Abstract
Aims: To conduct a lifetime cost-effectiveness analysis (CEA) of rasburicase compared with standard of care (SOC) for tumor lysis syndrome (TLS) in children with hematologic malignancies from the Chinese healthcare system perspective.
Materials and methods: The CEA was performed using a decision tree model with a lifetime horizon. The model explores the cost-effectiveness of rasburicase vs SOC for both preventing TLS and treating established TLS among pediatric patients with acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), and non-Hodgkin’s lymphoma (NHL). Both the prophylaxis-use model and treatment-use model incorporate long-term health states of the diseases: survival without TLS and death. The efficacy data of rasburicase and SOC were obtained from published literature. Drug costs, healthcare resource utilization (HRU), and adverse event (AE) management costs were obtained via a published study with clinical experts. Costs in US dollar and quality-adjusted life year (QALY) are reported, and incremental cost-effectiveness ratios (ICERs) were also calculated. Uncertainties due to parameter fluctuations in the model were assessed through one-way sensitivity analysis and probabilistic sensitivity analysis (PSA).
Results: During TLS prevention, compared with SOC, the ICER of rasburicase treatment in China are $17,580.04/QALY, $5,783.45/QALY, and $5,391.00/QALY for pediatric patients with AML, ALL, and NHL, respectively. For the established TLS treatment, compared with SOC, the ICERs of rasburicase treatment are $2,031.18/QALY, $1,142.93/QALY, and $990.37/QALY for pediatric patients with AML, ALL, and NHL, respectively.
Limitations: The clinical data for SOC are based on the published study in China, and the rasburicase prevention or treatment failure rate was either calculated based on the risk ratio or directly from the clinical study among non-Chinese pediatric patients. Another study limitation was the lack of utility data for pediatric patients with TLS and without TLS. Thus, the utility scores of pediatric cancer survivors were used as an alternative.
Conclusion: Rasburicase is estimated to be a cost-effective alternative to SOC in the prevention and treatment of TLS among Chinese pediatric patients with AML, ALL, and NHL.
Introduction
Tumor lysis syndrome (TLS) is a metabolic syndrome caused by the breakdown of malignant cells. It is characterized by hyperuricemia, hyperphosphatemia, hyperkalemia, and hypocalcemia. The consequences are potentially severe and include acute kidney injury, cardiac arrhythmias, seizures, and even deathCitation1. TLS is observed most frequently in hematologic malignancies such as acute leukemias and lymphomas after treatment. According to published studies, the incidence of clinical TLS for acute leukemias and lymphomas is ∼3–7% and 4–11%, respectivelyCitation2,Citation3. Various factors may trigger the incidence of TLS, and hematologic malignancies, such as high-grade acute lymphoblastic leukemia, are associated with the greatest risk of TLSCitation4. Leukemias and lymphomas are the most common pediatric cancers. Therefore, TLS has become most prevalent in children with hematologic malignancies, affecting ∼25% of pediatric patients after treatmentCitation4. The reported incidence rate of TLS among children with B-cell acute lymphoblastic leukemia was 26.4%Citation3.
According to guidelines released by the American Society of Clinical Oncology in 2008Citation5 and the guideline on behalf of the British Committee for Standards in Hematology published in 2015Citation1, the best management of TLS is prevention, which includes hydration and prophylactic rasburicase in high and intermediate risk pediatric patients. Hydration plus allopurinol can also be used for intermediate-risk patients. Allopurinol, whilst useful in the prophylactic setting, is not the drug of choice in established TLS, except in the presence of glucose-6-phosphate dehydrogenase (G6PD) deficiency or allergy to rasburicaseCitation5. There is no clinical guideline for managing TLS in China. Currently, standard of care (SOC) for preventing and managing TLS in China includes hydration, alkalization, allopurinol, furosemide, and low molecular dextran (DU). However, preventive care such as allopurinol cannot cure established TLS, due to the fact that it won’t reduce pre-existing uric acid levels in patients who already have hyperuricemiaCitation6. Thus, other treatment options should be considered when treating patients with established TLSCitation7. Once a patient’s condition exacerbates, hemofiltration (HF) is used in addition to SOC, because no effective treatments, such as rasburicase, are available in China.
