Abstract
Objective: This retrospective database analysis estimated the incremental effect that disease progression from non-clinically significant functional mitral regurgitation (nsFMR) to clinically significant FMR (sFMR) has on clinical outcomes and costs.
Methods: Medicare Fee for Service beneficiaries with nsFMR were examined, defined as those with a heart failure diagnosis prior to MR. Patients were classified as ischemic if there was a history of: CAD, AMI, PCI, or CABG. The primary outcome was time to sFMR, defined as pulmonary hypertension, atrial fibrillation, mitral valve surgery, serial echocardiography, or death, using a Cox hazard regression model. Annualized hospitalizations, inpatient hospital days, and healthcare expenditures were also modeled.
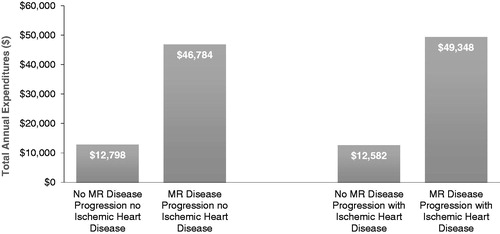
Results: Patients with IHD had higher risk (Hazard Ratio = 1.22 [1.14–1.30]) for disease progression compared to patients without. The progression cohort had significantly more annual inpatient hospitalizations (non-IHD = 1.32; IHD = 1.40) than the non-progression cohort (non-IHD = 0.36; IHD = 0.34), and significantly more annual inpatient hospital days (non-IHD = 13.07; IHD = 13.52) than the non-progression cohort (non-IHD = 2.29; with IHD = 2.08). The progression cohort had over 3.5-times higher costs vs the non-progression cohort, independent of IHD (non-IHD = $12,798 vs $46,784; IHD = $12,582 vs $49,348).
Conclusion: Treating FMR patients earlier in their clinical trajectory may prevent disease progression and reduce high rates of healthcare utilization and expenditures.
Introduction
Mitral regurgitation (MR) is the most common form of valvular heart disease in the US, and is defined as the backward flow of blood from the left ventricle into the left atrium during systoleCitation1–3. MR is especially prevalent in elderly populations, affecting about 10% of people aged 75 years and older (compared to only 1% of people aged 55–64)Citation1,Citation3–5. Two principal etiologies exist for patients with MR: (1) primary, or degenerative MR (DMR), and (2) secondary, or functional MR (FMR). DMR is the result of a primary, structural abnormality of the mitral valve (MV) and its supporting apparatus, whereas, with FMR, the mitral valve is generally normal in structure. Patients with FMR typically have left ventricular (LV) dysfunction from underlying cardiomyopathy, which results in inadequate leaflet coaptation causing MRCitation2,Citation5,Citation6. FMR is more common, and has a worse prognosis than DMR as a result of the associated LV dysfunctionCitation5,Citation7.
Diagnosing FMR is challenging because FMR is load-dependent. This can lead to variations in severity from study to studyCitation8. Additionally, given the secondary nature of FMR, treatment of this process is more complicated and therapies have yielded worse outcomesCitation2,Citation9. Management strategies for the treatment of FMR are complicated and largely dependent on a patient’s underlying pathology, which can be ischemic or non-ischemicCitation4,Citation6. Conclusive evidence supporting effective surgical interventions for FMR is lacking, rendering treatment pathways undefinedCitation2,Citation9,Citation10. The Cardiothoracic Surgical Trials NetworkCitation11 found no evidence of a statistically significant difference in mortality between surgical MV replacement and MV repair in patients with severe ischemic MR at 12 months. The mortality rate at 12 months was high in both groups: 14.3% in the repair group and 17.6% in the replacement group.
Given the prevalence of FMR and lack of consensus for therapy, the economic burden for FMR is high. A recent analysisCitation12 reported the largest direct annual-healthcare costs were associated with MV disease and not aortic valve (AV) disease. When scaled to the population of the US, the study revealed the combined annual costs of symptomatic and asymptomatic MV disease was $13.2 billion (compared to $10.2 billion for AV disease).
