Abstract
Objective: To develop an economic model to evaluate changes in healthcare costs driven by restricting usage of branded tyrosine kinase inhibitors (TKIs) through substitution with generic imatinib among chronic myeloid leukemia (CML) patients in a typical Oncology Care Model (OCM) practice, and examine the impact on Performance-Based Payment (PBP) eligibility.
Methods: An Excel-based economic model of an OCM practice with 1,000 cancer patients during a 6-month episode of care was developed. Cancer types and proportions of patients treated in the practice were estimated from an OCM report. All-cause healthcare costs were obtained from published literature. It was assumed that if a practice restricts usage of branded TKIs for newly-diagnosed CML patients, 80% of the market share of branded imatinib and 50% of the market shares of 2nd-gen TKIs would shift to generic imatinib. Among established TKI-treated patients, it was assumed that 80% of the market share of branded imatinib and no patients treated with 2nd-gen TKIs would shift to the generic.
Results: Four CML patients were estimated for a 1,000-cancer patient OCM practice with a total baseline healthcare cost of $51,345,812 during a 6-month episode. If the practice restricts usage of branded TKIs, the shift from 2nd-gen TKIs to generic imatinib would reduce costs by $12,970, while shifting from branded to generic imatinib lowers costs by $25,250 during a 6-month episode. Minimum reductions of $3,013,832 in a one-sided risk model and $2,372,010 in a two-sided risk model are required for PBP eligibility; the shift from 2nd-gen TKIs to generic imatinib would account for 0.4% and 0.5% of the savings required for a PBP, respectively.
Conclusions: This analysis indicates that the potential cost reduction associated with restricting branded TKI usage among CML patients in an OCM setting will represent only a small proportion of the cost reduction needed for PBP eligibility.
Introduction
With the objective of reducing cancer care costs in the US, the Center for Medicare and Medicaid Services (CMS) Innovation Center has implemented the Oncology Care Model (OCM)Citation1. A primary goal of the OCM under the Medicare Access and CHIP Reauthorization Act (MACRA) is to provide an alternative payment model (APM), which is based on a 6-month episode of cancer therapyCitation1–3. In addition to Medicare fee-for-service payments, the OCM incorporates a two-part payment system for participating practices: (1) a $160 per-beneficiary Monthly Enhanced Oncology Services (MEOS) payment, and (2) a Performance-Based Payment (PBP) based on a 6-month episode of careCitation1–3. Healthcare providers participating in the OCM are expected to deliver quality healthcare while reducing spending in targeted areas.
Chronic myeloid leukemia (CML) is a rare form of progressive cancer with approximately 8,400 new cases diagnosed each year in the US (0.5% of all new cancer cases)Citation4. It was estimated to affect 70,000 lives in 2010 in the US and is projected to affect up to 112,000 by 2020; its prevalence is predicted to remain increasing until 2050 when it is likely to reach a plateauCitation5. CML is categorized into three phases: the chronic phase, accelerated phase, and blast phase, the latter being the most severe phase of the diseaseCitation6. The National Comprehensive Cancer Network (NCCN) has recommended the first-generation (1st-gen) tyrosine kinase inhibitor (TKI), imatinib, and the second-generation (2nd-gen) TKIs, dasatinib, and nilotinib, as first line therapies for the treatment of chronic phase CMLCitation7. TKIs target against the BCR-ABL oncoprotein, which plays a primary role in the pathogenesis of CMLCitation6. The usage of TKIs for the treatment of CML has resulted in this cancer becoming a manageable, but chronic diseaseCitation7–10, which largely accounts for the aforementioned increasing prevalence over the next 30 years in the US. Thus, the majority of CML patients are considered as established on a TKI and chronically treated or newly-diagnosed. Despite having sustainable improved long-term outcomes, the clinical management of patients with CML can be associated with extensive healthcare resource utilization and costs, especially if progression occursCitation11; the incremental cost of CML progression has been estimated at $136,308 per patient per year vs patients without progressionCitation11.
