Abstract
Aim: The approved indication for denosumab (120 mg) was expanded in 2018 to include skeletal-related event (SRE) prevention in patients with multiple myeloma (MM). Therefore, a cost-effectiveness analysis was conducted comparing denosumab with zoledronic acid (ZA) for SRE prevention in patients with MM from the national healthcare system perspective in a representative sample of European countries: Austria, Belgium, Greece, and Italy.
Methods: The XGEVA global economic model for patients with MM was used to calculate incremental cost-effectiveness ratios (ICERs) for denosumab vs ZA over a lifetime horizon. Clinical inputs were derived from the denosumab vs ZA randomized, phase 3 study (“20090482”) in patients newly-diagnosed with MM, and comprised real-world adjusted SRE rates, serious adverse event (SAE) rates, treatment duration, dose intensity, progression-free survival (PFS), and overall survival (OS). Economic inputs comprised country-specific denosumab and ZA acquisition and administration costs, SRE and SAE management costs, and discount rates. Health utility decrements associated with MM disease progression, SRE and SAE occurrence, and route of administration were included.
Results: Estimated ICERs (cost per quality-adjusted life-year [QALY] gained) for denosumab vs ZA in Austria, Belgium, Greece, and Italy were €26,294, €17,737, €6,982, and €27,228, respectively. Using 1–3 times gross domestic product (GDP) per capita per QALY as willingness to pay thresholds, denosumab was 69–94%, 84–96%, 79–96%, and 50–92% likely to be cost-effective vs ZA, respectively.
Limitations: Economic inputs were derived from various sources, and time to event inputs were extrapolated from 20090482 study data.
Conclusions: Denosumab is cost-effective vs ZA for SRE prevention in patients with MM in Austria, Belgium, Greece, and Italy, based on often-adopted World Health Organization thresholds. This conclusion is robust to changes in model parameters and assumptions. Cost-effectiveness estimates varied across the four countries, reflecting differences in healthcare costs and national economic evaluation guidelines.
Introduction
The proliferation of malignant plasma cell clones in patients with multiple myeloma (MM) results in organ dysfunction and bone damageCitation1. Despite being rare among cancers—representing ∼1.1% of cases in Europe in 2018Citation2—the crude incidence of MM in 2018 in Austria, Belgium, Greece, and Italy per 100,000 people was 6.4, 8.9, 7.3, and 10.2, respectivelyCitation2. Furthermore, MM incidence is projected to increase by 29% by the year 2040, as the population of Europe ages, and life expectancies increaseCitation2.
Lytic bone lesions are a common feature of MM and are associated with substantial morbidity and mortalityCitation1,Citation3; most patients (∼80–90%) develop lytic bone lesions during the disease courseCitation4. Lytic bone lesions cause pain and reduce the structural integrity of bone, thereby increasing the risk of skeletal-related events (SREs)Citation3,Citation5. SREs include pathologic fracture, spinal cord compression, radiation to bone, and surgery to boneCitation3,Citation5. Most patients with MM (∼70–95%) will experience at least one SRE during their disease courseCitation6. Importantly, SREs are associated with a poor prognosis, and with substantial morbidity, pain, and reduced quality-of-lifeCitation3,Citation7–9. In addition to their negative impact on patients, SREs are associated with increased healthcare resource use (HRU), including emergency department visits, hospitalization, outpatient clinic attendance, and analgesic useCitation6,Citation10–13; therefore, SREs can increase MM direct healthcare costs by ∼75% vs those without SREsCitation12. Furthermore, multiple SREs, which patients often experience, are associated with greater HRU and costs than isolated eventsCitation11,Citation12.
Given their negative impact on patients and healthcare systems, it is important to prevent SREs using denosumab, or bisphosphonates such as zoledronic acid (ZA)Citation3,Citation14–16. Both agents are established treatments for patients with bone metastases from solid tumorsCitation17,Citation18; however, ZA was the standard of care in patients with MM before 2018, which is when the approved indication for denosumab was expanded to SRE prevention in adults with advanced malignancies involving boneCitation3,Citation16–19. Both denosumab and ZA are effective at preventing SREs in patients with MM, although they have different mechanisms of action and routes of administration (subcutaneous injection vs intravenous infusion, respectively)Citation20–22. For SRE prevention, denosumab (120 mg) is administered every 4 weeks (Q4W) and ZA (4 mg [dose adjusted]) is administered every 3–4 weeksCitation17,Citation18.
In addition to lytic bone lesions, most patients with MM (up to ∼60%) develop RICitation23, and worsening renal function is a common feature of MM that arises owing to both the disease process and its treatmentCitation1,Citation16,Citation23,Citation24. Unlike ZA, denosumab is not cleared by the kidneysCitation17; ZA requires dose adjustment for use in patients with renal impairment (RI) and it is not recommended for use in cases of severe RI (creatinine clearance [CrCl] < 30 mL/min)Citation18. Current European clinical practice guidelines, published before the approval of denosumab for use in patients with MM, recommend bisphosphonates for SRE prevention in patients with lytic bone lesionsCitation15,Citation16,Citation19. International guidelines also recommend bisphosphonates for patients without evidence of lytic bone lesions on conventional radiographsCitation3, and recently updated guidelines from the US suggest denosumab as an alternative to bisphosphonates for patients with MMCitation25,Citation26.
