Abstract
Aims: The overall cost and health-related quality of life (HRQoL) associated with current treatments for chronic kidney disease (CKD)-related anemia are not well characterized. A systematic literature review (SLR) was conducted on the costs and HRQoL associated with current treatments for CKD-related anemia among dialysis-dependent (DD) patients.
Materials and methods: The authors searched the Cochrane Library, MEDLINE, EMBASE, NHS EED, and NHS HTA for English-language publications. Original studies published between January 1, 2000 and March 17, 2017 meeting the following criteria were included: adult population; study focus was CKD-related anemia; included results on patients receiving iron supplementation, red blood cell transfusion, or erythropoiesis stimulating agents (ESAs); reported results on HRQoL and/or costs. Studies which included patients with DD-CKD, did not directly compare different treatments, and had designs relevant to the objective were retained. HRQoL and cost outcomes, including healthcare resource utilization (HRU), were extracted and summarized in a narrative synthesis.
Results: A total of 1,625 publications were retrieved, 15 of which met all inclusion criteria. All identified studies included ESAs as a treatment of interest. Two randomized controlled trials reported that ESA treatment improves HRQoL relative to placebo. Across eight studies comparing HRQoL of patients achieving high vs low hemoglobin (Hb) targets, aiming for higher Hb targets with ESAs generally led to modest HRQoL improvements. Two studies reported that ESA-treated patients had lower costs and HRU compared to untreated patients. One study found that aiming for higher vs lower Hb targets led to reduced HRU, while two other reported that this led to a reduction in cost-effectiveness.
Limitations: Heterogeneity of study designs and outcomes; a meta-analysis could not be performed.
Conclusions: ESA-treated patients undergoing dialysis incurred lower costs, lower HRU, and had better HRQoL relative to ESA-untreated patients. However, treatment to higher Hb targets led to modest HRQoL improvements compared to lower Hb targets.
Introduction
Chronic kidney disease (CKD) is a serious health disorder defined by impaired renal function or elevated albumin excretion, or bothCitation1,Citation2. Advanced renal impairment requires initiation of renal replacement therapy (dialysis or kidney transplant) for continued patient survivalCitation1,Citation3,Citation4.
The economicCitation5,Citation6, health-related quality of life (HRQoL)Citation7,Citation8, and clinical burdenCitation4,Citation9 of CKD is considerable, particularly among dialysis-dependent (DD) patientsCitation10. A significant direct contributor to the high burden of CKD is CKD-related anemia (defined by the World Health Organization as serum hemoglobin [Hb] levels ≤12 g/dL in women and ≤13 g/dL in men)Citation11, which affects more than half of patients with end-stage renal disease (ESRD)Citation12. Anemia is a serious complication of CKD, associated with increased cardiovascular comorbidity among other deleterious outcomesCitation13,Citation14.
CKD-related anemia can be managed with intravenous iron treatment, erythropoiesis stimulating agents (ESAs), and red blood cell transfusions (RBCT). While ESAs are an effective treatment for anemia, their use is limited by increased cardiovascular risk at higher dosesCitation15–19. In addition, a proportion of patients on dialysis (between 4.5% and 20%)Citation20–22 are hyporesponsive to ESAs (requiring substantially higher ESA doses or transfusions to manage their anemia).
Iron supplementation (administered intravenously or orally) can improve the efficacy of lower doses of ESA treatmentCitation23,Citation24, but there have been concerns regarding treatment safety and possible iron overloadCitation25. However, a recent randomized controlled trial (RCT) found that high-dose intravenous iron administered proactively and concomitantly with ESAs was non-inferior to lower doses of iron (administered reactively), and such higher doses of iron were not associated with higher risks of death, cardiovascular events, or infectionCitation26.
Blood transfusions remain an undesirable, last-resort option because of potential transfusion reactions and allosensitization limiting potential kidney transplantationCitation27–29. Therefore, there is a need for new additional treatment options.
In 2012, increasing awareness of the safety profile of ESAs prompted expert consensus—including the KDIGO guidelines group and regulatory agencies, such as the US Food and Drug Administration and the European Medicines Agency—to formulate recommendations against the use of ESAs to achieve Hb levels above 13 g/dL and repeated escalations of ESA dose in patients with ESA hyporesponsivenessCitation18,Citation30,Citation31.
The proportion of dialysis patients using ESAs, as well as the mean ESA dose, decreased between 2009 and 2013Citation32–37. The US temporal trends in Hb levels are different from other countries, with a sharper decrease in the US from ∼11.5 to 10.6 g/dL vs a smaller reduction in Europe and little to no change in Japan during the same periodCitation32–39. Moreover, lower doses of ESAs and iron than those prescribed in North America were administered in China and Japan from 2011 onwards. Much of these treatment disparities appear driven by differences in reimbursement policies as well as other factors (e.g. ethnic differences)Citation38,Citation40–42.
Given this treatment shift, promising novel approaches, and the existing regional differences in the use of ESAs, it is important to provide a global and up-to-date perspective on the impact of treating CKD-related anemia in patients undergoing dialysis. The literature on anemia of CKD is rich, and the economic and/or HRQoL burden of CKD-related anemia has been previously reviewed in three other systematic literature reviews (SLRs) studiesCitation43–45. However, of these, two were country-specific (JapanCitation43 and USCitation44) and did not focus specifically on patients with DD-CKDCitation43,Citation44. In addition, one was conducted prior to the publication of the 2012 KDIGO guidelinesCitation44. The third review, though recent, was restricted to only four randomized controlled trials (RCTs) on patients with DD-CKDCitation45. Due to their rigid protocols, RCTs may not be the most appropriate source of data for assessing costs and HRQoL. Thus, we conducted an SLR to provide an updated and comprehensive synthesis of the current literature on costs and HRQoL associated with current treatments for CKD-related anemia among patients with DD-CKD.