With the introduction of generic imatinib to the US market for the treatment of CML in 2016, there is an interest in understanding whether shifting patients from branded TKIs to generic imatinib, with its lower costs and manageable safety profile, may help OCM practices receive a PBP. Therefore, we developed an economic model to evaluate changes in healthcare costs driven by restricting usage of branded TKIs through generic substitution among CML patients in a typical OCM practice and additionally evaluated the impact of such changes on the eligibility of the practice for receiving a PBP.
Methods
Model description
An Excel-based economic model of an OCM practice that treats 1,000 cancer patients during a 6-month episode of care was developed. The model analyzed changes in costs associated with implementing substitution of branded imatinib and 2nd generation TKIs (dasatinib and nilotinib) with generic imatinib among patients with CML.
Model inputs and assumptions
The proportions of patients treated for various cancer types in the OCM practice were obtained from the first CMS annual report of the OCM, published February 1, 2018Citation3. The all-cause healthcare costs for each cancer type were obtained from published literature and publicly available sourcesCitation12–21, and inflation adjusted to 2018 USD. CML patients were stratified into newly-diagnosed/TKI treated patients and established TKI treated patientsCitation22. The percentages of newly-diagnosed and established CML patients treated with each of the TKIs (branded and generic imatinib, dasatinib and nilotinib) were estimated using 2018 market share dataCitation23. The TKI market shares for established TKI treated patients were estimated from the market shares of total TKI prescriptions shares. The 2018 Wholesale Acquisition Costs (WACs) for the TKIs were obtained from RedBookCitation24.
If an OCM practice implements the policy of restricting usage of branded TKIs as a cost-cutting measure, it was assumed that, among newly-diagnosed CML patients, 80% of the current market share of branded imatinib would shift to generic imatinib and 50% of the current market shares of 2nd-gen TKIs would shift to generic imatinib. Among established TKI treated CML patients, it was assumed that 80% of the current market share of branded imatinib would shift to the generic, whereas no patients treated with 2nd-gen TKIs would be switched to generic imatinib due to the lack of supporting evidence, physician and patient apprehension, and some patients already having used imatinib in prior lines of treatment, among other reasons.
Estimation of cost reductions associated with implementing a policy of restricting usage of branded TKIs among CML patients and the relationship with eligibility for a PBP
The TKI treatment costs for 6 months prior to (baseline period) and 6 months following (future episode) implementation of the policy restricting usage of branded TKIs were estimated using 30-day TKI WACs and estimates of CML patients in each TKI group treated in an OCM, based on market share data. Changes in costs driven by changes in TKI usage (i.e. branded to generic imatinib shift and 2nd-gen TKI to generic imatinib shift) were estimated for newly-diagnosed and established CML patients. Next, the relationship between the cost reductions achieved from restricting usage of branded TKIs and the total cost reduction threshold required for the OCM practice to receive a PBP using either a one-sided or two-sided risk model was determined. The one-sided risk model requires a 4% cost reduction threshold for PBP eligibility, while the two-sided risk model requires a 2.75% cost reduction thresholdCitation24. For both scenarios, a 100% performance multiplier was assumed and a CMS budget sequestration adjustment of −2% was applied, as specified by the OCM policyCitation25.
Sensitivity analysis
We also carried out a sensitivity scenario analysis in which the shift from a 2nd-gen TKI to generic imatinib was assumed to be 16% instead of 50% for newly-diagnosed CML patients, since 16% is the current estimated rate of Medicare Prior Authorization rejection. All other model inputs remained the same as in the default analysis.
Results
Estimated total healthcare costs in an OCM practice
Based on the number of patients treated for each cancer type in a typical OCM practice and the healthcare costs reported in the literature and other publicly available sources, the total all-cause healthcare costs of a 1,000-patient OCM practice for 6 months were estimated at $51,345,812 (). The number of CML patients treated in the 1,000-patient OCM practice was estimated to be four, with one newly-diagnosed/TKI treated patient and three established TKI treated patients. Thus, only 0.4% ($225,108) of the total all-cause healthcare costs of the OCM during 6 months were attributed to the treatment of CML patients.