Denosumab is superior to ZA for SRE prevention in patients with bone metastases from solid tumorsCitation27. The subsequent approval of denosumab for use in patients with MMCitation17 was based on the largest study in MM to date: an international, phase 3, double-blind, double-dummy, randomized study (“the 20090482 study”; NCT01345019)Citation20, which found denosumab to be non-inferior to ZA for delaying time to first on-study SRE (primary endpoint) in 1,718 patients with newly-diagnosed MM (hazard ratio [HR] = 0.98 [95% confidence interval (CI) = 0.85–1.14], p = 0.010)Citation20. Superiority of denosumab over ZA for SRE prevention—a secondary endpoint—was not demonstratedCitation20. As most first on-study SREs occurred within the first 3 months (60%), during which time denosumab and ZA may not have been able to take full effect, differences in efficacy of the two agents may not have been fully captured by the primary analysis; therefore, a post hoc landmark analysis was performed to capture differences in efficacy of the two agents over timeCitation20. This analysis suggested that denosumab is superior to ZA for preventing first SRE starting at 15 months (HR = 0.66 [95% CI = 0.44–0.98], p = 0.039)Citation20.
Denosumab is a fully human monoclonal antibody targeting the receptor activator of nuclear factor-κB (RANK) ligand (RANKL)Citation17,Citation22. In addition to its involvement in bone complications, there is evidence to suggest that RANKL–RANK signaling may play a role in MM pathogenesisCitation20,Citation28. This hypothesis was supported by the 20090482 study, which suggested that, when used in addition to anti-MM treatment, denosumab improved median progression-free survival (PFS) by 10.7 months vs ZA; PFS was a pre-specified, exploratory endpoint (HR = 0.82 [95% CI = 0.68–0.99], p = 0.036)Citation20. Overall survival (OS)—a secondary endpoint—was similar in both arms (HR = 0.90 [95% CI = 0.70–1.16], p = 0.41), although too few deaths had occurred at the time of analysisCitation20.
As modern healthcare systems have competing demands on HRU and spendingCitation29, it is increasingly important to demonstrate the value of novel treatments to multiple stakeholdersCitation30. Using clinical data from the 20090482 studyCitation20, an economic analysis conducted in the US concluded that denosumab was cost-effective vs ZA for SRE prevention in patients with MM from both a payer and societal perspectiveCitation31. However, there are no published European cost-effectiveness analyses of denosumab in this patient population, and cost-effectiveness results cannot be translated from one healthcare setting to another. Given the burden of SREs on patients and healthcare systems, the efficacy of denosumab in SRE prevention, and the potential of denosumab to extend PFS vs ZA in patients with MM without impairing renal function, it is important to evaluate the cost-effectiveness of denosumab vs ZA in this patient population. Using the same economic model structure as Raje et al.Citation31 in the US, we estimated the incremental cost-effectiveness ratio (ICER: incremental cost per quality-adjusted life-year [QALY] gained) for denosumab vs ZA from the national healthcare system perspective in four European countries (Austria, Belgium, Greece, and Italy).
Methods
Cost-effectiveness analysis
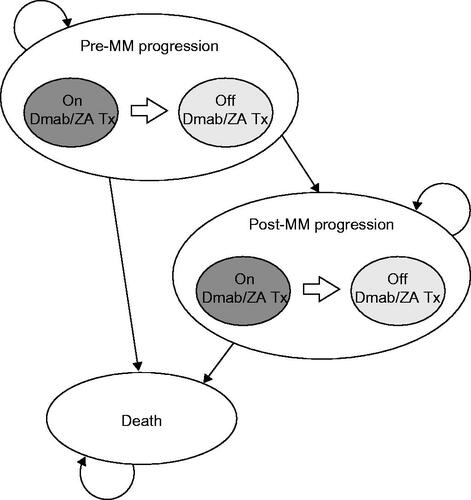
The cost-effectiveness of denosumab vs ZA in patients with MM was analyzed using a decision analytical model that was based on the XGEVA global economic model (X-GEM) for patients with MM developed by Raje et al.Citation31. The X-GEM is a partitioned-survival semi-Markov model. Briefly, the model included five health states, with 28-day cycles over a lifetime horizon (500 cycles, corresponding to ∼ 38 years). It took the same structure for both denosumab and ZA (). Clinical inputs were derived from the phase 3, randomized, controlled 20090482 studyCitation20, and other published sources, and cost and quality-of-life inputs were derived from various published sources. ICERs were calculated as the difference in total costs divided by the difference in QALYs between denosumab and ZA.
Model population
The model population comprised adults with newly-diagnosed MM from the 20090482 studyCitation20. Patients were enrolled over a 45-month period, with a median follow-up of 17.3 and 17.6 months in the denosumab and ZA arms, respectivelyCitation20,Citation31. Eligible patients had at least one lytic bone lesionCitation20. As patients with severe RI (CrCl < 30 mL/min) were not included in the 20090482 studyCitation20 owing to their ineligibility to receive ZACitation18, the base case analysis did not include patients with severe RI. Instead, a scenario analysis comparing denosumab with no treatment was performed to estimate the cost-effectiveness of denosumab in patients with severe RI.
Model clinical inputs
The following clinical outcomes were included in the model: SRE annual rates by treatment status and the distribution of SREs by type; PFS and OS rates; treatment-discontinuation and -compliance rates; and serious adverse event (SAE) rates (Supplementary Table S1)Citation20,Citation21,Citation31–33. The clinical inputs from the 20090482 studyCitation20 were used for all four countries.
Skeletal-related events
SREs comprised pathologic fracture, radiation to bone, surgery to bone, and spinal cord compression. Constant annual SRE rates by treatment status were calculated by dividing the total number of SREs experienced by patients in each arm of the 20090482 studyCitation20 by the number of patient-years of follow-upCitation31. SRE rates observed in the 20090482 studyCitation20 were adjusted based on real-world SRE ratesCitation32,Citation33 by multiplying them by a relative rate ratio (2.84)Citation31. The annual SRE rate for patients off denosumab/ZA treatment was estimated by dividing the SRE rate in the ZA arm of the 20090482 studyCitation20 by a relative rate ratio (0.57) derived from a recently updated, comprehensive, network meta-analysis by Mhaskar et al.Citation21; this was not available at the time of the US cost-effectiveness analysis. The cost-effectiveness analysis by Raje et al.Citation31 used a similar rate ratio (0.63), based on analysis of a much smaller data setCitation34 published earlier than the meta-analysis by Mhaskar et al.Citation21. The annual SRE rate for patients without SRE-preventive treatment was assumed to be the same as for those who had discontinued treatmentCitation31. The distribution of SRE types was calculated from the 20090482 studyCitation20; most SREs were pathologic fractures (81.6%)Citation31 (Supplementary Table S1).
