Abstract
Aims: To model implementation of a new treatment pathway leveraging long-acting antibiotics (LAs) for treatment of acute bacterial skin and skin structure infections (ABSSSIs) in a hospital emergency department (ED) with an observation unit, and to quantify health resource utilization and economic outcomes versus standard care (intravenous vancomycin).
Materials and methods: Discrete-event simulation was used to model implementation of the LA treatment pathway in the ED versus standard care from the US Medicare perspective. Model inputs were derived from published sources to simulate a real-world hospital ED with an observation unit. Outcomes included key ED metrics such as patient throughput rate and length of stay (LOS) and cost (estimated through reimbursed amounts in 2017 USD).
Results: Implementation of an LA pathway in the ED improved ABSSSI patient throughput rate by 350% (+5.8 dispositions/ED and observation unit day) and reduced LOS by 68% (−7.2 h/patient). These improvements in patient outcomes are driven by the reduced infusion time required for LA antibiotics and are greater for dalbavancin than oritavancin owing to the shorter infusion duration (30 min vs. 3 h).
Limitations: External validity of the model was not assessed. The model was limited to care received in EDs; therefore, certain clinical variables outside the ED were not captured for this analysis.
Conclusions: LA pathway implementation for ABSSSI treatment in the ED supported improved efficiency, which may translate to economic value. As EDs continue to focus on improving key metrics such as throughput rate and LOS, LA pathway implementation should be considered as a potential approach for abbreviated ABSSSI treatment in the ED.
Introduction
Emergency department (ED) visits and hospital admissions due to acute bacterial skin and skin structure infections (ABSSSIs) are increasingCitation1–3. The most common reason for hospital admission among patients with skin and skin structure infections, which alone accounted for almost 2% of hospital admissions in the United States during 2005–2011, is the need for intravenous (IV) antibiotic therapyCitation2,Citation4. In addition to the financial cost of ABSSSI treatment ($3.7 billion for cellulitis alone in 2013)Citation1, ABSSSIs impose a significant burden on ED resources, partly due to the increase in antibiotic-resistant bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA)Citation5, and to ED overcrowding due to increased demandCitation6.
Among the metrics available for the evaluation of ED efficiency, the most commonly used are patient throughput, time between patient arrival and evaluation by a physician (wait time), overall length of stay (LOS) in the ED, LOS in the ED after a decision to admit to hospital, and the rate of patients leaving without being seen by a physician or without treatmentCitation6,Citation7. Emergency department LOS is likely the single most important quality metric from a cost perspective and a key driver of direct medical costsCitation8. The Centers for Medicare and Medicaid Services (CMS) have added an ED LOS quality measure for hospital reporting purposes in recent years, highlighting the potential for LOS intervals to be tied to hospital reimbursementCitation9. Maintaining or shortening ED LOS for ABSSSIs may depend on efficient care processes or pathways in the ED, such that patients can be identified and shifted to outpatient settings to complete care rather than spending prolonged time in the ED or under observation.
Treatment of ABSSSIs in an outpatient setting may represent a cost- and resource-saving option. However, usual care for ABSSSIs with suspected MRSA supports empirical IV vancomycinCitation10,Citation11, which can lead to routine hospital admission or prolonged time in the ED or under observationCitation4,Citation12, impacting hospital costs. Current guidelines support the use of outpatient parenteral antibiotic therapy (OPAT) for suitable ABSSSI patients as an alternative to usual careCitation13. Outpatient infection management as an effective and safe option is further supported by a low ABSSSI mortality rate (1.1%)Citation14. Traditional OPAT, however, may pose a challenge for hospitals to coordinate, whereas treatment of ABSSSIs with long-acting antibiotics (LAs), such as dalbavancin or oritavancin, would be an abbreviated course of OPAT provided in the ED to complete an ABSSSI treatment course. While vancomycin requires 1 h infusions every 12 h over several days to complete treatmentCitation10, LAs are provided in a single dose: dalbavancin is given as a single 30 min infusionCitation15 and oritavancin as a single 3 h infusionCitation16.