Although the large clinical and economic burden of MR is well-documentedCitation3,Citation12,Citation13, there is limited literature regarding the incremental effect of disease progression (moving from non-clinically significant FMR to clinically significant FMR) on clinical outcomes and costs. Therefore, the primary objective of this retrospective analysis was to evaluate the clinical and economic burden of FMR disease progression in the Medicare population.
Methods
Data source
Data for this study was derived from the 5% Medicare Limited Dataset Standard Analytic Files (SAFs) for beneficiaries enrolled in fee-for-service (FFS) Medicare from January 1, 2011, through December 31, 2016. Medicare patients were selected because MR is more prevalent in elderly populationsCitation5. This data contains detailed information from claims submitted by providers, which include, but are not limited to, the following: source of admission and discharge destinations (including death), International Classification of Disease ICD-9 and ICD-10 diagnosis and procedure codes, revenue centers, CPT/HCPCS codes, and charges associated with those services. A separate file provides annual demographic and enrollment information for all Medicare beneficiaries.
Inclusion/exclusion criteria
Patients were included in this study if they were enrolled in Medicare Parts A and B and had at least one inpatient or two outpatient claims with a diagnosis for MR in any position on the claim between January 1, 2011, and October 1, 2015. The first-observed diagnosis of MR was defined as the index event. Patients were then included if they were 18 years or older and had at least 6-months of continuous enrollment before the index event (baseline period) and 6-months of continuous enrollment after (landmark period). Patients also had at least one inpatient or two outpatient claims for heart failure (HF) in any position on the claim during the baseline or landmark period.
Patients were excluded if, during the baseline or landmark period, they had a record of (1) clinically significant MR [defined in coding by the presence of pulmonary hypertension (PHTN), atrial fibrillation (AF), MV surgery, or serial echocardiography (sECHO) with two or more studies]; or (2) DMR (e.g. diagnosis of chordal rupture). The justification for two or more echocardiograms is based on the AHA/ACC clinical guidelinesCitation1 for valvular heart disease, which recommend that patients with severe MR have an echocardiogram as frequently as every 6 months to 1 year. Patients were also excluded if, at any time in the database, they had a record of the following: left-ventricular–assist devices (LVADs), heart transplantation, endocarditis of any valve, mitral stenosis, end-stage renal disease (ESRD), or rheumatic valvular disease.
Variables of interest
The primary outcome for this study was the time-to-disease progression (clinically significant FMR). A composite variable was created for clinically significant FMR, which required a patient to have a record of one or more of the following events in the post-landmark period: a diagnosis of PHTN or AF; a record of MV surgery; a record of sECHO during a 6-month time window; or death. Additional outcomes of interest included annualized inpatient hospitalizations (all-cause and cardiovascular-related), annualized inpatient hospital days (all-cause and cardiovascular-related), and annualized healthcare expenditures.
The key explanatory variable for time-to-disease progression was the presence of ischemic heart disease, defined as a record of coronary artery disease (CAD), acute myocardial infarction, coronary artery bypass grafting (CABG), or percutaneous coronary intervention (angioplasty) during either the baseline or landmark periods. For procedures, only one outpatient claim was required to meet the definition for ischemic heart disease (IHD). Annualized hospitalizations and expenditure outcomes accounted for both IHD as well as progression to clinically significant FMR.
Confounding variables of interest for this analysis included patient demographics (age, sex, region, index year), presence of certain cancers that could have been treated with cardiotoxic chemotherapy and/or radiation therapy (breast, lung, lymphoma), and comorbid conditions measured by the Elixhauser Comorbidity IndexCitation14 (ECI) score and the CHA2DS2-VAScCitation15 score. The ECI identifies 31 categories of comorbidities that are associated with mortality. The CHA2DS2-VASc score classification-algorithm is a risk assessment for stroke in patients with atrial fibrillation.
