefits-plan-design-work-in-canada-18252.
- Bauer G. Think tank evaluates current methods for drug coverage. 2011 [cited 2018 Sep 19]; Available from: https://www.benefitscanada.com/news/think-tank-evaluates-current-methods-for-drug-coverage-17965.
- Advocacy CC. Equality of access for Canadians to new medication. 2009 [cited 2016 Mar 1]; Available from: Centre for ADD/ADHD, http://caddac.ca/cms/CADDAC_pdf/Longacting_medications_policy_paper_March_1_Final.pdf.
- Canadian Agency for Drugs and Technologies in Health. Common drug review-CEDAC final recommendation: Paliperidone palmitate (Invega Sustenna- Janssen Inc.). 2011 [cited 2016 Mar 1]; Available from: https://www.cadth.ca/sites/default/files/cdr/complete/cdr_complete_Invega-Sustenna_April-29-11.pdf.