In a recent study, Revankar et al.Citation17 used discrete-event simulation (DES) to model hospital treatment pathways for patients, comparing alternative approved interventions for the treatment of ABSSSIs. The investigators concluded that modifications to the treatment pathway to increase the proportion of patients who receive a first dose of IV vancomycin in the ED, but who are then managed as outpatients (either by outpatient IV treatment or discharge with oral treatment) versus hospital admission, reduce the typical cost of ABSSSI treatment by 11–20%Citation17. However, the study focused on care delivery after the ED, whereas implementing an abbreviated pathway at the first “bottleneck” in hospital care delivery – the ED – may be highly important to improving downstream efficiency. To date, limited data exist on the effect of such treatment pathways and, in particular, the effect of implementing an LA pathway has not been assessed.
The objectives of this study were to model the implementation of an LA pathway (dalbavancin or oritavancin) in a hospital ED in comparison with standard care (IV vancomycin) and to quantify operational quality metrics and cost of LA pathway implementation from the US Medicare perspective.
Methods
Model characteristics
Model overview
Individual patient flow through the ED was captured by a DES model (). DES using AnyLogic 8.0.5 (AnyLogic, Oakbrook Terrace, IL) generates patients stochastically in an ongoing manner and is more reflective of real-world complexity than Markov cohort models, allowing accurate quantification of cost and resource use at the patient levelCitation18–20.
Figure 1. Overview of model steps and patient flow. All care pathways have the same basic flow through the model: patients enter ED; after registration, patients enter (1) waiting room, then (2) triage before being provided (3) an ED bed. Patients are then either discharged from ED to home, or to (4) observation or (5) are admitted to hospital; patients who go to (4) observation are either discharged home or (5) admitted to hospital; as patients flow through, the ED is modeled according to patient acuity; more severe patients follow an abbreviated path. Abbreviations. BCU, Biocontainment unit; DAL, Dalbavancin; ED, Emergency department; ORI, Oritavancin; VAN, Vancomycin. *Patients treated with DAL/ORI discharged home from ED. †Patients treated with VAN may go to observationCitation11 and then are either discharged home or admitted to hospital.
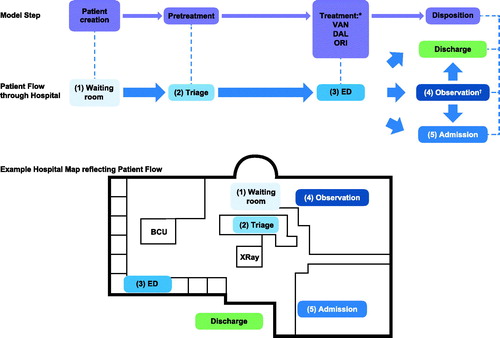
All variables in input data were treated with statistical independence. The model was nonterminating with a time horizon of 1 year and a warm-up period of 1 month, and patients did not persist in the model after treatment (recidivism was not modeled). Patients could not deviate from the process path or move between groups during the model run.
Patient flow through the model: patient pretreatment and treatment paths
After creation, patients underwent registration, triage and initial assessmentCitation21,Citation22 (Supplementary Figure S1), after which they were assigned to either the acute or nonacute patient pretreatment path according to their emergency severity index (ESI; Supplementary Figure S1). Acute patients received an ED bed quickly, while nonacute patients were first registered and triaged before receiving an ED bed. Patients were then divided according to ABSSSI status (ABSSSI vs. non-ABSSSI patients). The model assumed that patients were diagnosed with 100% specificity and sensitivity.
ABSSSI patients
The model assumed that all ABSSSI patients required IV antibiotic treatment for ABSSSI and were candidates for outpatient treatment with OPAT. The ABSSSI group included patients with cellulitis, abscess or postoperative wound infection. An assumption was made that all ABSSSI patients would receive at least one dose of IV antibiotics with anti-MRSA coverage before ED discharge. Any additional treatment for ABSSSI, including surgical interventions or follow-up antibiotic therapy upon leaving the model (due to ED or observation discharge, discharge home, or hospital admission), were not considered.
Following creation, ABSSSI patients were replicated and assigned either standard care or LA pathway treatment; vancomycin was considered the standard care IV antibiotic therapy because of its common useCitation23–25, and dalbavancin or oritavancin was the LA therapy considered. Standard care patients received the first dose of vancomycin in the ED, followed by discharge home, transfer to the observation unit or hospital admission. Patients who were sent to the observation unit were then either admitted to the hospital or discharged home. ABSSSI patients who received the LA pathway received treatment (dalbavancin or oritavancin) in the ED and subsequent discharge home (i.e. it was assumed that no LA pathway patients would be placed in the observation unit or admitted to the hospital). For the purposes of the model, treatment options were assumed to be of equivalent clinical efficacy up until the point of model exit.