Approved by the US Food and Drug Administration (FDA) in 2002, rasburicare is indicated for both pediatric and adult patients who are at risk of developing TLS or for the management of elevated uric acid levels. Rasburicase is a recombinant urate oxidase enzyme that can be administered to patients at risk of TLS to convert uric acid to a more soluble product, allantoin, which can be excreted by the kidneys more readily. Therefore, rasburicase is able to prevent or treat TLS in patients with malignancies. It has been widely used for both TLS prevention and treatment in developed countries. Economic studies showed that preventing and treating TLS with rasburicase are highly cost-effective in both pediatric and adult patients with hematological cancerCitation2. Studying patients with acute myeloid/lymphoid leukemia (AML/ALL) and non-Hodgkin’s lymphoma (NHL), Annemans et al.Citation2 found that prevention with rasburicase is cost-effective in children with incremental cost-effectiveness ratios (ICER) between €425 and €3,054 per life-year saved. In addition, treatment of established TLS with rasburicase has demonstrated cost savings among children and is highly cost-effective in adultsCitation2.
Currently, no published health economic studies have evaluated the cost-effectiveness of rasburicase in preventing and treating TLS among Chinese patients. Given the different socioeconomic conditions across countries, economic findings from other countries may not be applicable to the Chinese healthcare system. This study aimed to evaluate the cost-effectiveness of preventing TLS and treating established TLS with rasburicase compared with SOC under the Chinese healthcare environment.
Methods
The cost-effectiveness analysis was built based on a decision tree model from healthcare system perspective with a lifetime horizon. The model explores the cost-effectiveness of rasburicase relative to SOC for TLS prevention and treatment. Two long-term health states were considered in the model: surviving without TLS, and death. displays the TLS prevention pathways. Patients at risk of TLS can be prescribed to either rasburicase or SOC for preventing TLS. Each treatment branch was associated with a different probability of developing TLS. Those who were on rasburicase but still develop TLS will continue using rasburicase to treat established TLS. If they continue having TLS, HF will be used as the second-line treatment until they recover or die. The same treatment logic applied to patients on SOC. For those who were on SOC to prevent TLS but failed, they will continue using SOC plus HF until they recover or die. displays the TLS treatment pathways. Patients with TLS could either be given rasburicase or SOC plus HF. For rasburicase users, they could either recover or continue having TLS. For patients not responding to rasburicase, HF will be used as the second-line treatment until they recover or die. For patients on SOC plus HF, there is no second-line treatment available.
Figure 1. Decision tree models: (a) TLS prevention and (b) TLS treatment. Abbreviations. SOC, standard of care; HF, hemofiltration; TLS, Tumor lysis syndrome.
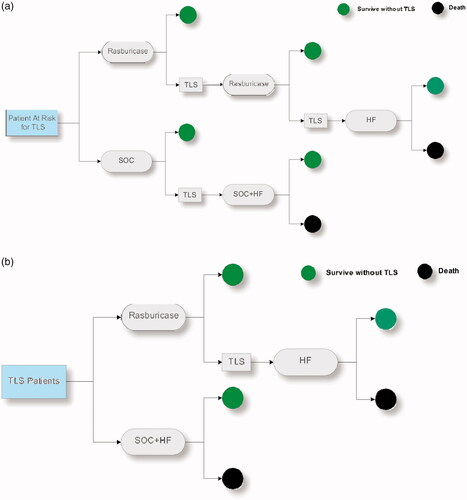
Figure 2. Tornado diagrams: One-way sensitivity analysis of ICER per QALY gained comparing rasburicase vs SOC for patients with AML during (a) TLS prevention and (b) TLS treatment. Abbreviations. SOC, Standard of Care; TLS, tumor lysis syndrome; AML, acute myeloid leukemia; QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratios.
