Abstract
Aim: To compare monthly healthcare resource utilization (HRU) and costs among adult patients with multiple myeloma (MM) receiving second or subsequent line of treatment (LOT) with carfilzomib or pomalidomide as monotherapy or in combination with dexamethasone.
Methods and materials: Adult MM patients who received carfilzomib or pomalidomide as second/subsequent LOT between 2006 and 2014 were selected from the MarketScan databases. LOT was determined using Medical/pharmacy claims using a published algorithm. For each patient, first LOT with carfilzomib or pomalidomide was defined as index LOT. Patients with first LOT as index LOT, who received other chemotherapy in combination with carfilzomib or pomalidomide, or who underwent stem cell transplant (STC) during index LOT were excluded. Monthly HRU and costs during index LOT were compared using inverse probability of treatment weights (IPTW) based on propensity scores for receipt of carfilzomib estimated by logistic regression with LOT, patient demographics, Charlson index, comorbidities, pre-index healthcare cost, and receipt of prior SCT as covariates.
Results: After weighting, baseline characteristics were well balanced among 114 carfilzomib and 144 pomalidomide patients. Mean (95% CI) numbers of outpatient visits per month were 7.1 (5.2–8.0) with carfilzomib and 4.7 (3.9–6.1) with pomalidomide (p = 0.006). Otherwise, there were no statistically significant differences between the groups in mean monthly HRU and costs or median time to therapy discontinuation. Mean (95% CI) monthly total healthcare costs were $19,776 (15,322–27,748) with pomalidomide and $17,321 (12,412–21,874) with carfilzomib (p = 0.522).
Limitations: Comparison of carfilzomib vs pomalidomide may be biased if there are unobserved factors not balanced by IPTW. The relatively small sample size limits the power of analyses to detect potential differences between treatment groups.
Conclusions: Monthly HRU and costs are similar among patients with relapse or refractory MM patients receiving carfilzomib or pomalidomide as monotherapy or in combination with dexamethasone.
Introduction
Multiple myeloma (MM) is a rare hematologic malignancy of plasma cells that is associated with a variety of complications, including—but not limited to—anemia, neutropenia, thrombocytopenia, bone loss and fractures, and kidney disease. In the US each year, ∼30,300 new cases of MM are diagnosed, and ∼12,600 persons die from the disease. Five-year survival for patients diagnosed with MM is ∼50%Citation1.
Current treatment guidelines recommend initiating therapy when patients become symptomatic with initial treatment individualized according to patient risk factors and transplant eligibilityCitation2. For patients who are refractory to or relapse following initial treatment, several treatment options have been approved in the last 5-years, giving patients and physicians the option to select treatment tailored based on individual patient characteristics. The National Comprehensive Cancer Network Clinical Practice Guideline recommends proteasome inhibitors (PIs: bortezomib, carfilzomib, and ixazomib), immunomodulators (IMiDs: lenalidomide, pomalidomide, and thalidomide), monoclonal antibodies (daratumumab, and elotuzumab), and histone deacetylase inhibitors (panobinostat) for patients with previously treated MMCitation2. Despite the multitude of options, the likelihood and duration of response decrease with each line of therapy (LOT), and the disease is ultimately fatalCitation2.
Carfilzomib was first approved in the US in July 2012 as monotherapy or with pre-dose dexamethasone and indicated for MM patients with ≥2 previous therapies, including bortezomib and IMiDs, who have demonstrated disease progression within 60 days after completing the last therapyCitation3. Pomalidomide was approved in February 2013, for MM patients who had relapsed within 60 days after receiving treatment, or were refractory to two prior therapies that included lenalidomide and PIsCitation4.