Non-ABSSSI patients
Non-ABSSSI patients were modeled by national average treatment duration rather than by specific treatments. It was assumed that non-ABSSSI patients would not utilize the observation unit. Once their specified treatment duration was reached, non-ABSSSI patients were either discharged home or admitted to the hospitalCitation26.
Model inputs and data sources
Patient inputs
Patient inputs for the model were presentation (arrival) rate of patients to the ED, the probability of presenting with an ABSSSI and the probability of being acute for ED pretreatment purposes. Based on prior ABSSSI modelingCitation26, the base model’s patient presentation rate followed a similar nonstationary Poisson process (shown in Supplementary Table S1) and, for a US ED, the average annual number of visits would be 36,342. This value was consistent with published estimates of annual ED visitsCitation27. Based on these assumptions, over an average day there were approximately 4.1 patient arrivals per hour. The probability of a patient having an ABSSSI was modeled as an independent Boolean random variable. Based on a 2013 report on US ED ambulatory visitsCitation28, 3.7% of patients in the base case would present with an ABSSSI and require IV antibiotic treatment. It was assumed that 10% of patients would present as acute patients, independent of ABSSSI status, based on prior modeling literatureCitation21,Citation22. Average values for patient model inputs and disposition probabilities are shown in .
Table 1. Model patient and resource inputs.
Resource inputs
Inputs for resource use in the ED, including nurses, physicians, triage nurses, registration clerks and ED beds, were obtained from Wang et al.Citation26 and Ross et al.Citation12 and the results of model validation. As part of model validation, increasing the number of triage nurses from one to two allowed for replication of the Wang et al. model results. Therefore, the current model used two triage nurses.
Average values for each parameter are shown in ; the exact values that were input into the model, according to ED and observation unit staffing levels at data timestamp, are shown in Supplementary Table S2. The triangular distribution of the average patient process times given in is presented in Supplementary Table S3. Average values for resource model inputs are shown in .
ABSSSI treatment
Standard care ABSSSI patients were initially treated with standard care (IV vancomycin) in the ED and were then admitted to the hospital (40%), discharged to the observation unit (30%) or discharged home (30%). If an observation bed was not available for patients sent to observation, those patients were admitted to the hospital. Observation time was determined by a cumulative density function (see Supplementary Table S4). Patients were checked by a nurse while in observation according to a triangular (0.5, 1, 4) distribution in hours (time spent with the patient was a random variate from a triangular [1, 2, 4] distribution measured in minutes). However, if the patient left observation before the nurse check occurred, no nurse check was required. After a patient remained in the observation unit for the specified time, the disposition changed to either admitted or discharged. The probability of admission across ABSSSI observation unit patients who received standard care was 0.1. It was assumed that patients treated with LA treatment pathways would be discharged home from the ED after receiving one dose of LA therapy in the ED (100% discharged home from ED).
Non-ABSSSI treatment
Non-ABSSSI treatment was modeled by national average treatment durations. The average of 90 min is consistent with median ED treatment times from the National Hospital Ambulatory Medical Care SurveyCitation32 once wait time is accounted for; it does not account for differences in type of infection and treatment. After ED treatment, it was assumed that 27% of patients would be admitted to the hospital and 73% of patients would be discharged home. Use of the observation unit for non-ABSSSI patients was not modeled.
Cost inputs
To evaluate the economic value of the different treatment scenarios, Medicare costs were estimated through the reimbursed amounts shown in .
Table 2. Model inputs: cost of ABSSSI treatment.
Model validation
Building from prior validation of the model of Revankar et al.Citation17, we performed model verification and face validation according to current best practice. Verification was performed through unit testing by a modeling expert in infectious disease simulation modeling (E.L.) and face validation by a currently practicing ED physician.
Model outcome metrics
Emergency department and observation setting metrics
All metrics used to measure the impact of LA pathway implementation in the ED and observation unit versus standard care for ABSSSI treatment over the 1 year model run are shown in . Key outcomes, based on CMS’s hospital quality rating for Medicare Timely and Effective Care metrics, included ED/observation unit throughput rate, ED wait time, ED/observation unit LOS and ED left-without-being-seen (LWBS) rateCitation9. The equations used to calculate the output metrics in are given in Supplementary Figure S2.
