Abstract
Objective: The standard of care for cancer-related venous thromboembolism (VTE) has been low molecular weight heparin (LMWH), but oral anticoagulants are also widely prescribed. This study compared VTE-related healthcare resource utilization and costs of cancer patients treated with anticoagulants.
Methods: Claims data from Humana Database (January 1, 2013–May 31, 2015) were analyzed. Based on the first anticoagulant received, patients were classified into LMWH, warfarin, or rivaroxaban cohorts. Characteristics were evaluated during the 6 months pre-index date (i.e. the first VTE); VTE-related resource utilization and costs were evaluated during follow-up. Cohorts were compared using rate ratios, and p-values and 95% confidence intervals were calculated. Healthcare costs were evaluated per-patient-per-year (PPPY) and compared using mean cost differences.
Results: A total of 2,428 patients (LMWH: n = 660; warfarin: n = 1,061; rivaroxaban: n = 707) were included. Compared to patients treated with LMWH, patients treated with rivaroxaban had significantly fewer VTE-related hospitalizations, hospitalization days, and emergency room and outpatient visits, resulting in an increase of $12,000 VTE-related healthcare costs PPPY with LMWH vs rivaroxaban. Patients treated with rivaroxaban had significantly lower VTE-related resource utilization compared to patients treated with warfarin; however, VTE-related costs were similar between cohorts. The higher drug costs ($1,519) were offset by significantly lower outpatient (−$1,039) and hospitalization costs (−$522) in rivaroxaban relative to the warfarin cohort.
Conclusions: Healthcare resource use and costs associated with VTE treatment in cancer patients are highest with LMWH relative to warfarin and rivaroxaban.
Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is one of the leading causes of morbidity and mortality in cancer patientsCitation1,Citation2. Research has shown that the presence of an active malignancy is a significant risk factor for recurrent VTE and that this risk is further increased by different forms of treatment, such as chemotherapy. Compared to the general population, cancer patients have a 4.1-fold higher risk of thromboembolism, and those undergoing chemotherapy have a 6.5-fold higher risk of thromboembolismCitation3. Tumor site and stage, patient characteristics, and treatment-related factors are all associated with an increased risk for VTE in cancer patients.
Anticoagulation is essential for the management of VTECitation4. Current treatment guidelines recommend low molecular weight heparin (LMWH) monotherapy for at least 3–6 months in cancer patients with VTECitation5. This recommendation is based on clinical trials in which LMWH was found to be superior to the vitamin K antagonist (VKA) warfarin in the prevention of recurrent VTE, with no difference in the risk of major bleedingCitation6,Citation7. More recently, direct oral anticoagulants (DOACs) such as rivaroxaban are also commonly prescribed for treatment of VTECitation8.
Prevention of recurrent VTE in cancer patients is crucial because VTE treatment is immensely expensive and resource-intensiveCitation9. Compared to cancer patients without VTE, cancer patients with VTE have been shown to have three times as many all-cause hospitalizations, more days spent in the hospital, and a significantly higher number of outpatient visitsCitation9. Cancer patients with VTE also have a high frequency of hospital readmission (5–14%), most of which occur within a couple of months of the initial hospitalizationCitation10. It has been estimated that all-cause healthcare costs of cancer are $30,538 higher for cancer patients with VTE compared to those without.
Given the importance of reducing recurrent VTE in cancer patients, research has been done to determine which of the available treatments is most effective based on various measures. For example, re-hospitalizations related to VTE recurrences have been shown to be lower in patients treated with rivaroxaban compared to patients treated with alternative anticoagulants such as LMWH or warfarinCitation11,Citation12. However, limited information exists on the costs associated with different anticoagulant treatments for VTE in patients with cancer. The objective of this study was to compare VTE-related healthcare resource utilization and costs associated with different anticoagulation therapies for VTE in a real-world setting.
Methods
Data source
Healthcare insurance claims from the Humana Database were used to conduct the analysis. The Humana database includes over 18 million insured lives of commercial and Medicare members in all census regions in the US, but predominantly in the Midwest and South. Over 9 million members from the database have both medical and pharmacy coverage. The present study spanned the period from January 2007 to June 2015, and data related to demographics, enrollment history, inpatient and outpatient claims, emergency-department visits, and pharmacy claims for commercial and Medicare Advantage Part D (MA-PD) members were extracted. The data included in the present study were de-identified and data collection complied with the requirements of the Health Insurance Portability and Accountability Act (HIPAA), to preserve the anonymity and confidentiality of participants.