Table 1. Cancer types treated in a typical OCM practice and associated all-cause healthcare costs.
TKI market shares and estimated number of CML patients with change in TKI drug usage
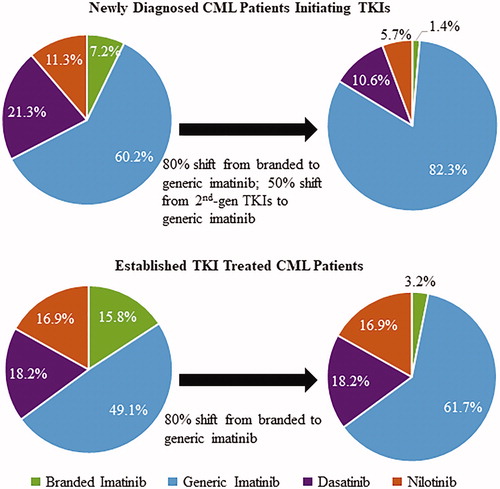
The economic model showed that, once the policy of restricting branded TKI usage is implemented, 1.4% of newly-diagnosed/TKI treated CML patients would be treated with branded imatinib, 82.3% with generic imatinib, 10.6% with dasatinib, and 5.7% with nilotinib in a future 6-month episode of care (). For established TKI treated CML patients, 3.2% would be treated with branded imatinib, 61.7% with generic imatinib, 18.2% with dasatinib, and 16.9% with nilotinib in a future 6-month episode of care (). Accounting for the number of CML patients in the OCM practice, the number of patients with a shift from branded to generic imatinib was estimated to be 0.43, and the number of newly-diagnosed patients with a shift from 2nd-gen TKIs to generic imatinib was estimated to be 0.17; thus, a total of 0.60 patients were estimated, on average, to have a change in TKI drug usage in a future episode of care.
Estimated cost reduction associated with restricting usage of branded TKIs
The 2018 30-day WACs of the TKI drugs and the 6-month episode treatment costs for CML patients in the OCM are shown in . It was estimated that, if the OCM practice restricts usage of branded TKIs, healthcare costs would be reduced by a total of $38,220 for a 6-month episode. The majority of this net cost reduction (66%; $25,250) would come from a branded to generic imatinib shift, while only 34% ($12,970) would come from 2nd-gen TKIs to generic imatinib shift ().
Table 2. TKI treatment costs of CML patients (newly-diagnosed and established) prior to (baseline episode) and after (future episode) implementing a policy of restricting usage of branded TKIs.
Table 3. Cost reductions associated with changes in TKI usage relative to the OCM cost reduction threshold requirement for a PBP.
Relationship of estimated cost reductions with eligibility for a PBP
illustrates cost reductions associated with changes in TKI usage relative to the OCM total cost reduction threshold requirement for PBP eligibility. For a 1,000-cancer patient OCM practice participating in a one-sided OCM risk model, a total cost reduction of $3,013,832 was estimated to be required to be eligible for a PBP. In this scenario, the cost reduction associated with a shift from 2nd-gen TKIs to generic imatinib amounts to only 0.4% of the required threshold before the practice is eligible for a PBP. For a 1,000-cancer patient OCM practice participating in a two-sided OCM risk model, a total cost reduction of $2,372,010 is required to be eligible for a PBP. In this case, the cost reduction associated with a shift from 2nd-gen TKIs to generic imatinib amounts to only 0.5% of the required threshold before the practice is eligible for a PBP.
Sensitivity analysis
In the sensitivity scenario analysis in which the shift from a 2nd-gen TKI to generic imatinib was assumed to be 16% instead of 50% for newly-diagnosed CML patients, the results were similar to that of the default analysis. In this scenario, it was estimated that, if the OCM practice restricts usage of branded TKIs, healthcare costs would be reduced by a total of $29,400 for a 6-month episode (). The majority of this net cost reduction (86%; $25,250) would come from a branded to generic imatinib shift, while only 14% ($4,150) would come from 2nd-gen TKIs to generic imatinib shift (). In an OCM practice with a one-sided risk model, the cost reduction associated with a shift from 2nd-gen TKIs to generic imatinib amounts to only 0.1% of the required threshold before the practice is eligible for a PBP; in a two-sided risk model it amounts to only 0.2%.