Table 3. ED and observation unit setting metrics.
Costs of ABSSSI treatment
Costs of ABSSSI treatment were estimated as reimbursed amounts () and the model accumulated costs as they were incurred by each patient. Costs of care for non-ABSSSI patients were not estimated because of variability in treatment and because they were not part of the original research question.
Statistical analysis
Main analysis
The experimental dataset was generated by assessing each outcome metric separately and comparing standard care (vancomycin treatment pathway) to the two alternative LA treatment pathways (dalbavancin and oritavancin). For each LA pathway, the simulation model generated 100 replicated simulations for each increment of the proportion of patients receiving LA pathway (increments of 5%, starting at 0% until 100% was reached).
This experimental dataset was used to assess the impact of LA pathways versus standard care for each outcome metric through linear regression modeling (Supplementary Figure S3D).
Sensitivity analysis
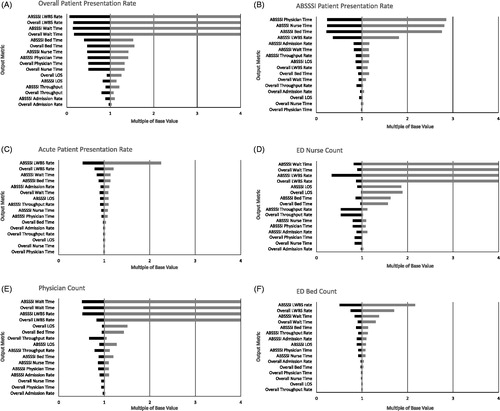
Deterministic one-way sensitivity analysis using key model inputs (overall patient presentation rate, ABSSSI presentation rate, acute presentation rate, ED nurse staffing, physician staffing and number of ED beds) was used to assess parameter uncertainty as well as robustness of the model (Supplementary Table S5). The base case treatment pathway (standard care for ABSSSI – vancomycin) was used as a worst-case scenario, as it was the care pathway that imposed the most burden on the ED. Because the input metrics have different units, the results of the sensitivity analysis were normalized to facilitate comparison. In the tornado diagrams shown in , the base case value was set to 1, and each value on the abscissa represents a multiple of the base value. Values greater than multiples of four were truncated. The number of observation beds was not included in the sensitivity analysis because only vancomycin-treated patients utilized observation beds and the number of ED beds was not expected to vary sufficiently to impact the observation bed count (observation beds vary with ED beds in a ratio of 1:6). Because LOS in the observation unit would impact LOS and other output metrics, we carried out a further sensitivity analysis to test the impact of the assumption that 100% of LA patients would be discharged home by varying the admission and observation unit rates in 5% increments based on the assumptions made for vancomycin (40% admitted to hospital, 30% transferred to observation, 30% discharged home).
Figure 2. One-way sensitivity analysis results: tornado diagrams of (A) patient presentation rate, (B) ABSSSI patient presentation rate, (C) acute patient presentation rate, (D) ED nurse count, (E) physician count and (F) ED bed count. To allow extreme values generated by the sensitivity analysis to be plotted on the same tornado diagram, results were normalized by the average result for the base scenario (which was set to 1). Abbreviations. ABSSSI, Acute bacterial skin and skin structure infection; ED, Emergency department; LOS, Length of stay; LWBS, Left without being seen.
Results
Key emergency department outcomes and cost of treatment for ABSSSI patients treated with a long-acting antibiotic pathway versus standard care
Key outcomes for the LA pathways and standard care for treatment of ABSSSIs are presented in . The absolute and relative differences between the two treatment pathways for key and additional outcomes of interest are presented in Supplementary Tables S6 and S7, respectively. Implementation of an LA pathway improved throughput rate for ABSSSI patients treated through an LA pathway by 3.5 times compared to standard care (+5.79 dispositions/ED and observation unit day [95% CI: 5.72, 5.87]; and Supplementary Table S7). Likewise, ED/observation unit LOS was decreased by 7.2 h per patient (95% CI: -7.40, -6.92) by use of an LA pathway (). For ABSSSI patients, ED wait time and LWBS rate were similar for standard care versus the use of LA pathways. A comparison of the LAs dalbavancin and oritavancin versus standard care showed that the improvements in the parameters noted above were driven mainly by the shorter infusion time required for dalbavancin (Supplementary Tables S6 and S7).