Study design
A retrospective longitudinal cohort design was used. To be included in the study, patients had to be newly diagnosed with cancer, and have at least one inpatient stay or two outpatient visits with their cancer diagnosis. Additionally, their first VTE (i.e. index date) must have occurred after 2013, and after the patient’s first cancer diagnosis. Patients that had a VTE diagnosis 30 days prior to cancer diagnosis were included in the study, as VTE can be an early sign of cancer. Patients were selected if they had one or more dispensings of the following anticoagulant agents within a week of their VTE diagnosis: LMWH, warfarin, or rivaroxaban. Patients were then classified into LMWH, warfarin, or rivaroxaban treatment cohorts (other anticoagulants were not included due to low utilization). Baseline characteristics were evaluated during the 6-month period prior to the index date. Patients that had been previously diagnosed with VTE or received anticoagulant treatment before the index date were excluded from the study.
The observation period lasted from the initiation of an anticoagulant therapy until the following: end of insurance coverage or end of data availability, whichever happened earlier. However, patients were allowed to reinitiate an anticoagulant therapy during the follow-up period.
Study endpoints
VTE-related resource utilization included the total number of outpatient visits, number of emergency room (ER) visits, number of hospitalizations, and number of hospitalization days. VTE-related healthcare costs included pharmacy costs, outpatient costs, ER visit costs, and hospitalization costs. VTE-related pharmacy costs were defined as dispensings for any anticoagulant therapies. VTE-related healthcare resource utilization and costs included all claims with a primary or secondary diagnosis for PE or DVT.
Statistical analysis
Patient demographics and baseline clinical characteristics between the respective cohorts were compared using descriptive statistical analyses. Patients’ baseline characteristics evaluated during the 6-month period prior to the index date were summarized using means, medians, and standard deviations for continuous variables, and frequencies (proportions) for categorical variables. Any differences in baseline and clinical characteristics were compared between cohorts using Pearson’s Chi-square tests for categorical variables and two sided t-tests for continuous variables.
Two comparisons between treatment cohorts were performed: LMWH compared to rivaroxaban, and rivaroxaban compared to warfarin. The inverse probability of the treatment weighting (IPTW) approach (which is based on propensity score [PS]) was used for each comparison to minimize the potential confounding between cohorts. The goal of weighting patients by the inverse probability of the treatment received helps create a pseudo sample that is independent of baseline coviariatesCitation13. Each patient was assigned a weight such that, in the weighted pseudo-population, the distribution of measured confounders was similar between the compared treatment cohorts. This process results in pseudo-populations where the sum of weights (i.e. the sample size) is different than that of the original population. For the corresponding calculation of IPTWs, the probability (i.e. the PS) of receiving LMWH (vs rivaroxaban; comparison 1) was first estimated using a multivariate logistic regression model conditional on the following baseline covariates: age, sex, type of cancer, very high risk and high risk cancer types for VTE, region, race, time-from-cancer to VTE diagnosis, time to anticoagulant initiation, setting of VTE diagnosis (inpatient, outpatient, or ER), type of VTE (DVT, PE, or both), treatment with an antineoplastic agent, and Charlson comorbidity index.
Comparisons between cohorts were performed using rate ratios (RR), and p-values and 95% confidence intervals (CI) were calculated using Poisson regression models. VTE-related healthcare costs were evaluated per-patient-per-year (PPPY) and compared using mean cost differences. The 95% CI for cost differences were based on bootstrap methods using 499 replications.
All the analyses in the study were performed with SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 2,428 cancer patients who developed a VTE and initiated an anticoagulant within 7 days of VTE diagnosis were identified, including 1,061 treated with warfarin, 707 with rivaroxaban, and 660 with LMWH. Overall, weighted cohorts were comparable in terms of baseline characteristics ().
Table 1. Patient characteristics after IPTWTable Footnotea.
The mean age of patients included in the study was 73 years, and about 50% of them were women. Of the included participants, 55–60% were diagnosed with DVT, 25–30% were diagnosed with PE, and 13–15% were diagnosed with both PE and DVT. Cumulative all-cause healthcare costs during the 6-month baseline period were comparable between the rivaroxaban vs warfarin cohort ($24,598 vs $25,440) and the rivaroxaban vs LMWH cohorts ($29,690 vs $30,254).