Methods
Data source
The following electronic databases were searched to identify relevant publications in English published between January 1, 2000 and March 17, 2017: the Cochrane Library, MEDLINE (including MEDLINE In-Process), EMBASE, National Institute for Health Research Economic Evaluation Database (NHS EED), and National Institute for Health Research–Health Technology Assessment (NHS HTA).
Literature search strategy and study selection process
The full search strategy is presented in Supplementary Table S1. Studies were included provided they met the following inclusion criteria: adult population (≥1 patient aged ≥18 years); disease area was CKD-related anemia; iron supplementation, RBCT, or ESAs (epoetin or darbepoetin) were among the included treatments; costs and/or HRQoL were among the outcomes investigated.
Studies were excluded when at least one of the following criteria were met: strictly pediatric population (all patients <18 years of age); study population was non-human (animal or cell-based studies); publication was a letter, an editorial, a comment, a case report, a biography, or an address (not a study in which new results are generated); article was not in English. Furthermore, studies that did not allow for the evaluation of incremental cost or HRQoL outcomes on patient outcomes from a particular treatment (head-to-head comparisons between different treatments, dose escalation studies, treatment switch studies, program evaluation studies, etc.) were also excluded.
The study selection process was conducted in two phases. In phase 1, titles and abstracts of studies identified from the electronic databases were reviewed for eligibility by two independent reviewers, based on the aforementioned inclusion and exclusion criteria. Discrepancies in the decision to include or exclude a study were resolved through discussion. Whenever consensus was not reached, a third independent reviewer was consulted. In phase 2, full texts of studies selected in phase 1 were reviewed for eligibility by two independent reviewers using the same aforementioned eligibility criteria. Discrepancies in the decision to include or exclude a study were resolved through discussion. Whenever consensus was not reached, a third reviewer was consulted. Once articles meeting these criteria had been identified, studies were retained if the patient population was DD or if results on the DD population were reported separately.
Data extraction
Data were extracted from the full publications by two independent team members. Discrepancies were resolved through discussion. Contingent on availability, the following variables were extracted from the included publications and reported in an Excel spreadsheet: author(s); year of publication, title, treatment(s) (ESAs, and/or RBCT and/or iron supplementation), country(ies) in which the study was conducted, study design, time period of data collection/analysis, Hb target/guideline(s) followed, dates of data collection, dose/dosing schedule, treatment duration, eligibility criteria (inclusion and exclusion criteria), number of patients included in the analysis, patient characteristics at study initiation (mean age, sex, CKD stage, type of dialysis and months on dialysis, comorbidities, and laboratory measurements), HRQoL outcomes (measured with: Kidney Disease Quality of Life [KDQOL] 36- and 12-Items surveys [KDQoL-36 and KDQoL-12, respectively], generic 36- and 12-items Short Form Surveys [SF-36 and SF-12, respectively], quality-adjusted life years [QALYs], Functional Assessment of Cancer Therapy: Fatigue (FACT-Fatigue), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, six-minute walking test, physical activity, Kidney Disease Questionnaire [KDQ]-13, KDQ-26, other measurements), and cost outcomes (healthcare resource utilization [HRU], cost per QALY, treatment-related costs, other direct costs, and indirect costs).
Analysis
Due to the heterogeneity of the study designs considered (RCTs, cross-sectional surveys, retrospective studies) and outcomes (different HRQoL outcomes, different definitions of cost outcomes), results were summarized in a narrative synthesis. Although most studies did not assess the clinical significance of their findings with minimal clinically meaningful differences (MCIDs), such thresholds were applied based on the literature, which are summarized in Supplementary Table S2.
Results
Search results
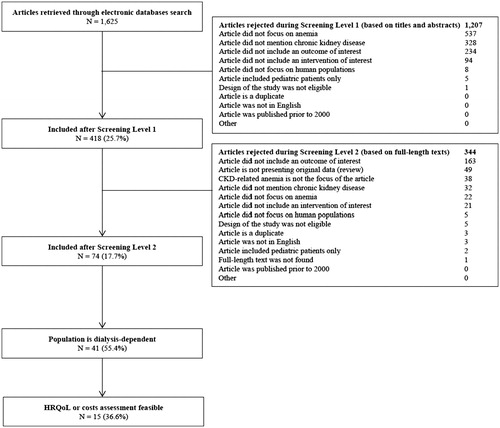
The electronic search strategy retrieved 1,625 unique publications. After screening by titles and abstracts, 418 full-length publications (25.7%) were assessed for eligibility, and 41 (9.8%) met the inclusion criteria. After eliminating studies that did not allow for the incremental evaluation of differences in costs or HRQoL outcomes of the treatment(s) of interest, 15 articles (36.6%) were included in this study ().
Figure 1. The PRISMA statement flow diagram. Abbreviations. CKD, chronic kidney disease; HRQoL, health-related quality of life.
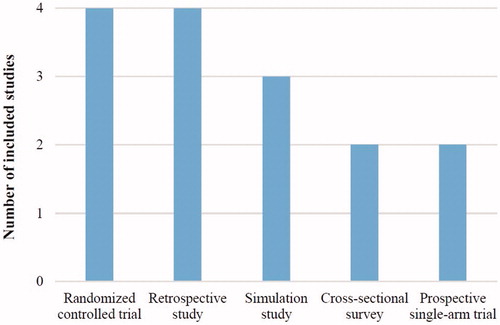
presents the characteristics of the included studies. Study designs included four (26.7%) RCTsCitation46–49, four (26.7%) retrospective studiesCitation50–53, three (20.0%) simulation studiesCitation54–56, two (13.3%) cross-sectional surveysCitation57,Citation58, and two (13.3%) prospective single-arm trials ()Citation59,Citation60. Six (40.0%) studies were real-world studies (non-simulated, non-interventional)Citation50–53,Citation57,Citation58, including three (50.0%)Citation51,Citation57,Citation58 published after 2012. Two publications were derived from the same study (included the same patients), but reported different results (different HRQoL instruments were used)Citation47,Citation48. Thus, these two publications were treated as independent studies in this narrative synthesis.