In the absence of head-to-head trials, real-world evidence (RWE) can be useful for conducting comparative analyses of the costs of novel treatments, including those for MM. It is important, however, that such analyses control for observed confounders, and utilize appropriate statistical methods to minimize selection bias that is inherent to observational research. A recent retrospective study by Chen et al.Citation5 using IMS PharMetrics Plus database compared healthcare outcomes and costs among MM patients receiving carfilzomib or pomalidomide as either monotherapy or in combination with other MM medications. Multivariate regression analyses were used to adjust for baseline demographic and certain clinical characteristics. The authors reported patients receiving pomalidomide had lower adjusted mean monthly costs during initial therapy. While this study provides important information on healthcare resource utilization (HRU) and costs in patients with MM, some limitations to the Chen et al. study may have impacted their findings. In particular, the comparison of carfilzomib vs pomalidomide in a heterogeneous population of patients receiving these therapies as either monotherapy or in combination with other MM therapies rather than as monotherapies may lead to conflation of the effects of carfilzomib and pomalidomide per se with those of other therapies used in the combinations. Also, if use of carfilzomib and pomalidomide as mono- vs combination therapy is associated with prognostic factors, then comparisons in patients receiving them as mono- or combination therapy may be more likely to be subject to confounding by unobserved factors than in a more homogeneous population receiving these treatments as monotherapy or in combination with dexamethasone. Finally, the multivariate regression analyses failed to adjust for LOT and other potentially important prognostic factors such as pre-index healthcare utilization and/or costs.
The objective of this study was to conduct a comparison of HRU and costs between patients receiving carfilzomib and pomalidomide as monotherapy or in combination with dexamethasonee. To control for differences in baseline demographic and clinical characteristics including LOT, we used stabilized inverse probability of treatment weights (sIPTW).
Methods
Study subjects
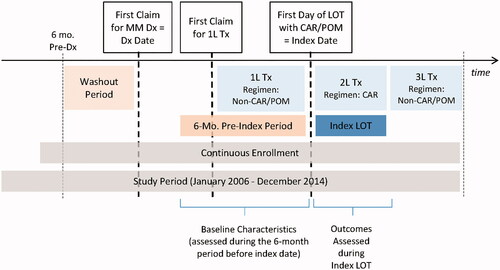
This study used Truven Analytics MedStat MarketScan Commercial Claims and Encounters (CCAE) and Medicare and Coordination of Benefits (MDCR) databases from January 1, 2006 to December 31, 2014 (study period). The databases contain enrollment information and information on the health insurance claims of employees of large, self-insured corporations and their dependents, along with a few commercial health plans (CCAE) and for Medicare-eligible persons (mainly retirees) who are also covered by self-insured employers (MDCR). The databases are fully de-identified and compliant with the Health Insurance Portability and Accountability Act of 1996. To identify the study population, patients with a confirmed diagnosis of MM (ICD-9-CM code 203.0x) who initiated second or subsequent LOT with carfilzomib or pomalidomide were selected. A confirmed diagnosis was defined as ≥1 inpatient or any combination of ≥2 inpatient professional service claims or non-diagnostic outpatient claims with a diagnosis of MM during the study period. For each patient, the entire follow-up was divided into LOTs by tracking medical and pharmacy claims for carfilzomib, pomalidomide, lenalidomide, thalidomide, bortezomib, and other MM chemotherapies on or after the date of the first MM diagnosis (diagnosis date). For each LOT, the regimen was defined by MM therapies received within 90-days of LOT initiation. The initiation of the second LOT was identified based on evidence of a new MM therapy received >90 days after initiation of the first LOT, or >90-day gap in therapy. The subsequent new LOTs were identified similarly. For each patient, the first LOT with carfilzomib or pomalidomide was defined as the index LOT and the date of initiation of this LOT was defined as the index date. A schematic of the study is shown in .
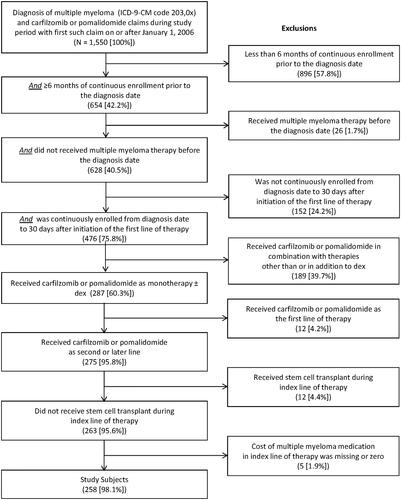
Subjects were excluded if they had any of the following: (1) Any gap in enrollment (≥1 day) during the period beginning 6 months prior to the diagnosis date and ending 1 month after initiation of the first MM therapy; (2) MM therapy or unclassified antineoplastic medication before diagnosis date; (3) age ≤18 years at initiation of MM therapy; (4) carfilzomib or pomalidomide as the first LOT; (5) carfilzomib or pomalidomide in combination with other chemotherapies (excluding dexamethasone) in the index LOT; (6) SCT during the index LOT; or (7) missing cost information for the index LOT.