Table 4. Key ED outcomes and cost of treatment for ABSSSI patients.
Among the additional metrics captured, number of patients treated per year was similar for standard care versus the use of LAs. Although annual nurse time was reduced by 18.03 days per year (95% CI: −19.21, −16.86), annual physician time was similar between standard care and the use of LA pathways (Supplementary Table S7).
Use of an LA pathway increased the cost of ABSSSI treatment versus standard care primarily because of the higher wholesale acquisition cost of the LA antibiotics compared with standard care ().
Key emergency department outcomes for all emergency department patients
As shown in Supplementary Tables S6 and S7, the improvement in ED outcomes for ABSSSI patients modestly improved outcomes for all patients (both ABSSSI and non-ABSSSI patients) presenting to the ED over the year modeled. All patients benefited from an 8.08% increase in throughput rate (+0.54 dispositions/ED and observation unit day).
Sensitivity of the model
Across the metrics evaluated, LWBS rate and wait time were the outputs most sensitive to changes in model inputs, likely because these outcomes occur early on in the patient’s ED stay. illustrates the model’s sensitivity to changes in presentation rate to the ED of all patients, ABSSSI patients and acute patients, respectively. LWBS and wait time for all patients and ABSSSI patients were highly sensitive to variation in total presentation rate. Bed time, nurse time and physician time were somewhat sensitive to the overall presentation rate and ABSSSI presentation rate (). As expected, ABSSSI resource use, including physician time, nurse time, bed time and LWBS rate, were moderately sensitive to changes in the presentation rate of ABSSSI patients to the ED; other parameters were not sensitive to this input, including ABSSSI LOS (). With the exception of the output LWBS rate, the model was relatively insensitive to the percentage of acute patients entered into the model (). illustrates the model’s sensitivity to variations in resource levels. Overall and ABSSSI wait time and LWBS rate were highly sensitive to reductions in nurse and physician staffing levels, such that reduction of nurse staffing by a single unit had a dramatic effect on these output metrics (). While LOS was insensitive to patient presentation parameters, it was impacted by a reduction in staff levels (). With the exception of LWBS rates and, to a lesser extent, wait times, the model was insensitive to small changes to the number of ED beds (). When the LA pathway hospital admission rate increased to that of standard care, results were consistent with the primary analysis. As the LA pathway observation unit rate increased to that of standard care, the relative difference in LOS between standard care and use of LA pathways decreased linearly. Once the observation unit rate was equivalent to that of standard care, the LOS difference reduced to approximately a difference in infusion durations (i.e. standard care 1 h, dalbavancin at 30 min and oritavancin at 3 h).
Discussion
In an increasingly cost-constrained healthcare environment, healthcare systems should consider care pathways that reduce burden and increase efficiency in the ED, or reduce the bottleneck from a patient flow perspective. Our model focused on implementing a new care pathway for ABSSSI treatment in the ED, as it is the most resource- and cost-intensive step in hospital care and thus the setting where an intervention to increase efficiency may be the most impactful. To improve antimicrobial stewardship in the treatment of ABSSSIs, it is important to monitor the use of antimicrobials and ensure selection of optimal antimicrobial drug regimens, including dose, route of administration and duration of treatmentCitation9. Our model is the first to assess key outcomes and cost through implementation of an LA pathway in the ED.
Previous models of ABSSSI treatment have used decision analytic modeling, a systematic and quantitative approach to making decisions under uncertainty via a decision tree or Markov modelCitation8,Citation39–43. We elected, in common with Revankar et al.Citation17, to use a DES model in which the operation of a system is modeled as a discrete sequence of events: each event occurs at a particular instant in time and can capture costs and time at the patient and resource levels. Similar to Revankar et al.Citation17, our results may support operational efficiency by leveraging LA, such as dalbavancin or oritavancin. Efficiency may translate to economic value and improved quality of care for Medicare patients. LA pathway implementation improved most ED outcomes versus standard care for ABSSSI patients, apart from wait time, LWBS rate and physician time. The intervention may not have affected wait time and LWBS rate because these outcomes occur early on in the ED bottleneck before initial treatment, and physician time because it is a generally limited resource in the ED regardless of the intervention. We found that the duration of the LA pathway infusion was correlated with the results, where dalbavancin (30 min infusion) improved ED outcomes versus standard care to a larger extent than oritavancin (3 h infusion).