Table 4. Sensitivity analysis: cost reductions associated with changes in TKI usage relative to the OCM cost reduction threshold requirement for a PBP.
Discussion
The findings of this economic model indicate that the cost reduction associated with restricting usage of branded TKIs among CML patients in an OCM setting will represent only a very small portion of the cost reduction threshold required before an OCM practice is eligible for a PBP. Furthermore, of the potential reduction in TKI costs, more than two-thirds was attributed to the shift from branded to generic imatinib. Only 0.4% and 0.5% of the total cost reduction required for PBP eligibility for a one-sided and two-sided risk OCM, respectively, are predicted to come from restricting usage of the 2nd-gen TKIs. This smaller proportion of the PBP eligibility cost threshold is largely due to the rarity of CML in routine clinical practice and the limited number of individuals affected by formulary management. However, if another cost reduction could be achieved among other cancer patients also, the combined cost reduction may help achieve the PBP eligibility cost threshold in the OCM model. In efforts towards reducing the overall cancer care cost, steps beyond the simple shifting from branded to generic drugs should be considered to achieve a potentially lower total healthcare cost while maintaining high-quality cancer care.
Identifying the most strategic and appropriate cost-cutting measures is of primary concern for OCM practices, since the PBP is directly connected to reduced spending in practice, as well as accountable delivery of high-quality cancer care. Having different treatment options available, especially in the case of CML, can be beneficial to both patient outcomes and providers’ budgets. For instance, CML progression, a very costly eventCitation11, has a high dependency on the genetic mutations that a patient may have or acquire during treatment, alongside other individual patient characteristicsCitation6,Citation7. These genetic mutations may differentially impact the response to treatment with the different TKI drugsCitation6,Citation7. The NCCN guidelines include recommendations of specific TKIs when particular genetic mutations/profiles are detected, and also provide recommendations for management of cytogenetic/hematologic resistance to TKIsCitation7. Furthermore, an economic analysis that evaluated the potential treatment responses and economic consequences of limiting access to 2nd-gen TKIs, while taking into account frequencies of genetic mutations that exhibit different sensitivities to the drugs impacting patient responses to therapy, found that open access to both dasatinib and nilotinib is associated with improved clinical and economic outcomes compared to restricted accessCitation26.
Another potential approach for an OCM to reduce the total cost of care of CML patients might be to improve the frequency of molecular monitoring, since several studies have reported routine molecular monitoring is associated with improved patient outcomes, which could lead to a reduction in healthcare costsCitation27–33. Two separate studies have also reported cost reductions associated with earlier and more frequent monitoring of CML patients to proactively manage TKI treatment responsesCitation31,Citation34. In a retrospective claims analysis of patients with CML (n = 1,431) from January 2006 through June 2015, it was reported that the healthcare savings associated with each additional molecular monitoring test performed was $2,918 per-patient-per-year (2015 USD)Citation31. In an economic analysis that examined the annual cost reduction associated with molecular monitoring vs non-monitoring of CML patients, it was estimated at $5,840 per patient (2015 USD)Citation34. An OCM practice is expected to find sustainable spending for cancer care, as well as improve the coordination and appropriateness of careCitation1. Restricting access to treatments that yield superior responses in specific patients based on their individual characteristics may not be clinically reasonable or cost-effectiveCitation35.
There are limitations to this economic analysis. Specific cancer type all-cause healthcare costs were obtained from multiple sources, as no single source had the necessary information across multiple cancers; assessments of costs may have varied between the sources. Inherent to any modeling analysis, the default setting may not be generalizable to all practices, as they vary in terms of cost structure, patient mix, focus on specific cancer types, and other factors influencing costs. Additionally, in this economic model we did not include the usage of newer 3rd-gen TKIs in the evaluation, since their usage in the current US market is still very limited.