On and off therapy periods
For each patient, the index LOT was separated into on- and off-therapy periods. Since carfilzomib and pomalidomide are administered in 28-day cycles with the last carfilzomib infusion occurring on day 16 and the last oral administration of pomalidomide on day 21, the on-therapy period was defined as (1) the index date through 12 days after the last infusion for patients receiving carfilzomib or through 7 days after the last day supply among patients receiving pomalidomide, (2) initiation of a new LOT, or (3) the end of continuous enrollment, whichever occurred first. The “off-therapy period” was defined as the remaining time in the index LOT. If the index LOT was the last LOT for the patient, and the last day on therapy was within 90 days before the end of the continuous enrollment period, the “on-therapy period” was defined as the entire duration of the index LOT.
Patient characteristics
Baseline patient characteristics were assessed as of index date and included: year of LOT initiation (2014 vs earlier); age (in years); gender; US Census region (South vs Northeast, North central, or West/unknown); and plan type (PPO vs comprehensive, HMO, or other). The following patient characteristics were also assessed based on claims during the 6 month period prior to the index date: (1) the Charlson Comorbidity Index–National Cancer Institute (NCI) Modification; (2) comorbid conditions including anemia, secondary malignancy (hematologic cancer other than MM or solid tumor), chronic obstructive pulmonary disease, chronic liver disease, chronic pain, chronic renal disease, coronary heart disease, dementia, diabetes, and hypertension; (3) number of emergency department visits, physician office or outpatient visits, outpatient pharmacy claims (i.e. prescriptions), hospitalizations, and inpatient days; and (4) outpatient, pharmacy, inpatient, and total healthcare costs. Costs were assessed based on total payments (including patient contributions). As the NCI modified Charlson Comorbidity Index does not include cancer in its calculation, history of solid tumor and hematologic cancer other than MM were included as separate comorbidity categories. The diagnosis codes, procedure codes, and medications used to identify comorbidities are provided in the Appendix.
Outcome measures
Measures of HRU included the number of emergency department visits, the number of physician office or outpatient hospital visits, the number of outpatient pharmacy claims (i.e. prescriptions), and the number of hospitalizations. Cost measures were classified as MM-related and non MM-related. MM-related costs included MM therapy medications, MM therapy administration, MM complications and adverse events (AEs), and other MM-related costs. Total MM-related and non-MM-related costs were also calculated. MM therapy medications included claims for corticosteroids. MM therapy administration claims were identified based on procedure codes for chemotherapy administration that were accompanied by a MM therapy claim on the same date. MM complications and AEs were identified based on the following criteria: (1) presence of one or more inpatient admission records with a primary diagnosis code for the complication or event; (2) two or more outpatient claims with a diagnosis code for the complication or event; (3) one or more outpatient claims with a procedure code related to the treatment of the complication or event; or (4) one or more outpatient pharmacy claims for a medication for the treatment of the complication or event. For each patient with a complication or an AE, the cost of the complication or AE was calculated as the sum of payments for all claims associated with the complication or AE during the evaluation period. Complications of MM that were considered in the analysis were identified based on complications reported in published guidelinesCitation2. AEs were identified based on package inserts for carfilzomib, pomalidomide, lenalidomide, thalidomide, and bortezomibCitation4,Citation6–9. “Other MM-related costs” included payments for claims with a diagnosis of MM that were not classified as costs of MM therapy medications or administration or MM complications or AEs.
The measures above were calculated separately over the entire index LOT, as well as for the corresponding on- and off-therapy periods. Measures of HRU and costs were reported on a monthly basis to account for differential follow-up. For total and MM-related healthcare costs, results were also reported by time since initiation of LOT. Time to therapy discontinuation was defined as the duration of the on-therapy period. Patients were censored if the LOT was the last line for the patient and the last day on therapy was within 90 days of the last day of continuous enrollment.