Table 1. Summary of included articles.
Four (26.7%) articles reported exclusively on costsCitation51–53,Citation55, eight (53.3%) reported on HRQoL onlyCitation46–50,Citation57–59, and three (20.0%) reported on both costs and HRQoLCitation54,Citation56,Citation60.
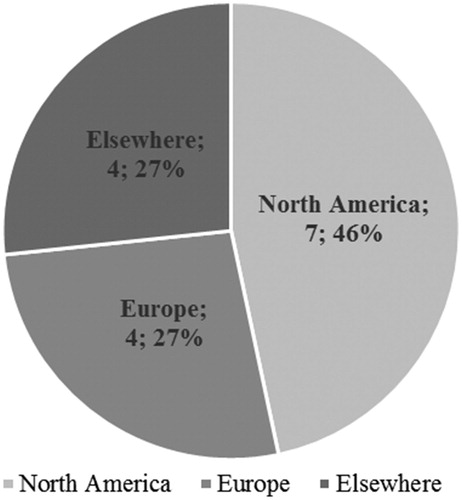
Seven out of 15 (46.7%) studies were conducted in North America exclusivelyCitation47,Citation48,Citation52–56, four (26.7%) were conducted in Europe exclusivelyCitation46,Citation51,Citation57,Citation60, and four (26.7%) were conducted elsewhere (including international collaborations; )Citation49,Citation50,Citation58,Citation59. Among the seven studies that reported cost outcomes, five (71.4%) were conducted in North America exclusivelyCitation52–56, and two (28.6%) in Europe exclusivelyCitation51,Citation60. Among the 11 studies that reported HRQoL outcomes, four (36.4%) were conducted in North America exclusivelyCitation47,Citation48,Citation54,Citation56, three (27.3%) in Europe exclusivelyCitation46,Citation57,Citation60, and four (36.4%) were conducted elsewhere (including international collaborations; )Citation49,Citation50,Citation58,Citation59.
Figure 3. Geographical distribution of studies. Note: “Elsewhere” includes international collaborations.
Among these 11 studies on HRQoL, disease-specific questionnaires, including the KDQoL (four studiesCitation49,Citation50,Citation57,Citation58 [36.4%]) and the KDQ (three studiesCitation46–48 [27.2%]), were most frequently used. Other HRQoL quantifying tools included QALYs (two studiesCitation54,Citation56 [18.2%]), and the Sickness Impact Profile (SIP) (two studiesCitation48,Citation60 [18.2%]); the SF-36Citation59, Functional Assessment of Chronic Illness Therapy (FACIT)-FatigueCitation59, six-minute walking testCitation48, and treadmill stress testCitation48, were each used in one study (9.1%).
All studies investigated ESAs and, in some of them, iron was administered concomitantlyCitation50,Citation57. No study investigated RBCT or iron supplementation as standalone treatment (). All six prospective studies (four RCTsCitation46–49 and two prospective single-arm trialsCitation59,Citation60) reportedly used concomitant iron supplementation with ESAs. A majority of studies (11 out of 15 [73.3%]) used data collected prior to 2011Citation46,Citation47,Citation49,Citation50,Citation52–56,Citation59,Citation60, the year prior to the change in recommended Hb target by the KDIGO. Among the four studies published or that collected data on or after 2011Citation48,Citation51,Citation57,Citation58, none presented data restricted to the US. Among the nine studies for which an Hb target was specified, targets ranged between 9.0–10.9 g/dLCitation54 and 13.5–16.0 g/dLCitation46. Six studies did not specify or explicitly used an Hb targetCitation50,Citation53,Citation55,Citation57,Citation58,Citation60.
Among the three prospective studies that provided both an Hb target and the Hb levels achieved at the end of the follow-up periodCitation46,Citation49,Citation59, only the high Hb target group in Foley et al.’sCitation49 study did not achieve the target.
Impact of treatments on HRQoL outcomes
Three studies assessed HRQoL in ESA-treated and ESA-untreated patients, two of which found a positive impact of treatment on HRQoL and one that reported no differenceCitation47,Citation57,Citation58. In Keown et al.’sCitation47 study, ESA treatment led to statistically and clinically meaningful improvements in HRQoL (MCID reached), as assessed using the KDQ (). A cross-sectional study conducted by Eriksson et al.Citation57 assessed HRQoL using KDQoL-36 in ESA/iron-treated vs non-treated patients. The authors reported no statistically significant difference in HRQoL ().Citation57 Okpechi et al.Citation58 found that ESA use weakly correlates with improvement on HRQoL (). The study suffered from a high ESA non-response rate. The authors report this is likely due to inadequate dosing of an ESA (hospital restrictions due to financial constraints) and, in some cases, poor compliance with treatmentCitation58.
Table 2. Summary of findings: HRQoL.