Interestingly, the improvement in ED outcomes was maintained when the analysis focused on all ED patients, albeit more modestly. Our results suggest that an intervention delivered to a smaller proportion of ABSSSI patients in the ED can potentially improve ED outcomes for all patients receiving care in the EDCitation17. Although our analysis was focused on the US Medicare perspective, there are additional considerations relevant to hospitals and patients.
From an operational perspective, the LA pathway intervention is well suited because it would not modify existing hospital infrastructure. However, implementing an LA pathway in the ED would rely on a coordinated effort in the ED, involving patient identification at the point of care and real-time support from an antibiotic stewardship program for ED physicians and hospitalistsCitation44. From the clinical perspective, OPAT has demonstrated effectiveness and safety; in terms of LA use in the outpatient setting, evidence similarly supports effective and safe treatmentCitation38,Citation45. From the ABSSSI patient perspective, patients are generally satisfied with ED treatment, yet most preferred to complete care at home and single-dose IV treatment for ABSSSI versus alterative regimens, highlighting that an LA pathway is aligned with patient preferencesCitation46. Further research is warranted to understand both hospital/health system perspectives and patient perspectives on LA pathway implementation.
We acknowledge that our model has limitations arising from both the model structure and the model parameters. For example, EDs are structurally different in their operations, and there is variability in the numbers of patients admitted and the number of healthcare personnel assigned to their care. However, we based our model on other validated ED models as well as literature to represent a current ED in the United States. From a clinical perspective, we did not model specific types of infection, which can be more or less resource consuming, and the model does not compartmentalize the costs and resource use associated with wound infections that can account for up to 20% of ABSSSI treatmentCitation45. In our model, patients could not move from one diagnosis category to another, were assumed to have been diagnosed correctly, did not have comorbidities represented, and were assumed to always be transferred home following treatment for the LA pathway. Changing any of these assumptions to better reflect clinical practice would likely have increased resource use observed in model results. Real-world implementation of an LA pathway by individual healthcare settings may result in the realization of less operational efficiency than modeled. We included ED outcomes as well as reimbursed amounts to approximate direct hospital ABSSSI treatment costs, which may be impactful for hospital administrators. Indeed, a recent cost analysis of ABSSSI admissions with a primary Medicare payer supported that hospitals were reimbursed on average 6–9% less than the cost of the admission, highlighting the potential need for operational efficiencyCitation47. However, because our model focused on the ED setting, cost offsets associated with hospital avoidance or LOS reduction (∼$1000 per US hospital day for ABSSSI)Citation45 were not modeled; further cost savings and cost-effectiveness may have been realized if accounted for in our model. As our model terminates at patient discharge from the ED/hospital, it does not model the potential longer-term beneficial effects of using an LA treatment pathway: use of LAs is expected to reduce the need for postdischarge ambulatory antibiotic therapy compared with standard treatment with vancomycin.
Our model did not include all possible ED outcomes, which may be important as health systems likely value ED outcomes differently, use different measures to evaluate ED performance or may not have access to data to analyze ED outcomes.
In addition, the external validity of the model has not been assessed because this requires a prospective study implementing an LA pathway versus standard care. Finally, we did not assess long-term outcomes associated with LA pathway versus vancomycin treatment beyond the duration of infusion because the model was limited to care received in the ED setting.
To support the external validity of the model, the results support potential future research such as a prospective study in the ED assessing the economic value of an LA intervention versus standard care for ABSSSI treatment.
Conclusions
Health systems should consider care pathways in the ED that improve care for ABSSSI treatment while also containing costs. Our results support improvement in ED outcomes for ABSSSI patients receiving treatment through an LA pathway versus standard care, including key ED metrics such as throughput rate and LOS. The use of LA pathways for ABSSSI patients also improved ED outcomes for all patients seen in the ED, suggesting that an intervention for a proportion of patients may affect a larger patient population in the ED. The ultimate value of an LA pathway for ABSSSI treatment, operationally or economically, may vary among hospitals. External validation of the model and/or a prospective study of the implementation of an LA treatment pathway in the ED is warranted.
Transparency
Declaration of funding
This study was sponsored by Allergan PLC (Dublin, Ireland).