VTE-related resource utilization
Overall, patients treated with rivaroxaban had lower VTE-related resource utilization relative to patients treated with LMWH: hospitalizations, hospitalization days, and outpatient visits were significantly lower in the rivaroxaban group compared to the LMWH group. The RR of VTE-related resource utilization in person-years for the rivaroxaban vs LMWH cohort was 0.72 (p < 0.001) for hospitalizations, 0.66 (p < 0.001) for hospitalization days, and 0.60 (p < 0.001) for outpatient visits ().
Figure 1. VTE-related healthcare resource utilization rates of the rivaroxaban, LMWH, and warfarin cohorts.
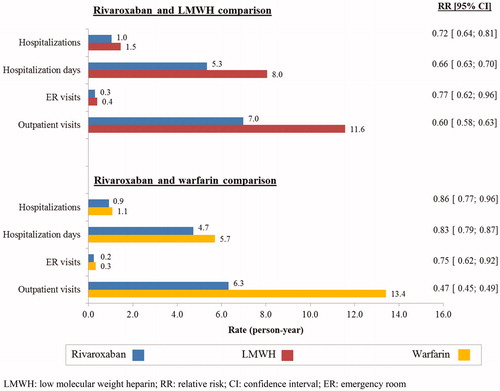
Similarly, patients treated with rivaroxaban had lower VTE-related resource utilization when compared to patients treated with warfarin. Fewer outpatient visits and fewer number of hospitalization days were observed in the rivaroxaban group compared to the warfarin group. The RR for VTE-related resource utilization in person-years for the rivaroxaban vs warfarin cohorts was 0.83 (p < 0.001) for hospitalization days and 0.47 (p < 0.001) for outpatient visits ().
VTE-related healthcare costs
Patients treated with rivaroxaban had significantly lower total VTE-related costs relative to patients treated with LMWH, with a mean cost difference of $12,000 PPPY (p < 0.001). This difference was mainly driven by a decrease in hospitalization costs by $6,993 (p < 0.001), ER visit costs by $333 (p < 0.001), outpatient visit costs by $2,479 (p < 0.001), and pharmacy costs by $2,200 (p < 0.001) for the rivaroxaban compared to the LMWH cohort ().
Table 2. VTE-related healthcare costs.
Total mean VTE-related costs PPPY were similar between the rivaroxaraban ($11,722) and warfarin ($11,819) cohorts, with a cost difference of $97 (p = 0.90). Pharmacy costs were significantly higher by $1,519 in the rivaroxaban group relative to the warfarin group (p < 0.001). This cost difference was offset by a significantly lower outpatient visit cost difference of −$1,039 (p = 0.024) and a slightly lower mean hospitalization cost difference of −$522 (p = 0.54; ).
Discussion
The results of this large administrative database analysis show that cancer patients with VTE who initiated rivaroxaban consumed significantly fewer healthcare resources for VTE-related disease management, such as days spent in the hospital and outpatient visits, when compared to patients initiated on LMWH or warfarin. The lower resource utilization also translated to significantly lower total VTE-related costs for the rivaroxaban cohort compared to patients treated with LMWH (mean cost difference = $12,000). Total VTE-related costs between the rivaroxaban and warfarin treatment cohorts were not significantly different (mean cost difference = $97) due to higher drug costs in the rivaroxaban cohort being offset by higher outpatient and hospital visit costs in the warfarin cohort. These findings may provide a better understanding of VTE treatment-related utilization of healthcare resources and corresponding costs in patients with cancer.
VTE events impose a significant economic burden on the healthcare system that is further amplified among patients with cancer. Using claims data, Khorana et al.Citation9 found that cancer patients with VTE incurred significantly higher total healthcare costs compared to cancer patients without VTE ($74,959 vs $41,691 per patient over the 12-month follow-up period; p < 0.0001). Another study found that the average cost of a hospitalization in patients with cancer was over $20,000 for DVT alone or concomitant DVT and PECitation14. Furthermore, recurrent VTEs are considerably more expensive to manage, with the additional total all-cause cost of care associated with them estimated to be $50,000 at 1 yearCitation15,Citation16.
In the existing literature, there is limited information on the healthcare resource use and costs associated with LMWH in patients with cancer. With respect to the rivaroxaban vs LMWH comparison, a previous analysis of our study population showed that LMWH is less effective than rivaroxaban in preventing recurrent VTE in patients with cancerCitation11. This is corroborated by the results of the SELECT-D trial, which demonstrated a 57% reduction in the risk of recurrent VTE at 6 months with rivaroxaban compared to LMWHCitation17. Besides the lower cost of rivaroxaban compared to LMWH, these findings suggest that a significant proportion of the cost differences observed in the current study may be driven by lower VTE recurrence rates with rivaroxaban. For instance, VTE recurrence rates were lower for rivaroxaban users than for LMWH users at 6 months (13.2% vs 17.1%; p = 0.06) and 12 months (16.5% vs 22.2%; p = 0.03), while major bleeding rates were similar between the cohorts in our previous analysisCitation11. Similarly, in our real-world analysis of a younger population of cancer patients, those who initiated LMWH had a 17% higher risk of recurrent VTE compared to rivaroxaban, but a similar risk of major bleedingCitation18.