Eight studies assessed the impact of different Hb targets or Hb levels on HRQoL, and the magnitude and clinical significance of changes varied across studies and HRQoL domainsCitation46,Citation48–50,Citation54,Citation56,Citation59,Citation60. presents the Hb targets for each study. Two RCTs reported that aiming for higher Hb targets using ESAs was associated with some improvements in HRQoL (improvements restricted to a few specific domains only [notably fatigue/vitality] and/or MCIDs not reached)Citation46,Citation49. Higher Hb targets were defined as 13.5–14.5 g/dL in the Foley et al.Citation49 study and 13.5–16.5 g/dL in the Furuland et al.Citation46 study, while lower Hb targets were defined as 9.5–11.5 g/dL in the Foley et al.Citation49 study and 9.0–12.0 g/dL in the Furuland et al.Citation46 study. Using the SIP, Moreno et al.Citation60 found clinically and statistically meaningful improvements for patients treated with epoetin. Using the SF-36 and FACIT-Fatigue, Kubota et al.Citation59 did not find improvements in patients treated with darbepoetin alfa (). Two cost-utility analyses showed that the gains in QALY with ESA became more limited above an Hb target of about 12 g/dL ()Citation54,Citation56. While no HRQoL domains were significantly improved in Kubota et al.’sCitation59 study, improvements in the fatigue domain appeared more important and nearly statistically significant. Akizawa et al.Citation50 reported that patients with Hb levels <8.0 g/dL had significantly lower HRQoL than those with Hb levels between 11.0 and 12.0 g/dL ().
Impact of treating anemia on cost outcomes
Two studies compared the cost or HRU of anemia management with ESAs vs no treatment, both of which found that non-treatment is associated with higher costs or HRUCitation52,Citation53. In a retrospective commercial claims analysis, Moyneur et al.Citation53 reported total direct costs of $13,199 per patient per month (PPPM) among no-ESA patients, and $11,312 PPPM among ESA patients in the 6-month period following initiation of dialysis (, univariate cost ratio = 0.86, p < 0.001). In a multivariate analysis, the same study observed that ESA-untreated patients incurred $1,515 higher direct costs PPPM during the first 6 months of dialysis compared to ESA-treated patients (multivariate cost ratio = 0.89, p = 0.027), and that higher sick leave costs in ESA-treated patients were offset by lower disability costs compared to untreated patients, thereby yielding similar indirect costs ()Citation53. Liu et al.Citation52 found that not achieving an 11 g/dL target has negative economic and clinical implications: average medical costs and HRU increased with longer time spent with Hb <11 g/dL, although it is not clear whether other severe comorbidities may have contributed to ESA resistance and, thus, resulted in a higher HRU burden (, ).
Figure 4. Relative cost outcomes among studies that compared treated vs untreated patients. Abbreviations. EPO, erythropoietin; Hb, hemoglobin; PPPM, per patient per month.
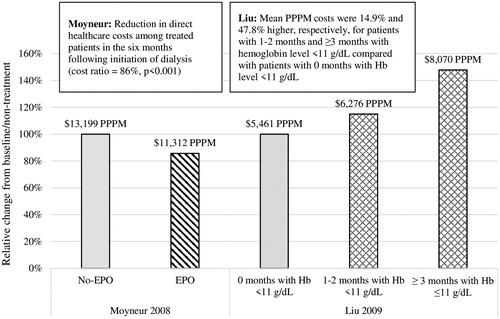
Table 3. Summary of findings: costs.
Four studies assessed the economic impact of different Hb targets, and conclusions were heterogeneousCitation51,Citation54,Citation56,Citation60. With respect to HRU, using data from 34 Spanish hemodialysis centers, Moreno et al.Citation60 reported in 2000 that normalizing Hb levels at a mean of 12.5 g/dL led to 58% and 69% reductions in hospitalizations and in lengths of hospital stays, respectively, after increasing epoetin dosage by 51% (). Hazara et al.Citation51 reported that the number of median weekly ESA doses per patient decreased in the UK after the publication of the KDIGO guidelines in 2012 (). Using 2006 data from Canadian databases, Clement et al.Citation54 showed that aiming for a high Hb target (>12 g/dL) incurred higher costs than aiming for lower Hb targets (). Using 2001 US data, Tonelli et al.Citation56 reported that the cost per QALY gained when aiming for a Hb target of 11.0–12.0 g/dL vs 9.5–10.5 g/dL was $55,295, whereas it was $613,000 when aiming for a target of 12.0–12.5 g/dL vs 11.0–12.0 g/dL ().
In addition, Pizzi et al.Citation55 reported in 2006 that poor adherence to clinical practice guidelines was associated with increased use of EPO. This resulted in an incremental cost of ∼ $250 PPPM ().
Discussion
CKD-related anemia is a condition for which the clinical management has substantially changed in the past decade. In addition, there are regional differences in the clinical management of CKD-related anemia, hence the need for a global and updated assessment of the cost and HRQoL burden of this condition. In this SLR, the economic and HRQoL burden of CKD-related anemia among patients with DD-CKD was qualitatively assessed in a narrative synthesis that included 15 studies.
All of these studies focused on ESAs, including some in which iron was administered concomitantly. With respect to HRQoL outcomes, two placebo-controlled RCTs showed that ESA treatment leads to statistically and clinically meaningful HRQoL improvementsCitation47,Citation48, a finding that was not corroborated by another real-world studyCitation57. Given the fundamental methodological differences between RCTs and real-world studies, the interpretation of this apparent discrepancy should be cautious. The overall gains in HRQoL associated with higher Hb targets generally appeared modest (MCID not systematically reached), and more pronounced for fatigue-related domainsCitation46,Citation49,Citation50,Citation54,Citation56,Citation58–60.
With respect to costs, studies showed that, in spite of the high acquisition cost of ESAs, non-treatment of CKD-related anemia entailed higher medical costs and HRU that outweighed ESA costs, highlighting the importance of treating this condition given its economic burdenCitation52,Citation53,Citation60. Aiming for Hb targets above 12 g/dL incurred higher HRU and costs, and translated into limited gains in QALY, and, thus, higher costs per QALY gainedCitation51,Citation54,Citation56.