Author contributions
All authors contributed equally to the study design, the acquisition and analysis of study data, and the development of this manuscript. All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Declaration of financial/other relationships
K.R.K. has disclosed that she is an employee of Allergan PLC. E.L. has disclosed that he has served as a consultant for Allergan PLC. S.H. has disclosed that he is an employee of Sterling Simulation. In their salaried positions, Sterling Simulation employees are precluded from receiving payment or honoraria directly from these organizations for services rendered. Sterling Simulation has received funding for the study from Allergan PLC. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
This work has not been published previously.
Supplementary Material
Download PDF (204.8 KB)Acknowledgements
Editorial support for development of this manuscript was provided by Moira Hudson, Todd J. Waldron and John E. Fincke at Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and funded by Allergan PLC.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Katelyn R. Keyloun, upon request.
References
- Peterson RA, Polgreen LA, Cavanaugh JE, et al. Increasing incidence, cost, and seasonality in patients hospitalized for cellulitis. Open Forum Infect Dis. 2017;4:ofx008
- Kaye KS, Patel DA, Stephens JM, et al. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS One. 2015;10:e0143276.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674.
- Talan DA, Salhi BA, Moran GJ, et al. Factors associated with decision to hospitalize emergency department patients with skin and soft tissue infection. West J Emerg Med. 2015;16:89–97.
- Cosgrove SE, Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36:1433–1437.
- Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med. 2011;12:192–197.
- Sorup CM, Jacobsen P, Forberg JL. Evaluation of emergency department performance – a systematic review on recommended performance and quality-in-care measures. Scand J Trauma Resusc Emerg Med. 2013;21:62.
- Ektare V, Khachatryan A, Xue M, et al. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18:1092–1101.
- Centers for Medicare and Medicaid Services. Medicare.gov [Internet]. 2014 [cited 2014 March 4]. Available from: http://www.medicare.gov/hospitalcompare/
- Vancocin HCl (vancomycin hydrochloride for injection USP). Full Prescribing Information. Baudette (MN): ANI Pharmaceuticals Inc.; 2017.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10–e52.
- Ross MA, Hockenberry JM, Mutter R, et al. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff. 2013;32:2149–2156.
- Pollack CV Jr, Amin A, Ford WT Jr, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med. 2015;48:508–519.
- Gunderson CG, Cherry B, Fisher A. Mortality of hospitalized patients with cellulitis: a systematic review and meta-analysis. Presented at: Hospital Medicine. 2018 Apr 8–11; Orlando (FL).
- Dalvance (dalbavancin). Full Prescribing Information. Parsippany (NJ): Durata Therapeutics US Ltd; 2018.
- Xydalba (dalbavancin). Full Prescribing Information. Amsterdam (Netherlands): Durata Therapeutics International BV; 2016.
- Revankar N, Ward AJ, Pelligra CG, et al. Modeling economic implications of alternative treatment strategies for acute bacterial skin and skin structure infections. J Med Econ. 2014;17:730–740.
- Caro JJ, Moller J, Getsios D. Discrete event simulation: the preferred technique for health economic evaluations? Value Health. 2010;13:1056–1060.
- York Health Economics Consortium. Discrete Event Simulation [Internet]. York, UK: 2016 [cited 2019 Feb 15]. Available from: https://www.yhec.co.uk/glossary/discrete-event-simulation/
- Standfield LB, Comans TA, Scuffham PA. An empirical comparison of Markov cohort modeling and discrete event simulation in a capacity-constrained health care setting. Eur J Health Econ. 2017;18:33–47.
- Day TE, Al-Roubaie AR, Goldlust EJ. Decreased length of stay after addition of healthcare provider in emergency department triage: a comparison between computer-simulated and real-world interventions. Emerg Med J. 2013;30:134–138.
- Morgareidge D, Cai H, Jia J. Performance-driven design with the support of digital tools: applying discrete event simulation and space syntax on the design of the emergency department. Front Architectural Res. 2014;3:250–264.
- Sulham K, Fan W, Werner R. Real-world prescribing patterns for the treatment of acute bacterial skin and skin structure infections in the United States: a retrospective database analysis. Value Health. 2015;18:A1–A307.
- Mistry RD, Shapiro DJ, Goyal MK, et al. Clinical management of skin and soft tissue infections in the U.S. emergency departments. West J Emerg Med. 2014;15:491–498.
- Lipsky BA, Moran GJ, Napolitano LM, et al. A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Wang J, Li J, Tussey K, et al. Reducing length of stay in emergency department: a simulation study at a community hospital. IEEE Trans Syst Man Cybern A. 2012;42:1314–1322.