Among patients with cancer, data comparing rivaroxaban and warfarin treatment for VTE is similarly sparse. However, as seen in the comparison of rivaroxaban and LMWH, the previous analysis of our study population demonstrated lower VTE recurrence rates with rivaroxaban treatment compared to warfarin in patients with cancer (13.2% vs 17.5% at 6 months and 15.7% vs 19.9% at 12 months; p < 0.05), again supporting the conclusion that lower VTE recurrence is the main driver of the lower costs observed with rivaroxaban in the current studyCitation11. Similar cost savings in the general VTE population have also been found in several claims-based studies. In a retrospective claims analysis, treatment with rivaroxaban resulted in a mean hospital-visit cost saving of $1,508 (95% CI = −$2,296; −$580; p = 0.002)Citation19. A different study using the MarketScan Commercial Claims and Encounters Database found that mean overall treatment costs per patient were lower for rivaroxaban users vs warfarin users (−$1,116, p = 0.0016)Citation20. Like in the present study, this cost difference was driven by lower inpatient (−$622) and outpatient (−$1,156) treatment costs, such that the higher pharmacy costs ($661) were fully offsetCitation20.
Research has shown that hospital length of stay (LOS) for recurrent VTE is considerably longer and more expensive than those for initial VTECitation10. These more frequent and longer hospital stays are reflected in the higher healthcare resource use and costs observed with LMWH in this study. These findings support the suggestion made by Smrke and GrossCitation21, who argued that, given the lower acquisition cost (i.e. ∼10-times lower) and ease of administration, DOACs like rivaroxaban are an attractive alternative to LMWH in patients with cancerCitation22. Indeed, despite guideline recommendations, real-world research shows that rivaroxaban is used as frequently, for longer, and with better persistence than LMWH, and is associated with less treatment switching than LMWH in the management of VTE in cancer patientsCitation8. Rivaroxaban also appears to be a viable alternative to warfarin. Several post-hoc studies of LOS have demonstrated the benefit of rivaroxaban in patients without cancer. In a sub-group analysis of the non-interventional XALIA study comparing rivaroxaban with standard anticoagulation (including treatment with VKA), adjusted LOS was 2.6 days shorter with rivaroxaban compared to standard anticoagulationCitation23. A post-hoc analysis of hospitalization and LOS data was also conducted in the North American sub-set of patients from the randomized EINSTEIN studies, which compared rivaroxaban with enoxaparin followed by VKA. It found that, among 382 hospitalized patients with VTE, the patients randomized to receive rivaroxaban had a significant 1.6-day mean reduction in LOS compared with the patients randomized to receive enoxaparin as a bridge to VKACitation24. These results were supported by a retrospective observational study of 113 patients (29% with cancer) with VTE that similarly found that the mean hospital LOS was significantly reduced in rivaroxaban vs LMWH/warfarin initiators (mean LOS difference [95% CI] = −1.78, [−3.46; −0.10], p = 0.039]), although the results did not account for all differences in treatment groupsCitation25.
The current study has several limitations that need to be considered. First, as mentioned earlier, anticoagulants other than LMWH, warfarin, and rivaroxaban were not included in the study due to low utilization, which may not be fully representative of the current treatment landscape. Second, the claims database used in the study may contain billing inaccuracies or omissions in coded procedures, diagnoses, and pharmacy claims. However, it is unlikely that this would have significantly impacted the results of our study, given the large sample size. Third, as with all claims data, the study results may be subject to residual confounding due to unmeasured confounders such as stage of cancer or physician selection bias for anticoagulant treatment. Fourth, cost results reflect those in the US, and it is unclear whether and to what extent these can be extrapolated to other countries. Fifth, analyses were performed under the assumption that mortality was evenly distributed across study cohorts. Finally, like all observational studies, adjustments in the multivariate analyses could only account for observable factors. Despite the aforementioned limitations, well-designed observational studies with appropriate statistical modeling provide important information that is generalizable, and offer a real-world perspective.