The probabilities of developing TLS after different treatment and death rates were obtained from published literature. The time horizon of this analysis was the lifetime of pediatric patients at risk of TLS. Each health state is also assigned a payoff (e.g. total cost or effectiveness), which represents the net value of a specific path.
Clinical inputs
Clinical data for rasburicase and SOC during TLS prevention and treatment period are summarized in . The efficacy of SOC was from a published studyCitation8. According to this study, the reported incidence rates of clinical TLS for patients with AML, ALL, and NHL who were treated with SOC during TLS prevention were 5.4%, 9.7%, and 9.4%, respectively. The risk ratio of the incidence of clinical TLS during prevention between urate oxidase and allopurinol is 0.77, which was derived from one controlled clinical trial reported by Wössmann et al.Citation3. The efficacy data of rasburicase during the prevention period were calculated based on this published risk ratio. The failure rate of TLS treatment using rasburicase is 8% during the treatment period, regardless of the underlying diseaseCitation9. HF failure rates for TLS treatment vary based on the underlying diseaseCitation8. Uncontrollable TLS will result in deathCitation10. Based on the failure rates displayed in , the final distribution of “survival without TLS” and “death” can be calculated accordingly. Besides acute adverse events (AE), the model also considered that 4% of patients have kidney damage due to HF, 11% of whom need a long-term treatmentCitation8.
Table 1. Failure rates of TLS during prevention and treatment for different hematologic malignancies.
Both rasburicase and SOC were safe and well tolerated by patients with low risk of developing significant severe AEsCitation9. There is also no significant difference between the safety profiles of rasburicase and allopurinol in the clinical trialCitation11. Therefore, drug induced AE for both SOC and rasburicase were assumed non-material and not included in the current analysis. HF may introduce significant short-term and long-term adverse reactions with relative high treatment costsCitation8 (). Due to the difference of treatment efficacy, patients receiving SOC or rasburicase had different HF utilization. The current CEA model explicitly considered the HF-induced AEs.
Table 2. Incidence rate of HF-induced AEs.
Cost and utility
Costs were calculated separately for different underlying diseases. All costs related to SOC were obtained from a published studyCitation8. displays the SOC-related costs for patients with different underlying diseases during both prevention and treatment periods. The costs related to rasburicase were provided by the manufacturer. During the 5-day TLS prevention period, patients should be given two vials of rasburicase per day. The estimated cost per vial is $250.83. displays the long-term costs of managing underlying disease reported by Chu et al.’sCitation8 study. Two management phases were considered: early-phase treatment and consolidation therapy. Each phase has a different treatment duration specific to the underlying disease. The treatment costs of AEs due to HF were also considered in the model ().
Table 3. Costs related to SOC for patients with AML, ALL, and NHL during both prevention and treatment periods.
Table 4. Long-term treatment costs and length of managing underlying diseases using early-phase treatment and consolidation therapy for patients without and with experiencing TLS.
Table 5. Treatment information for HF-induced AEs.
The utility score measures the quality-of-life corresponding to each health state on a scale of 0–1, where 0 indicates death and 1 indicates perfect health. Due to the data limitation, we used the childhood cancer survivor utility score to represent the utility score for pediatric patients surviving without TLS. The utility value used in the base-case analysis, 0.769, was retrieved from the study by Yeh et al.Citation12. The life expectancies of pediatric patients are the same, regardless of survivor’s previous TLS history. They were derived from the study by Annemans et al.Citation2 and were verified by Chu et al.’sCitation8 study ().
Table 6. Median life expectancy (years) for pediatric patients without TLS history and with cured TLS.
A deterministic model was run with the base-case input values. The total cost was converted into US dollars (1 Chinese Yuan = US$0.1511). A 3% annual discount rate was applied to both costs and outcomes. The incremental cost-effectiveness ratio (ICER) was calculated.