- Analysis of American Hospital Association Annual Survey data, 2014, for community hospitals [Internet]. 2014 [cited 2014 Jul 1]. Available from: https://www.ahadataviewer.com/additional-data-products/aha-survey/
- Rui P, Kang K, Albert M. National Hospital Ambulatory Medical Care Survey: 2013 Emergency Department Summary Tables [Internet]. 2013 [cited 2019 Feb 18]. Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf
- US Census Bureau. Annual Estimates of the Resident Population: for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios: April 1, 2010 to July 1, 2014 [Internet]. 2015. [cited 2019 Feb 18]. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=PEP_2017_PEPANNRES&src=pt
- Shaikh SB, Jerrard DA, Witting MD, et al. How long are patients willing to wait in the emergency department before leaving without being seen? West J Emerg Med. 2012;13:463–467.
- Orbactiv (oritavancin). Full Prescribing Information. Lincolnshire (IL): Melinta Therapeutics Inc.; 2018.
- Centers for Disease Control and Prevention. Quick stats: median emergency department (ED) wait and treatment times, by triage level – National Hospital Ambulatory Medical Care Survey, United States, 2010–2011. Morb Mortal Wkly Rep. 2014;63:421–440.
- Udeze C, Bookstaver B, Liu Y, et al. Hospital admission and unplanned revisits in patients with ABSSSIs. Presented at: Academy of Managed Care Pharmacy Nexus. 2016 Oct 3–6; National Harbor (MD).
- Brenner S, Zeng Z, Liu Y, et al. Modeling and analysis of the emergency department at University of Kentucky Chandler Hospital using simulations. J Emerg Nurs. 2010;36:303–310.
- Jerardi KE, Auger KA, Shah SS, et al. Discordant antibiotic therapy and length of stay in children hospitalized for urinary tract infection. J Hosp Med. 2012;7:622–627.
- Truven Red Book Online 2.0. [Internet] [cited 2017 June 22]. Available from: http://truvenhealth.com/Products/Micromedex
- Lodise TP, Fan W, Sulham KA. Economic impact of oritavancin for the treatment of acute bacterial skin and skin structure infections in the emergency department or observation setting: cost savings associated with avoidable hospitalizations. Clin Ther. 2016;38:136–148.
- Tice AD, Turpin RS, Hoey CT, et al. Comparative costs of ertapenem and piperacillin–tazobactam in the treatment of diabetic foot infections. Am J Health Syst Pharm. 2007;64:1080–1086.
- Shah NP, Reddy P, Paladino JA, et al. Direct medical costs associated with using vancomycin in methicillin-resistant Staphylococcus aureus infections: an economic model. Curr Med Res Opin. 2004;20:779–790.
- De Cock E, Sorensen S, Levrat F, et al. Cost-effectiveness of linezolid versus vancomycin for hospitalized patients with complicated skin and soft-tissue infections in France. Med Mal Infect. 2009;39:330–340.
- Bounthavong M, Hsu DI. Cost-effectiveness of linezolid in methicillin-resistant Staphylococcus aureus skin and skin structure infections. Expert Rev Pharmacoecon Outcomes Res. 2012;12:683–698.
- Stephens JM, Gao X, Patel DA, et al. Economic burden of inpatient and outpatient antibiotic treatment for methicillin-resistant Staphylococcus aureus complicated skin and soft-tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013;5:447–457.
- Jensen IS, Lodise TP, Fan W, et al. Use of oritavancin in acute bacterial skin and skin structure infections patients receiving intravenous antibiotics: a US hospital budget impact analysis. Clin Drug Investig. 2016;36:157–168.
- Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4:105–114.
- LaPensee KT, Fan W. Economic burden of hospitalization with antibiotic treatment for bacteremia/sepsis in the US. Presented at: IDWeek Annual Meeting. 2012 Oct 17–21; San Diego (CA).
- Almarzoky Abuhussain SS, Burak MA, Kohman KN, et al. Patient preferences for treatment of acute bacterial skin and skin structure infections in the emergency department. BMC Health Serv Res. 2018;18:932.
- Keyloun KR, Gillard P, Zhao Q, et al. Hospital cost and reimbursement for acute bacterial skin and skin structure infections: a retrospective observational analysis of admissions using 2014 Medicare claims data. Open Forum Infect Dis. 2016;3:1145.