Conclusions
This economic model indicates that the cost reduction associated with restricting usage of branded TKIs among CML patients in an OCM setting may represent only a small proportion of the cost reduction threshold required before an OCM practice is eligible for a PBP. Of the potential reduction in TKI costs, approximately two-thirds was attributed to the shift from branded to generic imatinib. Restricting usage of the 2nd-gen TKIs represented a much smaller proportion of savings.
Transparency
Declaration of funding
This study was funded by Bristol-Myers Squibb.
Declaration of financial/other relationships
EJJ is a consultant to Bristol-Myers Squibb, Pfizer, Takeda, Amgen, and Abbvie, and has received research grants from Pfizer, Takeda, Amgen, Abbvie, and Adaptive Biotechnologies. MFM and DM are full-time employees of Bristol-Myers Squibb. MLS and JL are employees of Novosys Health, which has received research funds from Bristol-Myers Squibb in connection with conducting this study and development of this manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
We would like to acknowledge and thank Scott Keating, an employee at Bristol-Myers Squibb, for reviewing and providing constructive feedback during the preparation of this manuscript.
References
- Centers for Medicare & Medicaid Services. Oncology Care Model (OCM). Baltimore, MD [updated 2018 August 8; cited 2019 Mar 4]. Available from: https://innovation.cms.gov/initiatives/Oncology-Care/.
- Centers for Medicare & Medicaid Services. Oncology Care Model (OCM) overview and application process. Baltimore, MD [updated 2015 Feb 19; cited 2019 Mar 4]. Available from: https://innovation.cms.gov/resources/OCMintro.html.
- Centers for Medicare & Medicaid Services. First annual report from the evaluation of the Oncology Care Model: Baseline period. Abt Associates. Bethesda, MD [updated 2018 Feb 1]. Available from: https://downloads.cms.gov/files/cmmi/ocm-baselinereport.pdf.
- American Cancer Society. Key statistics for chronic myeloid leukemia. Atlanta, GA: ACS. [cited 2019 Mar 4] 2018. Available from: https://www.cancer.org/cancer/chronic-myeloid-leukemia/about/statistics.html
- Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123–3127.
- Jabbour EJ, Hughes TP, Cortés JE, et al. Potential mechanisms of disease progression and management of advanced-phase chronic myeloid leukemia. Leuk Lymphoma. 2014;55:1451–1462.
- National Comprehensive Cancer Network Guidelines. Chronic myeloid leukemia. v.1.2019. Fort Washington, PA: NCCN; 2018.
- Jain P, Kantarjian H, Alattar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. Lancet Haematol. 2015;2:e118–e128.
- Kreys ED, Frei CR, Villarreal SM, et al. Evaluation of long-term chronic myeloid leukemia treatment practices with tyrosine kinase inhibitors in a national cohort of veterans. Pharmacotherapy. 2017;37:278–286.
- Brunner AM, Campigotto F, Sadrzadeh H, et al. Trends in all-cause mortality among patients with chronic myeloid leukemia: a surveillance, epidemiology, and end results database analysis. Cancer. 2013;119:2620–2629.
- Jabbour EJ, Lin J, Siegartel LR, et al. Evaluation of healthcare resource utilization and incremental economic burden of patients with chronic myeloid leukemia after disease progression to blast phase. J Med Econ. 2017;20:1007–1012.
- Millman Research Report: A multi-year look at the cost burden of cancer. Seattle, WA: Millman, Inc. 2017.
- Alemayehu B, Buysman E, Parry D, et al. Economic burden and healthcare utilization associated with castration-resistant prostate cancer in a commercial and Medicare Advantage US patient population. J Med Econ. 2010;13:351–361.
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128.
- Princic N, Song X, Lin V, et al. Healthcare costs associated with adult Philadelphia chromosome-negative (Ph-) acute lymphoblastic leukemia (ALL) in the US. Blood. 2016;128:5940.
- Matasar MJ, Dacosta Byfield S, Blauer-Peterson C, et al. Real-world health care utilization and costs among patients newly initiating systemic therapy for chronic lymphocytic leukemia (CLL) in the United States. Blood. 2016;128:5928.