Analyses
Stabilized IPTWs were used to derive average treatment effect (ATE) estimates for study outcomes in order to adjust for differences between groups in baseline characteristicsCitation10. With this approach, a propensity score is estimated for each patient, which represents a subject’s probability of treatment selection (e.g. carfilzomib or pomalidomide), conditioned on all observed baseline covariates. Weighting subjects by the inverse of the probability of treatment creates a synthetic sample in which treatment assignment is independent of these covariatesCitation10. If there are no residual differences in observed or unobserved baseline characteristics between treatment groups after weighting, this approach yields unbiased estimates of average treatment effects. In this analysis, the propensity score for receipt of carfilzomib was estimated using a multivariable logistic regression model conditioned on age, gender, region, plan type, LOT, Charlson comorbidity index, comorbidities, receipt of SCT, year of index LOT initiation, and pre-index healthcare utilization and costs, and number of months since last date with prior therapy. Because multiple outcome measures were examined which might have different relationships with baseline characteristics, we used a non-parsimonious model for calculating propensity scores. IPTWs were then calculated using the propensity scores. Stabilized weights were used to preserve the original sample size in the weighted population. The balance of baseline characteristics was evaluated by calculating standardized differences between groups in these characteristics for the unweighted and weighted populationsCitation10. Descriptive statistics on HRU and costs were reported by treatment group for the unweighted and weighted samples. Differences between treatment groups in mean healthcare utilization and costs were reported. p-values for the difference in means were calculated using the standard deviation of 2,000 bootstrap estimates. Kaplan-Meier estimates of time to therapy discontinuation for the unweighted and weighted samples were calculated for each group and the p-value for the log-rank statistics was reported. All analyses were conducted using SAS Proprietary Software, Release 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient selection and characteristics
There were 1,550 patients with a confirmed diagnosis of MM and with one or more claims for carfilzomib or pomalidomide between January 1, 2006 and December 31, 2014 (). There were two patients with evidence of receipt of carfilzomib or pomalidomide in 2012, and 840 and 974 patients in 2013 and 2014, respectively. Of these patients, 258 patients qualified for the study, including 114 who received carfilzomib and 144 who received pomalidomide.
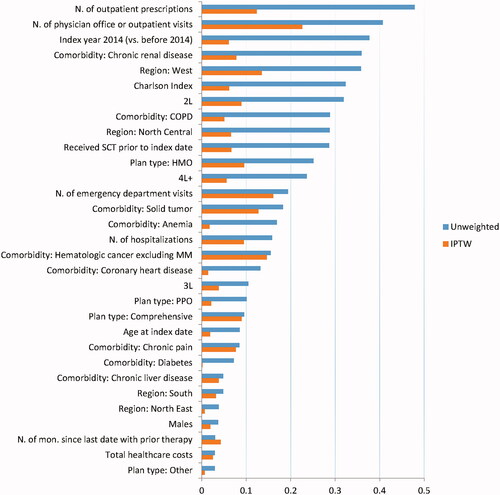
Patient characteristics at the index date for the unweighted and weighted populations are reported in . The sums of the stabilized IPTWs were 139 and 108 for the pomalidomide and carfilzomib groups, respectively. The corresponding effective sample sizes were 101 and 73 patients, respectivelyCitation11. Prior to weighting, the mean age was similar (carfilzomib 67 years and pomalidomide 68 years), as was the percentage of males (carfilzomib 59% and pomalidomide 57%). However, patients in the carfilzomib group were more likely to receive index treatment in second vs subsequent LOT, initiate LOT after 2013, be male, and have chronic renal disease, anemia, and diabetes. Patients in the pomalidomide group were more likely to have prior SCT, chronic pain, and coronary heart disease.
Table 1. Baseline patient characteristics for unweighted and weighted study population.
Standardized differences between treatment groups in baseline characteristics for the unweighted and weighted populations are depicted in . Prior to weighting, there were relatively large standardized differences in baseline characteristics, with most baseline characteristics having standardized differences ≥0.10 and many with differences ≥0.20. After weighting, the sample was well balanced for most baseline characteristics, with standardized differences <0.10 for most characteristicsCitation10,Citation12.