Progression-free and overall survival, and treatment discontinuation
PFS, OS, and treatment-discontinuation rates were derived from independent fits of parametric survival distributions to patient-level data from the 20090482 studyCitation20,Citation31. As OS and treatment-discontinuation rates were similar in both arms, data from the two arms were pooled for each of these parametersCitation20,Citation31. In the 20090482 studyCitation20, PFS was different in the two arms in a pre-specified exploratory analysis; therefore, PFS was modeled independently for denosumab and ZA. Of the distributions assessed, the generalized gamma function was selected for PFS and treatment-discontinuation data, and the Weibull function for OS data (Supplementary Figure S1), based on the Akaike Information Criterion, and visual inspection of fitCitation31. The long-term extrapolation of OS was corrected using country, gender, and age-specific life tables to ensure that the death rate of patients with MM was at least that observed in the general population of each countryCitation35–38.
Treatment compliance and serious adverse events
Treatment compliance was calculated as the ratio of the number of denosumab/ZA doses received by each patient to the number scheduled up to the last dose received in the 20090482 studyCitation20,Citation31. Constant annual rates for SAEs of special interest (hypocalcemia, osteonecrosis of the jaw, and RI) were calculated from the safety population of the 20090482 studyCitation20 using a similar method to the calculation of SRE ratesCitation31. SAEs were as defined in the 20090482 studyCitation20.
Model utility inputs
QALY decrements for the following clinical outcomes were included in the model: SREs by type, MM disease progression, SAEs by type, and route of administration (intravenous vs subcutaneous) (Supplementary Table S2)Citation31,Citation39–42. QALY decrements for SREs by typeCitation39 and by route of administrationCitation41 were derived from time trade-off studies involving participants from the general populations of the UK and Canada. As per Raje et al.Citation31, MM disease progression was associated with a 19.5% QALY decrement; the utility levels before and after MM disease progression were 0.800 and 0.644, respectively (based on van Agthoven et al.Citation42). SAE QALY decrements were based on Stopeck et al.Citation40.
Model economic inputs
Cost inputs were derived from national sources to reflect differences between the healthcare systems and economic wealth of the four countries: denosumab/ZA acquisition and administration costs, and SRE and SAE management costs ()Citation10,Citation43–55. The most recent estimates for drug costs were used (2018); all other costs referred to the year 2017Citation56.
Table 1. Model economic inputs by country.
Drug acquisition and administration costs
Denosumab and ZA were assumed to be administered Q4W, which is in line with the schedule used in the 20090482 studyCitation20 and the product labelsCitation17,Citation18. Denosumab/ZA acquisition costs were based on national listingsCitation47–49,Citation51. The cost of generic ZA was used in Greece, Italy, and Belgium. In Austria, a weighted-average cost was used because the distribution of sales of generic and originator ZA was available (IQVIA: RSÖ, DPMÖ, and DPMÖK databases 2018). Denosumab/ZA administration costs, which included a renal function monitoring cost for each ZA dose, were based on national sources. In Belgium, the cost of ZA administration was assumed to be twice that of denosumab, with the addition of a renal function monitoring cost. This assumption was made because the tariff system for intravenous drug administration in day clinics in Belgium has changed, with the current system not allowing estimation of the true cost of intravenous administration. A factor of two was selected because it was consistent with the ratio observed in Austria and Italy, and because it was a conservative estimate. The situation in Greece is different because payers do not incur a cost for denosumab administrationCitation50.
Skeletal-related event and serious adverse event management costs
Country-specific SRE management costs by SRE type for Austria and GreeceCitation53 were derived from a published study involving both patients with MM and solid tumors, whereas in BelgiumCitation54 only data on patients with bone metastases from solid tumors were available. As MM-specific HRU data were available for ItalyCitation10, a micro-costing approach was used, applying national unit costs to HRUCitation52. Country-specific SAE management costs were derived using a micro-costing approach in Austria, Belgium (data on file [Amgen]), and ItalyCitation52, and from national sources in GreeceCitation50.
Discount rates
Country-specific annual discount rates for costs and health outcomes were derived from local health technology assessment (HTA) guidelinesCitation43,Citation44,Citation46, except for Greece, where guidance from the National Institute for Health and Care Excellence (NICE [UK]) was used owing to a lack of local recommendationsCitation45 ().
Scenario analyses
To explore the sensitivity of model results to changes in the model parameters and assumptions, we conducted several scenario analyses, as well as one-way deterministic and multivariate probabilistic sensitivity analyses. The main alternative scenario analyses comprised: (1) including first and subsequent line (up to fourth line) anti-MM treatment costs in the model to account for a delay in initiating subsequent treatment lines due to the prolonged PFS observed with denosumab vs ZA (unlike the cost-effectiveness analysis by Raje et al.Citation31, our base case scenario did not include anti-MM treatment costs in the model); (2) comparing denosumab with no treatment for patients who may not be able to receive bisphosphonates, such as patients with severe RICitation18, by applying a rate ratio of 0.57Citation21 to the ZA SRE rate; and (3) using SRE rates from the post hoc 15-month landmark analysis in the 20090482 study, in which time to first SRE was longer in the denosumab arm than in the ZA arm (HR = 0.66 [95% CI = 0.44–0.98], p = 0.039)Citation20. For alternative scenario 3, the model time horizon was split in two: the first 14 months of treatment, and from 15 months after the start of treatment. Annual SRE rates were calculated by dividing the number of SREs observed by the number of patient-years at risk in each arm in each time period in the 20090482 studyCitation20 (Supplementary Table S3).