All studies included in this SLR investigated ESAs. No studies investigated RBCT or iron supplementation independently of ESAs. The lack of studies on iron supplementation alone may be attributable to the higher cost of ESAs compared to iron, which may have motivated more research concerning the economic and HRQoL impact of ESAs. Nonetheless, the effect of iron in anemia treatment is not negligible. Together with iron loss due to dialysis and impaired renal erythropoietic signaling (including a downstream effect on iron mobilization), patients with DD-CKD require iron supplementation to maintain the erythropoietic demand imposed by ESAs. However, iron homeostasis is frequently perturbed in CKDCitation61. Hepcidin secretion resulting from CKD-related inflammation interferes with iron absorption and mobilization, resulting in functional iron deficiency and ultimately leading to ESA hyporesponsivenessCitation61–64. Such hyporesponsiveness caused by inflammation (comorbidities) may in turn translate into higher costs and HRU as these patients need higher doses and more frequent administration of ESAs. The need to control the delicate balance of iron regulation in order to obtain an optimal erythropoietic response with ESAs explains the importance of iron as a concomitant treatmentCitation26.
With respect to HRQoL, two publications from the same RCT reported significant improvements in HRQoL following treatment with ESAs (compared to no treatment)Citation47,Citation48. However, a real-world study conducted by Eriksson et al.Citation57 reported non-significant differences in HRQoL between ESA/iron-treated vs ESA/iron-untreated patients. A possible explanation for this discrepancy is related to a general limitation of HRQoL studies: patients who did not fill in the HRQoL questionnaires may have different demographic and clinical characteristics than those who did, creating selection biasCitation57. However, Eriksson et al.Citation57 did not assess to what extent these characteristics were different, and did not report on the nature of these differences. Furthermore, as illustrated by Pizzi et al.Citation55, discrepancies may exist between the recommended management of CKD-related anemia and that observed in clinical practice.
While there is an association between Hb levels and HRQoLCitation65–68, this does not necessarily imply that aiming for unnecessarily high Hb targets with ESAs leads to better HRQoL improvements in patients with DD-CKD. In this review, the impact of higher Hb targets on overall HRQoL, if any, appeared modest. While two cost-utility analyses reported decreases in QALYs with Hb targets >12 g/dLCitation54,Citation56, two prospective studies found discrepant conclusions (improvements [Moreno et al.Citation60] vs no difference [Kubota et al.Citation59]), and two RCTs reported HRQoL improvements of uncertain clinical significanceCitation46,Citation49. These heterogeneous conclusions may be partially explained by the increased cardiovascular risk associated with higher doses of ESAs, which prevent more robust anemia correction. ESAs act as an exogenous source of erythropoietin (EPO) that may lead to levels exceeding those of endogenous EPO, which in turn could be responsible for increased cardiovascular risk.
In the overall assessment of HRQoL in this SLR, the impact on fatigue-related domains stood out from the other dimensions. Of 20 KDQoL-36 domains assessed, Foley et al.Citation49 observed that only “energy/fatigue” was significantly improved in patients treated to a higher Hb target. Furuland et al.Citation46 also observed significant improvements in the fatigue domain of KDQ, although improvements did not reach the MCID. While Kubota et al.Citation59 found no significant difference in HRQoL domain, only FACIT-fatigue was borderline significant. This is consistent with the idea that the most significant improvements in HRQoL observed following anemia treatment are related to physical symptoms and fatigue, whereas other domains show more modest improvementsCitation69,Citation70. While no studies directly assessed the impact of fatigue-related HRQoL on indirect costs such as work productivity, this may have significant consequences both on society and, more importantly, on patients, that warrant further researchCitation71. From a clinical perspective, the SONG-HD initiative aims to do just that, which is to better study, measure, and understand the impact of anemia on fatigueCitation72. The economic implications remain to be explored.
A recent meta-analysis conducted by Collister et al.Citation45 evaluated the impact of ESAs on the HRQoL of patients with CKD-related anemia (DD and non-dialysis-dependent [NDD] studies separately evaluated). The authors did not find clinically or statistically significant differences in HRQoL associated with higher Hb targets in patients with DD-CKD, and suggest that a positive impact of ESAs on HRQoL should be ruled outCitation44. Here, by examining study findings separately with regards to (1) treatment vs no treatment and (2) higher vs lower Hb targets, it appears that the conclusions drawn by Collister et al.Citation45 should be somewhat caveated. The data presented in the current study show that ESAs have an impact on HRQoL, but only when ESA-treated patients are compared to their untreated counterparts (the “total” impact). However, there appears to be a limited, if at all, benefit of aiming for unnecessarily high Hb targets using ESAs, when compared to aiming for lower Hb targets. This conclusion is consistent with the ability of ESAs to increase Hb levels in anemic patients, at the risk of increased cardiovascular risk when aiming to fully correct anemia. Interestingly, Collister et al.Citation45 also noted that the impact of ESAs on HRQoL appeared even more attenuated in patients with DD-CKD. While a meta-analysis has not been performed to quantitatively assess these differences, a similar SLR that focused on patients with NDD-CKD reached largely similar conclusionsCitation73.
Due to the high costs associated with the production of recombinant proteins (biologics)Citation74, ∼80% of patients with CKD-related anemia may not be treated with ESAsCitation12. In the present review, Moyneur et al.Citation54 showed that costs are higher in ESA-untreated patients (prior to the initiation of dialysis) compared to ESA-treated patients, suggesting the high acquisition costs of ESAs may be offset by lower medical costs. This is likely due to the alleviation of CKD-related comorbidities (among ESA-responsive patients), which could translate into lower HRU. However, since patients were grouped based on their pre-dialysis ESA treatment status in the Moyneur et al.Citation54 study, these results may also reflect other aspects of pre-dialysis disease management which could have had an impact on the economic burden of treatment following the initiation of dialysis.
In 2012, the KDIGO guidelines recommended that targeting Hb levels >13.0 g/dL with ESAs be avoided. Only four included studies, including three real-world studies, were conducted on or after 2011, showing that there is still a need for more up-to-date real-world evidence on patients with DD-CKD-related anemia.