HRU and costs
There were no statistically significant differences between groups in mean monthly total and MM-related healthcare costs for the unweighted or weighted samples (). In the unweighted population, mean (SE) monthly total healthcare costs were $18,905 ($1,433) with pomalidomide vs $18,972 ($1,543) with carfilzomib (p = 0.98). In the weighted population, mean (SE) monthly total healthcare costs were $19,776 ($3,079) with pomalidomide vs $17,321 ($2,307) with carfilzomib (p = 0.52). The annual hospitalization rate was higher among patients receiving pomalidomide vs those receiving carfilzomib (Unweighted: 59% vs 47%, p = 0.042; weighted: 60% vs 40%, p = 0.25). Mean monthly number of office and outpatient visits were greater for carfilzomib vs pomalidomide (Unweighted: 7.5 vs 4.5, difference 3.0, p < 0.001; weighted: 7.1 vs 4.7, difference 2.4, p = 0.006), as were the mean monthly administration costs (Weighted: $634 vs $6, difference $628, p < 0.001). Conversely, mean numbers of prescriptions per month were greater among pomalidomide patients vs carfilzomib patients in both the unweighted and weighted populations. These findings are consistent with the modes of administration of the two therapies (IV for carfilzomib and oral for pomalidomide). Total costs of carfilzomib and pomalidomide medications, chemotherapy administration, and adverse events or complications of MM were $15,067 (95% CI = 12,303–17,831) for carfilzomib and $14,027 (95% CI = 12,033–16,022) for pomalidomide in the unweighted population (p = 0.55). Corresponding values for the weighted samples were $13,471 (95% CI = 8,592–16,887) and $14,535 (95% CI = 11,140–21,145), respectively (p = 0.75).
Table 2. Healthcare resource utilization and costs for the unweighted and weighted study population.
During the follow-up time when patients were on therapy, mean (SE) monthly total healthcare costs were $20,259 ($2,302) with pomalidomide vs $21,038 ($1,543) with carfilzomib in the unweighted population (p = 0.78). Corresponding results for the weighted population were $20,656 ($2,504) with pomalidomide vs $18,986 ($3,198) with carfilzomib (p = 0.68). In the unweighted population monthly medication costs while on therapy were $11,418 with pomalidomide vs $10,795 with carfilzomib (p = 0.49). In the weighted population, monthly medication costs while on therapy were $11,275 with pomalidomide vs $10,612 with carfilzomib (p = 0.82). Unweighted and weighted monthly chemotherapy administration costs while on therapy were both $0 with pomalidomide vs $960 and $869 with carfilzomib (p < 0.001 for both unweighted and weighted). Total costs of carfilzomib and pomalidomide medications, chemotherapy administration, and adverse events or complications of MM during the on treatment period were $17,404 for carfilzomib and $15,343 for pomalidomide in the unweighted population (p = 0.21). Corresponding values for the weighted population were $15,774 and $15,291, respectively (p = 0.89).
During the follow-up time when patients were off therapy, mean (SE) monthly healthcare costs were $15,488 ($3,197) with pomalidomide vs $14,126 ($3,107) with carfilzomib (p = 0.76) in the unweighted population. After weighting, mean (SE) monthly healthcare costs were $17,753 ($6,472) with pomalidomide vs $12,477 ($4,007) with carfilzomib (p = 0.49).
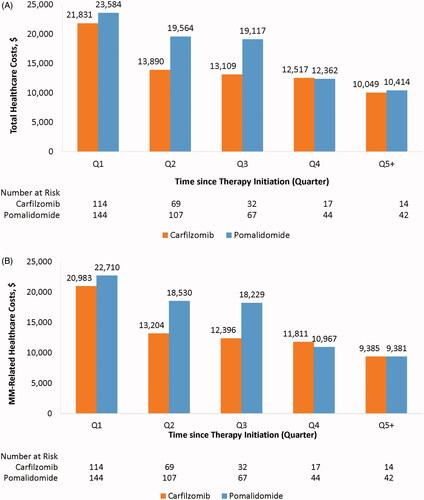
Based on the weighted sample, mean monthly total and MM-related costs were nominally higher during the first three quarters after the index date among patients receiving pomalidomide compared with patients receiving carfilzomib, although these differences were not statistically significant ().
Time to therapy discontinuation
Kaplan-Meier estimates of median time to therapy discontinuation for the unweighted sample were 4.8 months and 7.3 months for carfilzomib and pomalidomide, respectively (log-rank p = 0.0250). When adjusted for differences between the two groups with sIPTW, they were 5.4 months for carfilzomib and 5.7 months for pomalidomide (log rank p = 0.1617).