Additional scenarios included those commonly considered in cost-effectiveness analyses, comprising: (4) using shorter time horizons (10 and 20 years); (5) applying 0% discount rates to costs and health outcomes; and (6) considering other parametric distributions for survival models (modeling PFS using a Weibull distribution, applying an HR of 0.82Citation20, instead of using a generalized gamma distribution; a Weibull distribution was the second most plausible parametric fit). The model input parameters used in the scenario analyses are provided in Supplementary Table S3.
Sensitivity analyses
Deterministic sensitivity analyses
Sensitivity analyses of key model inputs were conducted to evaluate model robustness. The univariate deterministic sensitivity analysis involved varying each model parameter in turn to its lower and upper bound. The model input parameters used in the deterministic sensitivity analyses are provided in Supplementary Table S4.
Probabilistic sensitivity analyses
The multivariate probabilistic sensitivity analysis involved attributing probabilistic distributions to each of the parameters, and simulating 2,000 samples drawn from these distributions. Ranges and parameters for the probabilistic distributions were informed by uncertainty measures associated with the point estimates (standard errors or 95% CIs), or were varied by ±20% when no uncertainty measure was available. The model input parameters used in the probabilistic sensitivity analyses are provided in Supplementary Table S5.
Results
Base case
Results from the base case cost-effectiveness analysis by country are shown in . Discounted life-years ranged from 6.27 in Austria to 7.75 in Belgium, with no difference between denosumab and ZA. Denosumab was estimated to be associated with QALY gains vs ZA in all four countries: incremental QALYs gained were lowest in Austria (0.19) and highest in Belgium (0.24); they were 0.21 in Greece and 0.22 in Italy. Total costs over the time horizon were largely driven by SRE management costs, which ranged from €60,606 for denosumab in Italy to €183,019 for ZA in Austria. Total costs over the time horizon were higher for denosumab than for ZA in all four countries. Comparing denosumab with ZA, incremental costs incurred were lowest in Greece (€1,445) and highest in Italy (€5,858); they were €4,879 in Austria and €4,314 in Belgium. Although drug acquisition costs were higher for denosumab than for ZA (the difference ranged from €5,579 in Greece to €8,024 in Austria), these costs were partially offset by lower SRE management and drug administration costs for denosumab than for ZA (the difference ranged from –€2,668 in Austria to –€824 in Italy for SRE management costs, and from –€2,879 in Greece to –€431 in Italy for drug administration costs). The difference in SAE management costs between denosumab and ZA was negligible (<€100 in all four countries). The ICER (incremental cost per QALY gained) for using denosumab instead of ZA was €26,294, €17,737, €6,982, and €27,228 in Austria, Belgium, Greece, and Italy, respectively.
Table 2. Base case cost-effectiveness analysis (discounted) by country.
Alternative scenarios
Results from the alternative scenario cost-effectiveness analyses by country are shown in . When adding anti-MM treatment costs to the model, or comparing denosumab with no treatment, denosumab dominated in all four countries (i.e. denosumab was associated with fewer total costs and more QALYs than ZA or no treatment). The PFS benefit of denosumab vs ZACitation20 translated into cost savings for denosumab vs ZA when anti-MM treatment costs were included in the model. These savings ranged from €11,836 (Italy) to €29,661 (Belgium), and were largely driven by lower anti-MM drug acquisition costs for denosumab than for ZA. When comparing denosumab with no treatment, savings associated with denosumab ranged from €3,863 (Italy) to €34,207 (Austria), and were largely driven by lower SRE management costs for denosumab than for no treatment. Using SRE rates from the 15-month landmark analysisCitation20 resulted in lower ICERs than in the base case (ranging from €3,586 [Greece] to €23,905 [Italy] per QALY). Shortening the model time horizon and modeling PFS using a Weibull distribution resulted in higher ICERs than in the base case (ranging from €9,811 [Greece] to €38,874 [Italy] per QALY using a 10-year time horizon; ranging from €10,359 [Greece] to €40,929 [Italy] per QALY when modeling PFS using a Weibull distribution). Finally, applying a 0% discount rate to costs and health outcomes resulted in slightly lower ICERs than in the base case (ranging from €5,681 [Greece] to €22,744 [Italy] per QALY).
Table 3. Alternative scenario cost-effectiveness analyses (discounted) by country.
Sensitivity analyses
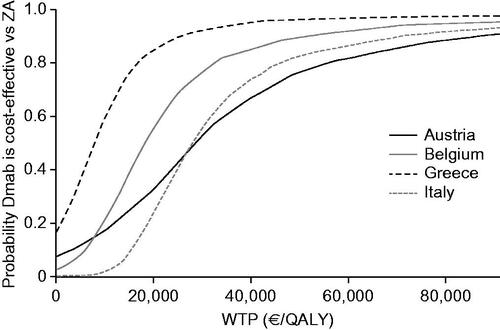
Univariate deterministic sensitivity analyses, which accounted for uncertainty around the estimated values of key model inputs, showed that the ICERs were relatively stable and remained within acceptable limits. ICERs varied the most in Austria and the least in Greece. The highest and lowest ICERs were: €56,910 and €4,391 per QALY (Austria); €31,968 and €6,671 per QALY (Belgium); €18,899 per QALY and dominance (Greece); and €43,362 and €18,771 per QALY (Italy). Annual SRE rates and utility levels after MM disease progression were the main drivers of uncertainty (). By using 1–3-times the gross domestic product (GDP) per capita of each country per QALY gained as willingness-to-pay (WTP) thresholds (), the results of the multivariate probabilistic sensitivity analysis, which accounted for uncertainty associated with several key model inputs simultaneously, estimated denosumab as 69–94%, 84–96%, 79–96%, and 50–92% likely to be cost-effective vs ZA in Austria, Belgium, Greece, and Italy, respectively ().