Sensitivity analysis
One-way sensitivity analysis and probabilistic sensitivity analysis (PSA) were performed to assess CEA results variation due to parameter uncertainty. The one-way sensitivity analysis was conducted with ±5% modifications of the base-case values for variables in the model (). Variables in the one-way and PSA included rasburicase and SOC preventive failure rates, rasburicase treatment failure rate, HF treatment failure rate, percentage of patients with kidney damage, percentage of patients with kidney damage that need long-term treatment, patient life expectancy without TLS, patient life expectancy with TLS cured, and the cost per vial of rasburicase. These variables were selected for sensitivity analysis as they were potentially the most impactful factors to final CEA results.
Table 7. Sensitivity analysis parameters.
The PSA was performed on the basis of 1,000 simulations, sampling values from pre-defined distributions for parameters with reported standard errors in the analysis (). Beta distribution was used for rasburicase and SOC preventive failure rates, rasburicase treatment failure rates, HF treatment failure rates, percentage of patients with kidney damage, and percentage of patients with kidney damage that need long-term treatment. Gamma distribution was used for patient life expectancy without TLS, patient life expectancy with TLS cured, and cost per vial of rasburicase. Other variables with uncertainties that can impact the analysis of results were excluded in the PSA, as standard errors of these variables were not reported in the corresponding studies. Results of the PSA were presented as Cost-Effectiveness Acceptability Curve (rasburicase vs SOC).
Microsoft Office Excel 2016 was used for modeling and analysis.
Results
Base-case results
The results from the base-case analyses for each underlying disease are presented in and .
Table 8. Treatment outcomes, costs, and ICER per QALY gained for rasburicase and SOC during TLS prevention.
Table 9. Treatment outcomes, costs, and ICER per QALY gained for rasburicase and SOC during TLS treatment.
Preventive use
For patients with AML, prevention with rasburicase vs SOC resulted in 0.2 total quality- adjusted life-years (QALYs) gained at an incremental cost of $2,648.54. The ICER was $17,580.05/QALY. For patients with ALL, prevention with rasburicase vs SOC resulted in 0.49 total QALYs gained at an incremental cost of $2,828.91. The ICER was $5,783.46/QALY. For patients with NHL, prevention with rasburicase vs SOC resulted in 0.5 total QALYs gained at an incremental cost of $2,818.53. The ICER was $5,391.00/QALY.
Treatment use
For patients with AML, treatment with rasburicase vs SOC resulted in 2.7 total QALYs gained at an incremental cost of $5,514.42. The ICER was $2,031.18/QALY. For patients with ALL, treatment with rasburicase vs SOC resulted in 4.95 total QALYs gained at an incremental cost of $5,657.13. The ICER was $1,142.93/QALY. For patients with NHL, treatment with rasburicase vs SOC resulted in 4.3 total QALYs gained at an incremental cost of $5,407.35 The ICER was $990.37/QALY.
One-way sensitivity analysis
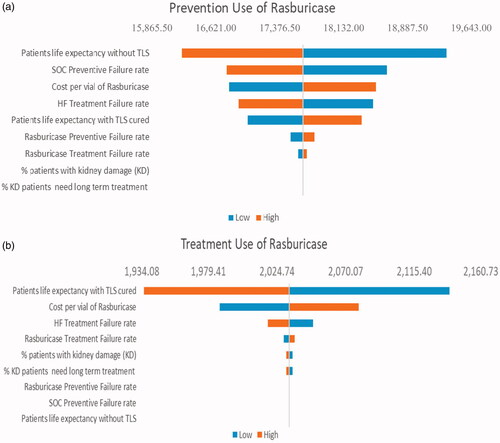
The results of the one-way sensitivity analysis comparing rasburicase with SOC for patients with underlying AML, ALL, and NHL are shown in the tornado graphs shown in . In TLS prevention, the most influential factor is the “life expectancy without TLS” in AML, ALL, and NHL. In TLS treatment, the most influential factor is the “life expectancy with TLS cured” in AML, ALL, and NHL.