Discussion
In this retrospective, observational study using large health insurance claims databases, there was no statistically significant difference in mean monthly total healthcare costs between patients receiving carfilzomib monotherapy or with dexamethasone vs pomalidomide monotherapy or with dexamethasone after adjusting for baseline characteristics using IPTW. Results were similar when attention was focused only on costs of medications, chemotherapy administration, and treatment of adverse events and complications of MM while patients were on therapy.
These findings differ from a recent retrospective study comparing healthcare outcomes and costs in MM patients receiving carfilzomib or pomalidomide as mono- or combination therapy using health insurance claims data from the IMS PharMetrics Plus databaseCitation5, which reported that pomalidomide recipients had lower adjusted mean monthly costs during initial therapy and longer adjusted median time to next treatment.
In contrast with the prior study by Chen et al.Citation5, which included patients receiving carfilzomib or pomalidomide in combination with other MM medications besides dexamethasone, our study focused only on patients receiving carfilzomib or pomalidomide as monotherapy or in combination with dexamethasone. Since the choice of monotherapy vs combination therapy may be related to patient prognosis, our focus on more homogeneous patients may reduce the likelihood of confounding by unobserved factors. Also, while the prior study used multivariable regression analysis to control for potential confounders, it failed to adjust for some potentially important factors including LOT and pre-index healthcare costs. While we did not examine the association between these factors and outcomes, we did find that these factors were associated with the receipt of carfilzomib vs pomalidomide, and controlled for them using sIPTW.
Before weighting, patients in the carfilzomib group were more likely than those in the pomalidomide group to be receiving earlier LOTs and have a higher Charlson Comorbidity index score. Carfilzomib patients also had shorter median time to discontinuation and lower likelihood of having received a prior stem cell transplant. LOT is a prognostic factor and earlier lines of therapy are expected to be associated with a longer time to next treatment; however, this assumes that all other factors are balanced. This was not the case between patients treated with carfilzomib and pomalidomide, which underlines the necessity to weight the comparison groups to minimize the effect of other patient characteristics. After weighting, the distribution of patients by line of therapy, and median time to therapy discontinuation were similar for patients receiving carfilzomib and pomalidomide in the weighted samples (5.4 months vs 5.7 months, p-value = 0.1617). Mean monthly costs of carfilzomib administration per LOT were $681 in the unweighted population and $634 in the weighted population. The $681 represents the average monthly costs for the entire LOT including both on- and off-treatment periods. The monthly cost of administration for patients receiving carfilzomib during the on-treatment period only was $960 for the unweighted population and $886 for the weighted population. The average cost for claims with CPT code 96409 (Injection and Intravenous Infusion Chemotherapy and Other Highly Complex Drug or Highly Complex Biologic Agent Administration) among carfilzomib patients was $192. This suggests that patients received ∼4.7 claims per month for administration of carfilzomib. This result is consistent with the recommended frequency of carfilzomib dosing of once per week for 3 weeks or twice per week for 3 weeks with 1 week off: i.e. 3–6 doses per month. Costs for other services besides administration of carfilzomib are likely included in other MM-related costs
Limitations of our study should be noted. First, comorbid conditions were not assessed by thepresence of confirmed diagnosis, but based only on the presence of diagnosis/procedure codes. As a result, patients with additional hematologic and/or solid malignancies may include those who received workup procedures but who did not have clinically confirmed conditions. As with all studies using health insurance claims data, identification of study subjects, assessment of their baseline characteristics, and measurement of study outcomes are subject to possible coding errors in enrollment information and coding of diagnosis and procedure codes on health insurance claims. However, we have no reason to believe that the extent of the errors would differ across treatment groups or otherwise bias the comparison of carfilzomib and pomalidomide. Although patients in the MarketScan databases are geographically diverse, they may not be representative of all patients in the US. This study only covered data up to 2014, and, therefore, the sample size was relatively small and the power to detect differences between groups was limited. Further research may be warranted when additional patients have received these treatments in clinical practice. Although we attempted to control for baseline characteristics using IPTW, data on some clinical characteristics such as genetic abnormalities and β2-microglobulin or International Staging System (ISS) were unavailable, and the possibly of residual confounding based on these unobserved factors must be recognized. Additionally, although patients in the two groups were well matched on observed characteristics after IPTW weighting, some residual differences persisted after weighting, and these residual differences could have biased our findings.