Figure 2. Main drivers of uncertainty in cost-effectiveness analysis by country. The ICER is the cost (€) per QALY gained derived from univariate analyses employing the lower and upper bounds of top five key model inputs. Cross-hatching represents situations in which Dmab is dominant vs ZA (Dmab is associated with more QALYs and fewer overall costs than ZA). The ranges for the model parameters are provided in Supplementary Table S4. Abbreviations. Dmab, denosumab; ICER, incremental cost-effectiveness ratio; i.v., intravenous; MM, multiple myeloma; QALY, quality-adjusted life-year; SRE, skeletal-related event; ZA, zoledronic acid.
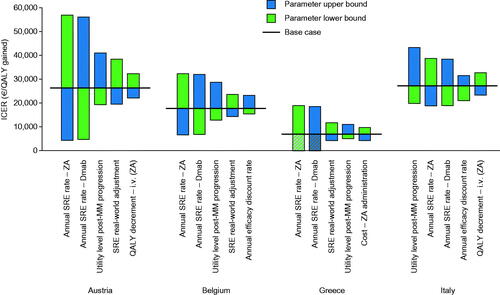
Figure 3. Cost-effectiveness acceptability curves by country. The model input parameters are provided in Supplementary Table S5. Abbreviations. Dmab, denosumab; QALY, quality-adjusted life-year; WTP, willingness to pay; ZA, zoledronic acid.
Discussion
This cost-effectiveness analysis compared denosumab with ZA for SRE prevention in patients with MM in four European countries (Austria, Belgium, Greece, and Italy) by populating an economic model (the X-GEM)Citation31 with clinical inputs from a large, phase 3, randomized, controlled studyCitation20. In our base case analysis, the ICER (incremental cost per QALY gained) for using denosumab instead of ZA ranged from €6,982 in Greece to €27,228 in Italy; the ICERs in Austria and Belgium were €26,294 and €17,737, respectively. In the absence of country-specific WTP thresholdsCitation58, WTP thresholds of 1–3-times GDP per capita per disability-adjusted life-year (DALY) avoided are often used to assess healthcare interventionsCitation59. These thresholds have been adopted by the World Health Organization (WHO) initiative ‘Choosing Interventions that are Cost-Effective’ (WHO-CHOICE)Citation60. Adopting these thresholds, and assuming that the WTP for a QALY is the same as for a DALY, denosumab was very cost-effective vs ZA in all four countries analyzed. Although this assumption is frequently made in the literature, we recognize that it is not without limitationsCitation61. Furthermore, MM is classified as a rare disease in EuropeCitation2,Citation62, and, in some healthcare systems, orphan drugs benefit from more flexible HTA processes to help improve patient accessCitation63–65.
The alternative scenario and sensitivity analyses demonstrated that estimated ICERs were robust to changes in model parameters. Considering the three main alternative scenarios, estimated ICERs were more favorable than in the rather conservative base case. The longer PFS observed with denosumab vs ZA in the 20090482 studyCitation20 led in the model to later initiation and shorter duration of subsequent anti-MM treatment lines, which resulted in lower incremental costs, making denosumab dominant vs ZA in this scenario. At first line, however, longer PFS was associated with slightly higher anti-MM treatment costs, although the influence of PFS was limited because regimens most commonly used at first line are typically administered for a fixed number of cyclesCitation66. Using SRE rates from the post hoc 15-month landmark analysisCitation20 in the model resulted in more favorable ICERs than in the base case because of significantly fewer SREs with denosumab vs ZA from 15 months after the start of treatmentCitation20. It was important to compare denosumab with no treatment for SRE prevention because some patients with MM, such as those with severe RI, are unable to receive ZACitation18,Citation67,Citation68; denosumab was dominant vs no treatment in this scenario.
As country-specific economic inputs were aligned with local HTA submission guidelines, the model inputs and results of our cost-effectiveness analysis reflected differences between the healthcare systems and economic wealth of the four countries analyzedCitation58,Citation69,Citation70. Total costs were slightly higher for denosumab than for ZA across all four countries, which were driven by higher acquisition costs for denosumab vs ZA; the latter is now available as a generic versionCitation47–49,Citation51. The higher acquisition costs for denosumab vs ZA were partially offset, however, by lower SRE management and drug administration costs. Healthcare costs and clinical management can vary substantially between countries, therefore justifying the need for country-specific cost-effectiveness analysesCitation58. Of note, estimated management costs for SREs and SAEs vary substantially between the four countries; for example, the cost of managing an episode of hypocalcemia ranged from €319 in GreeceCitation50 to €14,272 in Belgium. A potential explanation for these differences resides in differences in diagnosis-related group classification and costing. Furthermore, in Greece, denosumab can be administered at community pharmacies without cost to payers, therefore leading to a substantially lower administration cost vs ZACitation50,Citation71. Another source of variation in cost and quality-of-life estimates across countries resides in the different annual discount rates considered by national health authoritiesCitation43–46; for example, annual discount rates for health outcomes vary from 1.5% in Belgium to 5.0% in AustriaCitation43,Citation44.
Because patient and disease characteristics, treatment approaches and effects, and economic model structures and assumptions are different across studies, it is difficult to compare our results directly with published cost-effectiveness studies evaluating denosumab in either the US healthcare settingCitation31, or in patients with bone metastases from solid tumorsCitation33,Citation40,Citation71–81. There were three main differences between our model and the one used by Raje et al.Citation31 in the US: (1) anti-MM treatment costs were included as a scenario analysis, but not in the base case; (2) the main comparator for denosumab was ZA, with a scenario analysis with no treatment as the comparator (a weighted average of the two was used by Raje et al.Citation31); and (3) we limited the analysis to the payer perspective only. We elected not to include anti-MM treatment costs in our base case because of the rapidly evolving and heterogeneous treatment landscape for relapsed MM in EuropeCitation66,Citation82, and because of variations between list prices and net prices, the latter not being publicly available.