- Meyers J, Yu Y, Kaye JA, et al. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy. 2013;11:275–286.
- Knopf KB, Divino V, McGarry L, et al. Economic burden of tyrosine kinase inhibitor treatment failure in chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15:e163–e171.
- Henry J, Kaiser Family Foundation. The facts on Medicare spending and financing [2018 Jun 22; cited 2019 Mar 4]. Available from: https://www.kff.org/medicare/issue-brief/the-facts-on-medicare-spending-and-financing/
- Fisher MD, Fernandes AW, Olufade TO, et al. Patient characteristics and costs in recurrent or refractory head and neck cancer: retrospective analysis of a community oncology database. Clin Ther. 2018;40:562–573.
- Farr AM, Zhao ZY, Song X, et al. Medical costs by disease stage in Medicare patients with metastatic melanoma. JCT. 2017;08:913–992.
- Bristol-Myers S. Data on file: Cost calculator. Princeton, NJ; 2018.
- Symphony Health: Patient Transactional Data. Phoenix, AZ: Symphony Health; 2018.
- Truven health analytics. Red Book. Ann Arbor, MI: Truven Health Analytics [cited 2019 Mar 4]. Available from: http://www.micromedexsolutions.com/micromedex2/4.34.0/WebHelp/RED_BOOK/Introduction_to_REDB_BOOK_Online.htm
- Centers for Medicare & Medicaid Services. Oncology Care Model Webinar. Bethesda, MD [2016 Apr 20; cited 2019 Mar 4]. Available from: https://innovation.cms.gov/Files/slides/ocm-performancemethod-slides.pdf
- Jabbour EJ, Makenbaeva D, Lingohr-Smith M, et al. Impact of genetic mutations and health plan access to therapies on treatment response and drug costs related to tyrosine kinase inhibitor treatment among patients with chronic myelogenous leukemia. Am J Clin Oncol. 2018;41:213–217.
- Haque R, Shi J, Chung J, et al. Medication adherence, molecular monitoring, and clinical outcomes in patients with chronic myelogenous leukemia in a large HMO. J Am Pharm Assoc. 2017;57:303–310e.2.
- Guérin A, Chen L, Dea K, et al. Association between regular molecular monitoring and tyrosine kinase inhibitor therapy adherence in chronic myelogenous leukemia in the chronic phase. Curr Med Res Opin. 2014;30:1345–1352.
- Di Bella NJ, Bhowmik D, Bhor M, et al. The effectiveness of tyrosine kinase inhibitors and molecular monitoring patterns in newly diagnosed patients with chronic myeloid leukemia in the community setting. Clin Lymphoma Myeloma Leuk. 2015;15:599–605.
- Saleh MN, Haislip S, Sharpe J, et al. Assessment of treatment and monitoring patterns and subsequent outcomes among patients with chronic myeloid leukemia treated with imatinib in a community setting. Curr Med Res Opin. 2014;30:529–536.
- Latremouille-Viau D, Guerin A, Gagnon-Sanschagrin P, et al. Health care resource utilization and costs in patients with chronic myeloid leukemia with better adherence to tyrosine kinase inhibitors and increased molecular monitoring frequency. JMCP. 2017;23:214–224.
- Goldberg SL, Chen L, Guerin A, et al. Association between molecular monitoring and long-term outcomes in chronic myelogenous leukemia patients treated with first line imatinib. Curr Med Res Opin. 2013;29:1075–1082.
- Goldberg SL, Cortes JE, Gambacorti-Passerini C, et al. First-line treatment selection and early monitoring patterns in chronic phase-chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2017;92:1214–1223.
- Jabbour EJ, Siegartel LR, Lin J, et al. Economic value of regular monitoring of response to treatment among US patients with chronic myeloid leukemia based on an economic model. J Med Econ. 2018;21:1036–1040.
- Ai J, Tiu RV. Practical management of patients with chronic myeloid leukemia who develop tyrosine kinase inhibitor-resistant BCR-ABL1 mutations. Ther Adv Hematol. 2014;5:107–120.