Statistical analyses
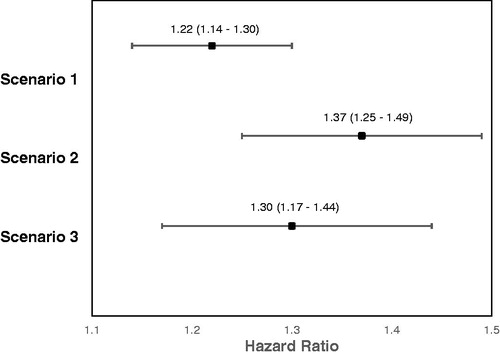
The outcome variable “time-to-clinically significant FMR” was dichotomous: patients either progressed to clinically significant FMR or they did not. If a patient’s FMR-status was still non-clinically significant at the end of data availability or health-plan enrollment, their data was censored. We considered three scenarios of disease progression with varying specificity: (1) death, MR surgery, PHTN, AF, or sECHO; (2) MR surgery, PHTN, AF, or sECHO; and (3) MR surgery, PHTN, or AF.
The time-to-clinically significant FMR for all three scenarios was analyzed using the proportional hazard Cox regression model with IHD as the key explanatory variable. Annualized inpatient hospitalizations and hospital days were modeled using a generalized linear model (GLM) with negative binomial distribution, while annualized expenditures were modeled using a GLM with log-gamma distribution. For both sets of GLM models, the key explanatory variables were IHD and disease progression to clinically significant FMR. Follow-up days were included in all GLM models as an offset because individual patients had different follow-up times (from which the number of hospitalizations, hospital days, and expenditures were calculated). Estimated marginal means were derived from the GLM models and estimated per year. Patient demographics (age, sex, region) and comorbid conditions (ECI and CHA2DS2-VASc) were covariates in both the Cox and GLM regressions. All statistical analyses were performed using the Instant Health Data platform from Boston Health Economics.
Results
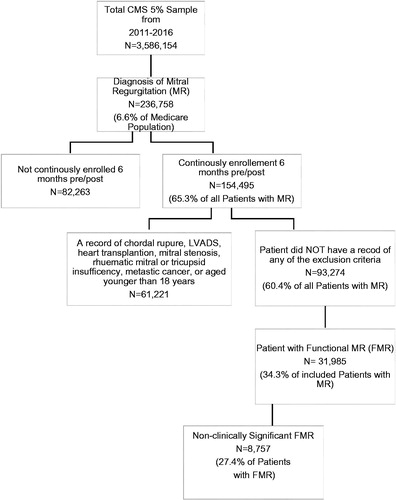
Of the total Medicare 5% sample from January 1, 2011, through December 31, 2016 (3,586,154 patients), 236,758 patients (6.6%) had a diagnosis of MR, and 31,985 patients (13.5% of the total MR population) met all inclusion and exclusion criteria for FMR. Out of the 31,985 patients with FMR, 8,757 (27.4%) patients had no evidence of clinically significant FMR in the landmark period ().
Of the 8,757 Medicare patients with non-clinically significant FMR, 4,797 (54.8%) progressed to clinically significant FMR from any time after the landmark period (6 months after MR diagnosis) to the end of data availability or the end of health-plan enrollment. displays summary statistics for patient demographics and comorbid conditions for patients progressing to clinically significant FMR. Those who progressed were older at the time of their MR diagnosis, with an average age (SD) of 76.18 (11.42) years vs 72.28 (11.40) years for those that did not progress. In both cases (progression versus no progression), females comprised the majority of patients (58.16% vs 59.82%). Over 80% of the sample was Caucasian (83.24% vs 81.26%), and most resided in the South (44.27% vs 45.01%). Patients that progressed to clinically significant disease were sicker at the time of diagnosis, with an average (SD) ECI score of 9.57 (3.03) compared to a score of 8.54 (2.98) for patients that did not progress. Furthermore, a greater percentage of patients in the progression cohort had a higher incidence of arrhythmias (53.24% vs 46.87%), chronic pulmonary disease (57.87% vs 47.65%), fluid and electrolyte disorders (58.10% vs 49.57%), peripheral vascular disease (46.47% vs 36.44%), and renal failure (44.74% vs 33.86%) ().