Both our cost-effectiveness analysis and the one by Raje et al.Citation31 have the advantage of assessing lifetime value, using clinical outcome data from a large, well-designed, phase 3, randomized, controlled study in MM that directly compared denosumab with ZACitation20; furthermore, SRE rates were adjusted using real-world dataCitation32,Citation33. We recognize, however, that there is uncertainty around true SRE rates in the real world due to a lack of robust data in patients with MM. Therefore, we conservatively tested a wide range of values for the SRE real-world adjustment factor in the deterministic sensitivity analysis (values ranging from 1 [no correction] to 4; base case value = 2.84), and found that the model results were relatively robust to this range. However, further research into the real-world incidence of SREs in patients with MM would be warranted to reduce this uncertainty. Our study also considered pertinent healthcare costs resulting from denosumab/ZA administration and RI, therefore providing a comprehensive assessment of real-world value. Additionally, discount rate and cost inputs in our study were country-specific, therefore accounting for variability in treatment approaches across the healthcare settings analyzed. There are, however, limitations inherent to health economic evaluations, which relate to model assumptions and the quality of model inputsCitation83. As explained by Raje et al.Citation31, some costs (e.g. SRE management costs in BelgiumCitation54) and utility data were derived from studies in patients with solid tumors instead of MM, although we do not believe that this will have had a substantial impact on our results. The need to extrapolate survival data using parametric survival models beyond the 20090482 studyCitation20 follow-up is also a common source of uncertainty. However, uncertainty surrounding these parameters was explored by performing extensive scenario and sensitivity analyses.
Conclusions
In our study, denosumab was found to be cost-effective vs ZA for SRE prevention in patients with MM in Austria, Belgium, Greece, and Italy due to its efficacy in SRE prevention and potential PFS benefit without compromising renal function. This conclusion was based on often-adopted WHO cost-effectiveness thresholds, and was robust to changes in model parameters and assumptions. Cost-effectiveness estimates varied across the four countries analyzed, reflecting differences in healthcare costs and national HTA guidelines. Denosumab represents an attractive value proposition for SRE prevention in patients with MM in the four countries included in this analysis.
Transparency
Declaration of funding
This study was funded by Amgen.
Declaration of financial/other relationships
ET has received grants, personal fees, and non-financial support from Amgen, Celgene, and Janssen-Cilag; received personal fees and non-financial support from Takeda; and received personal fees from Bristol-Myers Squibb, GlaxoSmithKline, Novartis, and Roche. AJ is an employee of Amgen. AC, MC, and DB are employees of Amgen and hold Amgen stock. LK has consulted for Amgen, and is the founder and chief economist at Epiphany, which has received funding from Amgen. WW is a part-time employee of Oncotyrol and has participated in steering committees for Amgen; participated in advisory boards and consulted for Amgen, Bristol-Myers Squibb, Celgene, CTI, Gilead, Janssen, Merck, Mundipharma, Novartis, Pfizer, Roche, Sandoz, Takeda, and The Binding Site; presented lectures for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Gilead, Janssen, Multiples Myelom und Lymphom Selbsthilfegruppe Österreich, Mundipharma, Novartis, Roche, Sandoz, Takeda, and The Binding Site; participated in speaker bureaus for Gilead; and received research funding from Amgen, Bristol-Myers Squibb, Bundesland Tirol Programm: “Translational research”, Celgene, European Commission (FP7 – OPTATIO), Janssen, Novartis, Oncotyrol, Roche, and Takeda. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose
Supplemental Material
Download MS Word (147.3 KB)Acknowledgements
The authors would like to thank Cornelia Moser (Austria), Valery Fikkert (Belgium), John Relakis (Greece), and Serena de Fazio (Italy) for their assistance with collecting data inputs (all are employees of Amgen). Oxford PharmaGenesis, Oxford, UK, received funding from Amgen for medical writing support, which was provided by Jack Dean.
References
- Hartley-Brown MA, Sullivan DM, Baz R. State-of-the-art management of complications of myeloma and its treatment. Adv Hematol. 2010;2010:343089.
- World Health Organization. International Agency for Cancer Research. Global Cancer Observatory. 2018. [cited 2018 Oct 31]. available from: http://gco.iarc.fr/
- Terpos E, Morgan G, Dimopoulos MA, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. JCO. 2013;31:2347–2357.
- Hameed A, Brady JJ, Dowling P, et al. Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastas. 2014;7:33–42.
- Terpos E, Christoulas D, Gavriatopoulou M, et al. Mechanisms of bone destruction in multiple myeloma. Eur J Cancer Care. 2017;26:e12761.
- von Moos R, Strasser F, Gillessen S, et al. Metastatic bone pain: treatment options with an emphasis on bisphosphonates. Support Care Cancer. 2008;16:1105–1115.
- Cocks K, Cohen D, Wisloff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43:1670–1678.
- Terpos E, Berenson J, Cook RJ, et al. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia. 2010;24:1043–1049.
- Costa L, Badia X, Chow E, et al. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–889.
- Ashcroft J, Duran I, Hoefeler H, et al. Healthcare resource utilisation associated with skeletal-related events in European patients with multiple myeloma: results from a prospective, multinational, observational study. Eur J Haematol. 2018;100:479–487.
- Nash Smyth E, Conti I, Wooldridge JE, et al. Frequency of skeletal-related events and associated healthcare resource use and costs in US patients with multiple myeloma. J Med Econ. 2016;19:477–486.
- Bhowmik D, Hines DM, Intorcia M, et al. Economic burden of skeletal-related events in patients with multiple myeloma: analysis of US commercial claims database. J Med Econ. 2018;21:622–628.