Figure 3. Tornado diagram: One-way sensitivity analysis of ICER per QALY gained comparing rasburicase vs SOC for patients with ALL during (a) TLS prevention and (b) TLS treatment. Abbreviations. SOC, Standard of Care; TLS, tumor lysis syndrome; ALL, acute lymphoid leukemia; QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratios.
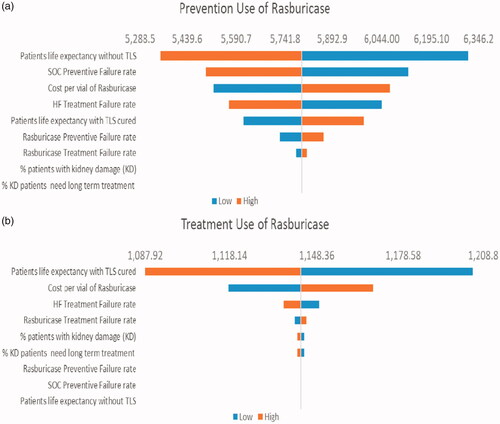
Figure 4. Tornado diagram: One-way sensitivity analysis of ICER per QALY gained comparing rasburicase vs SOC for patients with NHL during (a) TLS prevention and (b) TLS treatment. Abbreviations. SOC, Standard of Care; TLS, tumor lysis syndrome; NHL, non-Hodgkin's lymphoma; QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratios.
Figure 5. (a). Cost-effectiveness acceptability curve (rasburicase vs SOC) for patients with (a) AML, (b) ALL, and (c) NHL during TLS prevention and TLS treatment. Abbreviations. SOC, Standard of Care; TLS, tumor lysis syndrome; ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; NHL, non-Hodgkin's lymphoma; WTP, willing to pay.
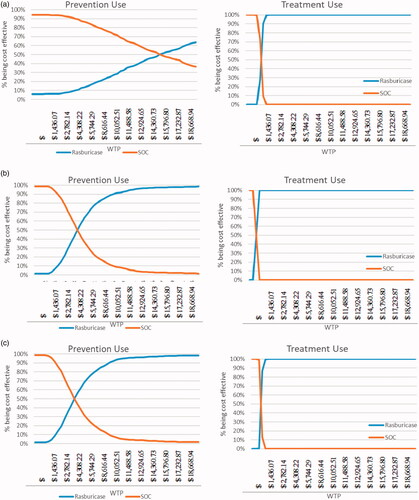
Probabilistic sensitivity analysis
The willingness-to-pay (WTP) value per QALY gained is set to be 3-times China’s gross domestic product (GDP) per capita, based on recommendations by the Commission on Macroeconomics and Health of the World Health Organization (WHO)Citation13. According to China’s National Bureau of Statistics, China’s per capita GDP was ∼$7,972.90 in 2017. Based on the WHO recommended WTP threshold, rasburicase is cost-effective vs SOC for TLS prevention with the probability of 66% for AML and nearly 100% for both ALL and NHL. The PSA results further demonstrated the cost-effectiveness of rasburicase is much more pronounced in the treatment-use scenario, achieving 100% cost-effective at WTP below 50% per capita GDP ().
Discussion
The present study compared the costs and effectiveness of using rasburicase and SOC for pediatric patients during the TLS prevention and treatment periods. Current clinical practice in China for managing TLS does not distinguish prevention and treatment explicitly: Most patients at risk are subject to SOC from the start. HF is used when TLS is established. The prophylaxis use scenario of this CEA is particularly relevant as the modeled pathways are closely mirrored with actual care delivery. It is worth noting that the ICERs of prophylaxis rasburicase use, compared with SOC, are cost-effective ($17,580.05/QALY, $5,783.46/QALY, and $5,391.00/QALY for pediatric patients with AML, ALL, and NHL, respectively). These data support rasburicase as an efficient clinical choice in TLS prevention in China. For treatment use, rasburicase is highly cost-effective compared with SOC with ICER at $2,031.18/QALY, $1,142.93/QALY, and $990.37/QALY for pediatric patients with AML, ALL, and NHL, respectively. These findings are consistent with previously reported research outcomes in the EUCitation2.