New effective treatment options with other modes of action appear necessary to help patients with CKD-related anemia meet their needs, and several agents are currently under study. Furthest in development is the class of hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs), currently in phase 3 development in the US and recently approved in China. Unlike ESAs, which act as an exogenous source of EPO, HIF-PHIs stimulate endogenous EPO production and co-ordinate the erythropoietic response at a whole-body system regulation levelCitation75. This new drug class has demonstrated the ability to achieve and maintain target Hb levels in short-term phase II clinical studiesCitation76–81, and elicited an EPO response closer to endogenous levels observed at moderate altitude, but several orders of magnitude lower than that observed following conventional ESA treatmentCitation76,Citation80,Citation82–85.
Limitations
This literature summary review should be considered in light of several limitations. First, it is possible that, despite the scope of the systematic search strategy used in the current SLR, a few studies have been missed in the manual review. For example, studies comparing treated and untreated patients that did not mention specific names of treatments may have been omitted. Second, due to the heterogeneity in study designs and choices of outcomes, this review could not include a meta-analysis to quantitatively assess the significance of the results, which highlights the need to standardize the outcomes reported in CKD studies. This also limited our ability to consider potentially relevant factors, such as ethnicity and comorbidities, in our interpretation. Third, the most accurate MCID for HRQoL may vary from one study to another due to population differences and other factors. For example, studies that included sicker patients at baseline are more likely to report higher improvements compared to studies in which patients were relatively healthy at baseline, hence the need to adapt the MCID from one study to anotherCitation86. For evaluating all studies on a similar scale, a rigid cutoff based on the literature was applied. Fourth, the current SLR did not include any studies in which iron or RBCT was investigated as standalone treatment, a situation that may be attributable to the exclusion of certain types of study design that did not allow the economic or HRQoL burden of anemia treatment to be evaluated. Finally, the included studies’ quality of reporting and risk of bias was not assessed, because of the high heterogeneity in study designs.
Conclusion
This literature review highlighted that the economic burden of CKD-related anemia is substantial among patients who are on dialysis and may be alleviated by currently available treatment options. However, the included studies reported that aiming for higher Hb targets above ∼12 g/dL resulted in modest—and expensive—HRQoL improvements of uncertain clinical significance, which could reflect the cardiovascular complications associated with higher doses of ESAs.
Transparency
Declaration of funding
This study was funded by Akebia Therapeutics, Inc (Akebia). The study sponsor was involved in all aspects of the research presented in this manuscript.
Declaration of financial/other interests
BS, RPF, WCW, and PEP received consulting fees from Akebia not related to the work presented in this manuscript. GS is an employee and stockholder of Akebia. AB is a former employee and current shareholder of Akebia. MS and HBK are employees of Otsuka Pharmaceutical Development & Commercialization, Inc., a collaboration partner of Akebia in the development of vadadustat for the treatment of anemia secondary to chronic kidney disease. SR, PT-L and PL are employees of Analysis Group Inc., which has received consulting fees from Akebia. A JME peer reviewer on this manuscript discloses membership on commercialization advisory boards for Akebia in March and November 2018, as well as consulting for Otsuka. Another peer reviewer discloses serving on an advisory board for, and receiving speaker fees from, Roche; serving on an advisory board for Astellas; and receiving speaker fees at meetings supported by Vifor-fresenius. The remaining peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Parts of the material in this manuscript were presented at the 2018 ERA-EDTA Annual Congress (Copenhagen, Denmark, May 24–27, 2018) and the 2018 American Society of Nephrology Kidney Week (San Diego, CA, October 23–28, 2018).
Supplemental Table 2
Download MS Word (13.3 KB)Supplemental Table 1
Download MS Word (14.8 KB)Acknowledgements
Editorial assistance was provided by Analysis Group, Inc. The authors thank Bostjan Ceh, Sunil Navani, Zalmai Hakimi, Jing Wang-Silvanto (of Otsuka Pharmaceutical Europe Ltd), and Andrew Garnham (of Clear Health Economics, London) for editorial comments, and Marie-Hélène Lafeuille, Farzin Khosrow-Khavar and Laure-Anne Damasse (of Analysis Group, Inc.) for help with initial search strategy design, study inclusion (FK-K and L-AD), and execution.
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
- Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018.
- United States Renal Data System. Chapter 1: CKD in the General Population 2017 [cited 2017 November 15]. Available from: https://www.usrds.org/2017/view/v1_01.aspx
- United States Renal Data System. 2017. USRDS Annual Data Report: Executive Summary 2017 [cited 2018 May 29]. Available from: https://www.usrds.org/2017/download/v1_00_ExecSummary_17.pdf
- Wang V, Vilme H, Maciejewski ML, et al. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol. 2016;36:319–330.
- Vekeman F, Yameogo ND, Lefebvre P, et al. Healthcare costs associated with nephrology care in pre-dialysis chronic kidney disease patients. J Med Econ. 2010;13:673–680.
- Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–1301.
- Gorodetskaya I, Zenios S, McCulloch CE, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68:2801–2808.
- Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant. 2017;32:ii121–ii128.
- Braun L, Sood V, Hogue S, et al. High burden and unmet patient needs in chronic kidney disease. Int J Nephrol Renovasc Dis. 2012;5:151–163.
- World Health Organization. Worldwide prevalence of anaemia 1993–2005 - WHO Global Database on Anaemia. 2008 [cited 2018 October 12]. Available from: http://apps.who.int/iris/bitstream/handle/10665/43894/9789241596657_eng.pdf;jsessionid=736CD9E264C858484E244909B38B47DD?sequence=1
- Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9:e84943.