Because the MarketScan databases do not include information on clinical outcomes such as disease progression or mortality, it was not possible to compare survival for patients receiving carfilzomib or pomalidomide. We used time to discontinuation as a proxy measure of clinical outcome, as patients are likely to continue therapy until disease progression or adverse events. Because the precise treatment discontinuation date is not available in databases, patients were censored for time to discontinuation if the last claim for treatment was within 90 days of the end of continuous enrollment. To the extent that some patients may interrupt treatment for >90 days, we may have under-estimated the treatment time (because some patients who were considered in this analysis to have stopped treatment in fact only interrupted treatment). If the likelihood of treatment interruption differs between the treatment arms, the comparison might be biased. Also, because it is not possible to distinguish in the dataset between health plan disenrollment and death, censoring on disenrollment may result in an over-estimation of actual time to discontinuation (because some patients who were censored died and, therefore, discontinued treatment). If the proportion of patients who died, as opposed to dis-enrolled alive, differed between the treatment arms, this could also bias the comparison. The comparison of time to discontinuation must, therefore, be interpreted cautiously.
Patients in the carfilzomib group had a higher mean number of office and outpatient visits than those in the pomalidomide group. We were unable to assess the differences between groups on any indirect consequences (positive or negative) associated with additional visits.
Conclusions
Monthly HRU and costs do not vary significantly for MM patients receiving carfilzomib or pomalidomide as monotherapy or in combination with dexamethasone for the second or subsequent LOT in typical clinical practice. In the absence of conclusive evidence of difference in outcomes and costs, therapy decisions should be made on the basis of individual patient characteristics and clinical considerations to generate the best outcomes for patients.
Transparency
Declaration of funding
This study was sponsored by Amgen Inc.
Declaration of financial/other interests
MH is an employee of PAI and TED is a partner at PAI; AS was employed by PAI during the conduct of this study. PAI received funding from Amgen Inc. for study design, data programming, and analyses. SP is an employee of, and owns stock in, Amgen Inc. The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a JME peer reviewer has disclosed that they have received research funding from Takeda and Amgen; are a member of speaker’s bureaus for Takeda, Amgen, Celgene, Janssen, and BMS; and are a consult for PharmaMar. The authors and JME peer reviewers on this manuscript have no other relevant financial or other relationships apart from those disclosed.
Acknowledgements
None reported.
Previous peer-reviewed presentation
58th ASH Annual meeting and exposition at San Diego, December 3–6, 2016.
References
- American Cancer Society. Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2017. Accessed 2017 July. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf.
- National Comprehensive Cancer Network. The NCCN Guidelines® Version 3. Multiple Myeloma. 2017 [cited 2017 Apr 10]. Available from: https://www.nccn.org/professionals/physician_gls/PDF/myeloma.pdf.
- Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825.
- Celgene Corporation. Highlights of Prescribing Information for Pomalyst. 2016 [cited 2017 Jul 7]. Available from: https://media.celgene.com/content/uploads/pomalyst-pi.pdf.
- Chen CC, Parikh K, Abouzaid S, et al. Real-World treatment patterns, time to next treatment, and economic outcomes in relapsed or refractory multiple myeloma patients treated with pomalidomide or carfilzomib. JMCP. 2017;23:236–246.
- Amgen. Highlights of Prescribing Information for Kyprolis. [cited 2017 Jul 7]; 2012. Available from: http://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/kyprolis/kyprolis_pi.ashx.
- Celgene Corporation. Highlights of Prescribing Information for Revlimid. 2015 [cited 2017 Jul 7]. Available from: https://media.celgene.com/content/uploads/revlimid-pi.pdf.
- Celgene Corporation. Highlights of Prescribing Information for Thalomid. [cited 2017 Jul 7]; 2017. Available from: https://media.celgene.com/content/uploads/thalomid-pi.pdf.
- Millennium Pharmaceuticals. Highlights of Prescribing Information for Velcade. [cited 2017, July 7]; 2017. Available from: http://www.velcade.com/files/pdfs/velcade_prescribing_information.pdf.
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statist Med. 2009;28:3083–3107.
- Kish L. Survey sampling. New York (NY): Wiley; 1965.
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679.