These results can be affected by variations in input values, including life expectancy without TLS, life expectancy with TLS cured, rasburicase prevention failure rate, SOC prevention failure rate, rasburicase treatment failure rate, HF treatment failure rate, and percentage of patients with kidney damage. Although all these factors will affect the final result, the range of ICERs comparing rasburicase with SOC for all three underlying diseases estimated from the one-way sensitivity analysis did not exceed the societal WTP threshold recommended by the Commission on Macroeconomics and Health of the World Health OrganizationCitation13.
The present study is subject to the limitations of the data and assumptions used to estimate the range for the variables used in the modeling. The clinical data for SOC are based on published data from expert opinion, and the factor used to derive the rasburicase prevention failure rate was based on non-Chinese pediatric patients. While they are the best available data, the clinical data of SOC is only used as the base in Chinese patients, and the risk ratio may not change much among patient groups, which is acceptable and feasible from methodological perspective. Another study limitation is that utility data were unavailable for either Chinese pediatric patients with and without TLS, or Chinese pediatric cancer patients. Thus, the utility scores of pediatric cancer survivors in western countries were used as an alternative.
In the present study, we showed that rasburicase was cost-effective compared with other treatment options based on the criterion recommended by the Commission on Macroeconomics and Health of WHO. The actual WTP threshold adopted by a specific decision-maker based on local socioeconomic conditions may determine the best treatment option for particular patients.
Conclusion
Among Chinese pediatric patients with hematologic malignancies who are at great risk of developing TLS, rasburicase is estimated to be cost-effective compared with the current SOC treatment options. It demonstrates both clinical and economic benefits not only in preventing TLS, but in treating established TLS for pediatric patients with AML, ALL, and NHL.
Transparency
Declaration of funding
The study was funded by Sanofi, China.
Declaration of financial/other interests
YH, WZ, and TZ are employees from WPP Health, which received funds for conducting the analyses and drafting the manuscript from Sanofi China. XY and LL are employees of Sanofi. The authors and JME peer reviewers on this manuscript have no other relevant financial or other relationships to disclose apart from those mentioned here. No potential conflict of interest was reported by the authors.
Acknowledgements
None reported.
References
- Jones GL, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661–671.
- Annemans L, Moeremans K, Lamotte M, et al. Pan-European multicentre economic evaluation of recombinant urate oxidase (rasburicase) in prevention and treatment of hyperuricaemia and tumour lysis syndrome in haematological cancer patients. Support Care Cancer. 2003;11:249–257.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160–165.
- Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21:18–26.
- Coiffier B, Altman A, Pui C-H, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. JCO. 2008;26:2767–2778.
- Held-Warmkessel J. Preventing & managing tumor lysis syndrome. Oncol Times. 2010;32:1–7.
- Alakel N, Middeke JM, Schetelig J, et al. Prevention and treatment of tumor lysis syndrome, and the efficacy and role of rasburicase. OTT. 2017;10:597–605. 10.2147/OTT.S103864
- Chu M, Zhang T, Yao X, et al. The current status and disease burden of pediatric tumor lysis syndrome, published in the 23rd Pediatric Conference of the Chinese Medical Association, 2018, October 27–28, Xiamen, China.
- ELITEK (rasburicase) for injection, for intravenous use Initial U.S. Sanofi. 2002.
- Zhengren H. Acute tumor lysis syndrome (analysis of 130 cases). J Clin Hematol. 2005;18:52–54.
- Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998–3003.
- Yeh JM, Hanmer J, Ward ZJ, et al. Chronic conditions and utility-based health-related quality of life in adult childhood cancer survivors. J Natl Cancer Inst. 2016;108:2016–2019.
- Commission of Macroeconomics and Health. Macroeconomics and health: investing in health for economic development. Rev Panam Salud Pública. 2002;12:143–144.