- Rao M, Pereira BJ. Optimal anemia management reduces cardiovascular morbidity, mortality, and costs in chronic kidney disease. Kidney Int. 2005;68:1432–1438.
- Weiner DE, Tighiouart H, Vlagopoulos PT, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803–1810.
- Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:192019–192032.
- Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084.
- Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098.
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Inter. 2012;2(Suppl.):279–335.
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590.
- Macdougall IC, Cooper AC. Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2002;17:39–43.
- Luo J, Jensen DE, Maroni BJ, et al. Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68:763–771.
- Gilbertson DT, Peng Y, Arneson TJ, et al. Comparison of methodologies to define hemodialysis patients hyporesponsive to epoetin and impact on counts and characteristics. BMC Nephrol. 2013;14:44.
- Roger SD, Tio M, Park HC, et al. Intravenous iron and erythropoiesis-stimulating agents in haemodialysis: a systematic review and meta-analysis. Nephrology (Carlton). 2017;22:969–976.
- Hougen I, Collister D, Bourrier M, et al. Safety of intravenous iron in dialysis: a systematic review and meta-analysis. CJASN. 2018;13:457–467.
- Vaziri ND. Safety issues in iron treatment in CKD. Semin Nephrol. 2016;36:112–118.
- Macdougall IC, White C, Anker SD, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380:447–458.
- Scornik JC, Bromberg JS, Norman DJ, et al. An update on the impact of pre-transplant transfusions and allosensitization on time to renal transplant and on allograft survival. BMC Nephrol. 2013;14:217.
- Tanhehco YC, Berns JS. Red blood cell transfusion risks in patients with end-stage renal disease. Semin Dial. 2012;25:539–544.
- Kidney Disease: Improving Global Outcomes. Chapter 4: Red cell transfusion to treat anemia in CKD. Kidney Int Suppl (2011). 2012;2:311–316.
- US Food and Drug Administration. FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. 2011 [cited 2018 February 7]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm259639.htm
- European Medicines Agency. Abseamed - epoetin alfa. 2019 [cited 2018 February 14]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000727/WC500020661.pdf
- Birnie K, Caskey F, Ben-Shlomo Y, et al. Erythropoiesis-stimulating agent dosing, haemoglobin and ferritin levels in UK haemodialysis patients 2005-13. Nephrol Dial Transplant. 2017;32:692–698.
- Chertow GM, Liu J, Monda KL, et al. Epoetin alfa and outcomes in dialysis amid regulatory and payment reform. J Am Soc Nephrol. 2016;27:3129–3138.
- Coritsidis GN, Maglinte GA, Acharya A, et al. Anemia management trends in hospital-based dialysis centers (HBDCs), 2010 to 2013. Clin Ther. 2014;36:408–418.
- Evans M, Suttorp MM, Bellocco R, et al. Trends in haemoglobin, erythropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013. Nephrol Dial Transplant. 2016;31:628–635.
- Thamer M, Zhang Y, Kaufman J, et al. Major declines in epoetin dosing after prospective payment system based on dialysis facility organizational status. Am J Nephrol. 2014;40:554–560.
- Wang C, Kane R, Levenson M, et al. Association between changes in CMS reimbursement policy and drug labels for erythrocyte-stimulating agents with outcomes for older patients undergoing hemodialysis covered by fee-for-service medicare. JAMA Intern Med. 2016;176:1818–1825.
- Fuller DS, Bieber BA, Pisoni RL, et al. International comparisons to assess effects of payment and regulatory changes in the United States on anemia practice in patients on hemodialysis: the dialysis outcomes and practice patterns study. J Am Soc Nephrol. 2016;27:2205–2215.
- Liu H, Yao Y, Cao Y, et al. Anemia management trends in patients on peritoneal dialysis in the past 10 years. Int J Clin Exp Med. 2015;8:18050–18057.
- McFarlane PA, Pisoni RL, Eichleay MA, et al. International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int. 2010;78:215–223.
- Ryu SR, Park SK, Jung JY, et al. The prevalence and management of anemia in chronic kidney disease patients: result from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). J Korean Med Sci. 2017;32:249–256.
- Zuo L, Wang M, Hou F, et al. Anemia management in the China dialysis outcomes and practice patterns study. Blood Purif. 2016;42:33–43.
- Akizawa T, Okumura H, Alexandre AF, et al. PUK26 - burden of illness associated with anaemia in chronic kidney disease in japan: a literature review. Value Health. 2017;20:A492.
- van Nooten FE, Green J, Brown R, et al. Burden of illness for patients with non-dialysis chronic kidney disease and anemia in the United States: review of the literature. J Med Econ. 2010;13:241–256.
- Collister D, Komenda P, Hiebert B, et al. The effect of erythropoietin-stimulating agents on health-related quality of life in anemia of chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2016;164:472–478.
- Furuland H, Linde T, Ahlmen J, et al. A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transplant. 2003;18:353–361.
- Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: a new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int. 2010;14:168–173.
- Muirhead N, Keown PA, Churchill DN, et al. Dialysis patients treated with Epoetin alpha show improved exercise tolerance and physical function: a new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int. 2011;15:87–94.
- Foley RN, Curtis BM, Parfrey PS. Erythropoietin therapy, hemoglobin targets, and quality of life in healthy hemodialysis patients: a randomized trial. Clin J Am Soc Nephrol. 2009;4:726–733.
- Akizawa T, Pisoni RL, Akiba T, et al. Japanese haemodialysis anaemia management practices and outcomes (1999–2006): results from the DOPPS. Nephrol Dial Transplant. 2008;23:3643–3653.
- Hazara AM, Owen SJ, Bhandari S. The impact of lowering haemoglobin targets on patterns of erythropoiesis-stimulating agent use in patients on haemodialysis. Blood Purif. 2016;41:287–292.