- Bhowmik D, Song X, Intorcia M, et al. Examination of burden of skeletal-related events in patients naive to denosumab and intravenous bisphosphonates therapy in bone metastases from solid tumors population. Curr Med Res Opin. 2019;35:513–523.
- Nishida H. Bone-targeted agents in multiple myeloma. Hematol Rep. 2018;10:7401.
- Coleman R, Body JJ, Aapro M, et al. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25:iii124–iii137.
- Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica. 2015;100:1254–1266.
- European Medicines Agency. XGEVA (denosumab) summary of product characteristics. 2018. [cited 2018 Nov 29]. Available from: https://www.ema.europa.eu/documents/product-information/xgeva-epar-product-information_en.pdf
- European Medicines Agency. Zometa (zoledronic acid) summary of product characteristics. 2018. [cited 2018 Nov 29]. Available from: https://www.ema.europa.eu/documents/product-information/zometa-epar-product-information_en.pdf
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv52–iv61.
- Raje N, Terpos E, Willenbacher W, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–381.
- Mhaskar R, Kumar A, Miladinovic B, et al. Bisphosphonates in multiple myeloma: an updated network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD003188.
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692.
- Qian Y, Bhowmik D, Bond C, et al. Renal impairment and use of nephrotoxic agents in patients with multiple myeloma in the clinical practice setting in the United States. Cancer Med. 2017;6:1523–1530.
- Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. JCO. 2016;34:1544–1557.
- Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. JCO. 2018;36:812.
- National Comprehensive Cancer Network (NCCN). NCCN guidelines. Multiple myeloma (version 2, 2019). 2018. [cited 2018 Nov 30]. Available from: https://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092.
- Raje NS, Bhatta S, Terpos E. Role of the RANK/RANKL pathway in multiple myeloma. Clin Cancer Res. 2019;25:12–20.
- Calabro GE, La Torre G, de Waure C, et al. Disinvestment in healthcare: an overview of HTA agencies and organizations activities at European level. BMC Health Serv Res. 2018;18:148.
- Garrison LP, Jr, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017;20:213–216.
- Raje N, Roodman GD, Willenbacher W, et al. A cost-effectiveness analysis of denosumab for the prevention of skeletal-related events in patients with multiple myeloma in the United States of America. J Med Econ. 2018;21:525–536.
- Hechmati G, Cure S, Gouepo A, et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ. 2013;16:691–700.
- Cristino J, Finek J, Jandova P, et al. Cost-effectiveness of denosumab versus zoledronic acid for preventing skeletal-related events in the Czech Republic. J Med Econ. 2017;20:799–812.
- Henk HJ, Teitelbaum A, Perez JR, et al. Persistency with zoledronic acid is associated with clinical benefit in patients with multiple myeloma. Am J Hematol. 2012;87:490–495.
- Statistik Austria. Life tables. 2017. [cited 2018 Nov 20]. Available from: http://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/sterbetafeln/index.html
- European Health and Life Expectancy Information System. Life tables – Greece. 2015. [cited 2018 Nov 20]. Available from: http://www.eurohex.eu
- National Institute for Statistics (Italy). Life tables by single age group and gender. 2016. [cited 2018 Nov 20]. Available from: http://demo.istat.it/tvm2016/
- Statistics Belgium (STATBEL). Life expectancy and life tables. 2017. [cited 2018 Nov 30]. Available from: https://statbel.fgov.be/en/themes/population/life-expectancy-and-life-tables#panel-12
- Matza LS, Chung K, Van Brunt K, et al. Health state utilities for skeletal-related events secondary to bone metastases. Eur J Health Econ. 2014;15:7–18.
- Stopeck A, Rader M, Henry D, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. 2012;15:712–723.
- Matza LS, Cong Z, Chung K, et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Prefer Adher. 2013;7:855–865.
- van Agthoven M, Segeren CM, Buijt I, et al. A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised phase III study. Eur J Cancer. 2004;40:1159–1169.
- Walter E, Zehetmayr S. Guidelines zur gesundheitsökonomischen evaluation konsensuspapier [Guidelines for health-economic evaluation consensus paper]. Wien Med Wochenschr. 2006;156:628–632. [German].
- Cleemput I, Neyt M, Van de Sande S, et al. Belgian guidelines for economic evaluations and budget impact analyses. 2nd ed. Health Technology Assessment. Brussels (Belgium): Belgian Healthcare Knowledge Centre (KCE). 2012. KCE Report 183C. D/2012/10.273/54.
- National Institute for Health and Care Excellence (United Kingdom). Processing and methods guidance [PMG9]. Guide to the methods of technology appraisal. 2013. [cited 2018 Nov 20]. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781
- Capri S, Ceci A, Terranova L, et al. Guidelines for economic evaluations in Italy: recommendations from the Italian Group for Pharmacoeconomic Studies. Drug Info J. 2001;35:189–201.
- Warenverzeichnis (Austria). Official list price of drugs. 2018. [cited 2018 Nov 20]. Available from: https://warenverzeichnis.apoverlag.at/
- National Institute for Health and Disability Insurance – INAMI–RIZIV (Belgium). Reimbursed pharmaceutical drugs: list and reference files. 2018. [cited 2018 Nov 20]. Available from: https://www.inami.fgov.be/fr/themes/cout-remboursement/par-mutualite/medicament-produits-sante/remboursement/specialites/Pages/specialites-pharmaceutiques-remboursables-listes-fichiers-reference.aspx#.WRA3UlWLS70
- Ministry of Health (Greece). Corrected price list for medicines for human use, November 2017. 2018. [cited 2018 Nov 20]. Available from: http://www.moh.gov.gr/articles/times-farmakwn/deltia-timwn/5313-laquo-diorthwtiko-deltio-timwn-farmakwn-anthrwpinhs-xrhshs-noembrioy-2017-raquo
- Ministry of Health (Greece). Decree 1702/B/01.08. 2011. [cited 2018 Nov 20]. Available from: http://www.moh.gov.gr/articles/ken-eswteriko/710-ypoyrgikes-apofaseis-egkyklioi-g-g?fdl=2431
- Codifa (Italy). 2018. [cited 2018 Nov 28]. Available from: https://www.codifa.it/
- Official Gazette of the Italian Republic. General series. Ordinary supplement. 2013. [cited 2018 Nov 30]. Available from: http://www.gazzettaufficiale.it/
- Pereira J, Body JJ, Gunther O, et al. Cost of skeletal complications from bone metastases in six European countries. J Med Econ. 2016;19:611–618.