Conclusions
This real-world claims analysis, which is representative of current clinical practice patterns in the US, showed that cancer patients with VTE who initiated rivaroxaban had significantly lower VTE-related healthcare resource utilization and costs as compared to patients initiated on LMWH, and lower resource utilization and corresponding medical costs that were offset by higher rivaroxaban costs compared to generic warfarin. A cost-effectiveness analysis of these agents using data from, for instance, the SELECT-D trialCitation17, may be warranted in patients with cancer.
Transparency
Declaration of funding
Financial support for this research was provided by Janssen Scientific Affairs, LLC (JSA).
Declaration of financial/other relationships
MS, KRM, and AAK have received research grants from JSA. FL, DL, and PL are employees of Groupe d’analyse, Ltée, a consulting company that has received research grants from JSA. DM is an employee of JSA. JS was an employee of JSA at the time this study was conducted. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Third party agreement
The data that support the findings of this study are available from Humana, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Any researchers interested in obtaining the data used in this study can access the database through Humana, under a license agreement, including the payment of the appropriate license fee.
Notation of prior publication/presentation
Parts of this manuscript were presented at the 58th American Society of Hematology (ASH) Annual Meeting & Exposition, December 3–6, 2016, San Diego, CA, as a poster presentation.
Supplemental Material: Table - Patient Characteristics before IPTW
Download MS Word (34.8 KB)Acknowledgements
Medical writing support was provided by a professional medical writer, Christine Tam, an employee of Groupe d’analyse, Ltée, which has received funding from JSA.
References
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634.
- Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107:17–21.
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–815.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488.
- Lyman GH, Bohlke K, Falanga A, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. JOP. 2015;11:e442–444.
- Lee AY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677–686.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153.
- Khorana AA, McCrae KR, Milentijevic D, et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer‐associated thrombosis. Res Pract Thromb Haemost. 2017;1:14–22.
- Khorana AA, Dalal MR, Lin J, et al. Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon Outcomes Res. 2013;5:101–108.
- Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. JMCP. 2007;13:475–486.
- Streiff MB, Milentijevic D, McCrae K, et al. Effectiveness and safety of anticoagulants for the treatment of venous thromboembolism in patients with cancer. Am J Hematol. 2018;93:664–671.
- Streiff M ,Milentijevic D ,McCrae K, et al. editors. Recurrent VTE in cancer patients treated with anticoagulation. ASCO Annual Meeting Proceedings; 2016 June 3–7; Chicago, IL.
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statist Med. 2015;34:3661–3679.
- Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653.
- Lefebvre P, Laliberte F, Nutescu EA, et al. All-cause and disease-related health care costs associated with recurrent venous thromboembolism. Thromb Haemost. 2013;110:1288–1297.
- Lin J, Lingohr-Smith M, Kwong WJ. Incremental health care resource utilization and economic burden of venous thromboembolism recurrence from a U.S. payer perspective. JMCP. 2014;20:174–186.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). JCO. 2018;36:2017–2023.
- Khorana AA, McCrae K, Milentijevic D, et al. The risk of recurrent VTE and major bleeding in a commercially-insured population of cancer patients treated with anticoagulation. Am J Hematol. 2018;94:E58–E61.
- Merli GJ, Hollander JE, Lefebvre P, et al. Costs of hospital visits among patients with deep vein thrombosis treated with rivaroxaban and LMWH/warfarin. J Med Econ. 2016;19:84–90.
- Coleman CI, Baugh C, Crivera C, et al. Healthcare costs associated with rivaroxaban or warfarin use for the treatment of venous thromboembolism. J Med Econ. 2017;20(2):200–203.
- Smrke A, Gross PL. Cancer-associated venous thromboembolism: a practical review beyond low-molecular-weight heparins. Front Med (Lausanne). 2017;4:142.
- GoodRx. 2016 [cited 2016, October 6]. Available from: https://www.goodrx.com/
- Mantovani LG, Haas S, Kreutz R, et al. Healthcare resource use in XALIA: a subgroup analysis of a non-interventional study of rivaroxaban versus standard anticoagulation for deep vein thrombosis. Eur J Intern Med. 2019;61:29–33.
- Bookhart BK, Haskell L, Bamber L, et al. Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. J Med Econ. 2014;17:691–695.
- Hom L, Sobieraj DM. The impact of initiating rivaroxaban versus low-molecular weight heparin plus warfarin in patients admitted to the hospital for venous thromboembolism. Int J Cardiol. 2015;198:87–88.