- Liu J, Guo H, Gilbertson D, et al. Associations of anemia persistency with medical expenditures in Medicare ESRD patients on dialysis. Ther Clin Risk Manag. 2009;5:319–330.
- Moyneur E, Bookhart BK, Mody SH, et al. The economic impact of pre-dialysis epoetin alpha on health care and work loss costs in chronic kidney disease: an employer's perspective. Dis Manag. 2008;11:49–58.
- Clement FM, Klarenbach S, Tonelli M, et al. An economic evaluation of erythropoiesis-stimulating agents in CKD. Am J Kidney Dis. 2010;56:1050–1061.
- Pizzi LT, Patel NM, Maio VM, et al. Economic implications of non-adherence to treatment recommendations for hemodialysis patients with anemia. Dial Transplant. 2006;35:660–671.
- Tonelli M, Winkelmayer WC, Jindal KK, et al. The cost-effectiveness of maintaining higher hemoglobin targets with erythropoietin in hemodialysis patients. Kidney Int. 2003;64:295–304.
- Eriksson D, Goldsmith D, Teitsson S, et al. Cross-sectional survey in CKD patients across Europe describing the association between quality of life and anaemia. BMC Nephrol. 2016;17:97.
- Okpechi IG, Nthite T, Swanepoel CR. Health-related quality of life in patients on hemodialysis and peritoneal dialysis. Saudi J Kidney Dis Transpl. 2013;24:519–526.
- Kubota M, Hiramatsu M, Yamakawa M, et al. Darbepoetin alfa (KRN321) is safe and effective when administered subcutaneously once every 2 or 4 weeks to patients on peritoneal dialysis in Japan. Clin Exp Nephrol. 2011;15:884–892.
- Moreno F, Sanz-Guajardo D, Lopez-Gomez JM, et al. Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialysis patients. Spanish Cooperative Renal Patients Quality of Life Study Group of the Spanish Society of Nephrology. J Am Soc Nephrol. 2000;11:335–342.
- Ganz T, Nemeth E. Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol. 2016;36:87–93.
- Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634.
- Tsukamoto T, Matsubara T, Akashi Y, et al. Annual iron loss associated with hemodialysis. Am J Nephrol. 2016;43:32–38.
- Wish JB, Aronoff GR, Bacon BR, et al. Positive iron balance in chronic kidney disease: how much is too much and how to tell? Am J Nephrol. 2018;47:72–83.
- Finkelstein FO, Story K, Firanek C, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:33–38.
- Lucca U, Tettamanti M, Mosconi P, et al. Association of mild anemia with cognitive, functional, mood and quality of life outcomes in the elderly: the "Health and Anemia" study. PLoS One. 2008;3:e1920.
- Thein M, Ershler WB, Artz AS, et al. Diminished quality of life and physical function in community-dwelling elderly with anemia. Medicine (Baltimore). 2009;88:107–114.
- Rizzo M, Iheanacho I, van Nooten FE, et al., editors. Poster MP198: A systematic literature review of the humanistic burden of anaemia associated with chronic kidney disease. ERA-ERDTA; 2014; Amsterdam.
- Leaf DE, Goldfarb DS. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int. 2009;75:15–24.
- Johansen KL, Finkelstein FO, Revicki DA, et al. Systematic review of the impact of erythropoiesis-stimulating agents on fatigue in dialysis patients. Nephrol Dial Transplant. 2012;27:2418–2425.
- Horigan AE. Fatigue in hemodialysis patients: a review of current knowledge. J Pain Symp Manage. 2012;44:715–724.
- Ju A, Unruh M, Davison S, et al. Establishing a core outcome measure for fatigue in patients on hemodialysis: a Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) consensus workshop report. Am J Kidney Dis. 2018;72:104–112.
- Pergola PE, Thompson-Leduc P, Rochette S, et al. Economic and humanistic burden of non-dialysis-dependent patients with chronic kidney disease-related anemia: A systematic review. Academy of Managed Care Pharmacy (AMCP) Managed Care & Specialty Pharmacy Annual Meeting 2018; 2018 Apr 23-26; Boston, MA.
- Engelberg AB, Kesselheim AS, Avorn J. Balancing innovation, access, and profits–market exclusivity for biologics. N Engl J Med. 2009;361:1917–1919.
- Locatelli F, Del Vecchio L, Luise MC. Current and future chemical therapies for treating anaemia in chronic kidney disease. Expert Opin Pharmacother. 2017;18:781–788.
- Akizawa T, Tsubakihara Y, Nangaku M, et al. Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol. 2017;45:127–135.
- Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233.
- Chen N, Qian J, Chen J, et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373–1386.
- Haase VH, Chertow GM, Block GA, et al. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. 2019;34:90–99.
- Holdstock L, Meadowcroft AM, Maier R, et al. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27:1234–1244.
- Provenzano R, Besarab A, Wright S, et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912–924.
- Bottcher M, Lentini S, Arens ER, et al. First-in-man-proof of concept study with molidustat: a novel selective oral HIF-prolyl hydroxylase inhibitor for the treatment of renal anaemia. Br J Clin Pharmacol. 2018;84:1557–1565.
- Brigandi RA, Johnson B, Oei C, et al. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: a 28-day, phase 2a randomized trial. Am J Kidney Dis. 2016;67:861–871.
- Hara K, Takahashi N, Wakamatsu A, et al. Pharmacokinetics, pharmacodynamics and safety of single, oral doses of GSK1278863, a novel HIF-prolyl hydroxylase inhibitor, in healthy Japanese and Caucasian subjects. Drug Metab Pharmacokinet. 2015;30:410–418.
- Meadowcroft AM, Cizman B, Holdstock L, et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J. 2019;12:139–148.
- Angst F, Aeschlimann A, Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. J Clin Epidemiol. 2017;82:128–136.