- Body JJ, Chevalier P, Gunther O, et al. The economic burden associated with skeletal-related events in patients with bone metastases secondary to solid tumors in Belgium. J Med Econ. 2013;16:539–546.
- Statistics Belgium (STATBEL). Consumer price index and health index. 2017. [cited 2018 Nov 20]. Available from: https://statbel.fgov.be/en/open-data/consumer-price-index-and-health-index
- European Central Bank. Euro reference exchange rate: Swiss Franc (CHF). Average: 1 January 2017 to 31 December 2017. [cited 2018 Nov 20]. Available from: https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-chf.en.html
- European Commission. Eurostat. Gross domestic product at market prices. 2017. [cited 2018 Nov 2]. Available from: https://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=en&pcode=tec00001&plugin=0&tableSelection=1
- European Network for Health Technology Assessment. Methods for health economic evaluations – a guideline based on current practices in Europe. 2015. [cited 2018 Nov 5]. Available from: https://www.eunethta.eu/wp-content/uploads/2018/03/Methods_for_health_economic_evaluations.pdf
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull WHO. 2015;93:118–124.
- Hutubessy R, Chisholm D, Edejer TT. Cost Eff Resour Alloc. 2003;1:8.
- Robinson LA, Hammitt JK, Chang AY, et al. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan. 2017;32:141–145.
- Orphanet. Orphanet report series: rare diseases collection. Rare disease registries in Europe. 2018. [cited 2018 Nov 8]. Available from: https://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf
- Nicod E, Annemans L, Bucsics A, et al. HTA programme response to the challenges of dealing with orphan medicinal products: process evaluation in selected European countries. Health Policy. 2019;123:140–151.
- Institute for Clinical or Economic Review (ICER). Assessing the effectiveness and value of drugs for rare conditions: a technical brief for the ICER orphan drug assessment & pricing summit. 2017. [cited 2018 Nov 5]. Available from: https://icer-review.org/wp-content/uploads/2017/02/ICER_Assessing-the-Value-of-Drugs-for-Rare-Conditions_051017.pdf
- National Institute for Health and Care Excellence (United Kingdom). NICE and NHS England consultation on changes to the arrangements for evaluating and funding drugs and other health technologies assessed through NICE’s technology appraisal and highly specialised technologies programmes. 2017. [cited 2018 Nov 5]. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/board-paper-TA-HST-consultation-mar-17-HST-only.pdf
- Raab MS, Cavo M, Delforge M, et al. Multiple myeloma: practice patterns across Europe. Br J Haematol. 2016;175:66–76.
- Kim C, Hernandez RK, Cyprien L, et al. Patterns of bisphosphonate treatment among patients with multiple myeloma treated at oncology clinics across the USA: observations from real-world data. Support Care Cancer. 2018;26:2833–2841.
- Lebret T, Casas A, Cavo M, et al. The use of bisphosphonates in the management of bone involvement from solid tumours and haematological malignancies – a European survey. Eur J Cancer Care. 2017;26:e12490.
- Allen N, Liberti L, Walker SR, et al. A comparison of reimbursement recommendations by European HTA agencies: is there opportunity for further alignment? Front Pharmacol. 2017;8:384.
- European Commission. Mapping of HTAs methodologies in EU and Norway. 2017. [cited 2018 Nov 5]. Available from: https://ec.europa.eu/health/sites/health/files/technology_assessment/docs/2018_mapping_methodologies_en.pdf
- Yfantopoulos J, Christopoulou A, Hatzikou M, et al. The importance of economic evaluation in healthcare decision-making – a case of denosumab versus zoledronic acid from Greece. Third-party payer perspective. Forum Clin Oncol. 2013;4:25–31.
- Koo K, Lam K, Mittmann N, et al. Comparing cost-effectiveness analyses of denosumab versus zoledronic acid for the treatment of bone metastases. Support Care Cancer. 2013;21:1785–1791.
- Dellis A, Papatsoris A. Cost-effectiveness of denosumab as a bone protective agent for patients with castration resistant prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2016;16:5–10.
- Shapiro CL, Moriarty JP, Dusetzina S, et al. Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases: CALGB 70604 (Alliance). JCO. 2017;35:3949–3955.
- Snedecor SJ, Carter JA, Kaura S, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J Med Econ. 2013;16:19–29.
- Snedecor SJ, Carter JA, Kaura S, et al. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther. 2012;34:1334–1349.
- Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17:1–386.
- Xie J, Diener M, Sorg R, et al. Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clin Breast Cancer. 2012;12:247–258.
- Xie J, Namjoshi M, Wu EQ, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. JMCP. 2011;17:621–643.
- Carter JA, Botteman MF. Health-economic review of zoledronic acid for the management of skeletal-related events in bone-metastatic prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2012;12:425–437.
- Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eur J Hosp Pharm. 2013;20:227–231.
- Cavo M, Terpos E, Bargay J, et al. The multiple myeloma treatment landscape: international guideline recommendations and clinical practice in Europe. Expert Rev Hematol. 2018;11:219–237.
- Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR Good Research Practices Task Force report. Value Health. 2015;18:161–172.