Table 1. Patient demographics.
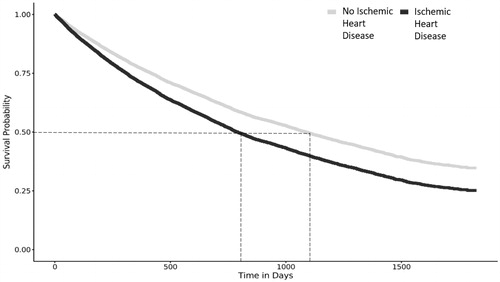
displays the hazard ratios (HRs) and confidence intervals (CIs) for the time-to-clinically-significant disease progression for the three scenarios of interest. We observed that, for scenario 1 (death, MR surgery, AF, PHTN, sECHO), patients with ischemic heart disease were at a higher risk (HR [CI] = 1.22 [1.14–1.30]) for MR progression compared to patients without IHD (see for survival curve). When removing death from the list of variables (scenario 2), we also found that patients with ischemic heart disease were at a higher risk (HR = 1.37 [1.25–1.49]) for disease progression compared to patients without. Finally, when removing death and sECHO from the list of variables (scenario 3), the findings were similar (HR = 1.30 [1.17–1.44]).
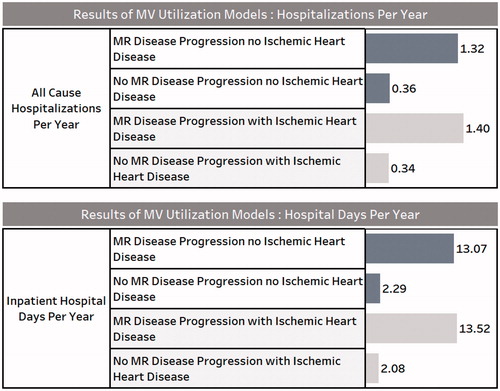
The results of the multivariable models for annualized inpatient hospitalizations and annualized hospital days for patients that progressed to clinically significant FMR vs those that did not (sub-set by those with and without IHD) are shown in . The progression cohort had significantly more inpatient hospitalizations per year (non-ischemic = 1.32; ischemic = 1.40) than the non-progression cohort (non-ischemic = 0.36; ischemic = 0.34). The progression cohort had significantly more inpatient hospital days per year (non-ischemic = 13.07; ischemic = 13.52) than the non-progression cohort (non-ischemic = 2.29; ischemic = 2.08).
displays the costs of disease progression. The cost-differences were over 3.5-times higher for the disease progression cohort vs the non-progression cohort, independent of IHD (non-ischemic, $12,798 vs $46,784; ischemic, $12,582 vs $49,348).
Discussion
We observed that patients with IHD were at a higher risk for disease progression compared to patients without IHD, even when considering only outcomes of MR surgery, AF, and PHTN (scenario 3). A recent observational study of patients with acute decompensation with HF that assessed the association of FMR with etiology of HF and preserved or reduced EF corroborates this observationCitation16. Compared to patients with non-ischemic FMR, patients with ischemic FMR were found to be at higher risk for HF-related readmissions post-discharge and all-cause mortality. Therefore, an increased risk of disease progression could be linked to a worse prognosis in patients with ischemic vs non-ischemic FMR. Furthermore, FMR with an ischemic origin tends to be a more common etiology than non-ischemic FMRCitation5, which has potential implications for the treatment of patients with ischemic FMR that has yet to progress.
While primary valve surgery is not currently recommended for patients with progressive FMR or for those with asymptomatic, severe FMR, those with ischemic FMR may benefit from surgery when concurrent revascularization is planned for significant CADCitation2. Some studiesCitation17–20 have demonstrated a therapeutic benefit of combining MV surgery with CABG in patients with ischemic FMR compared to CABG alone. However, the CTSN randomized trialCitation18,Citation19 comparing CABG alone to CABG plus MV repair failed to demonstrate a benefit in mortality and LV reverse remodeling between the two groups at 1 and 2 years. Another CTSN studyCitation21 comparing surgical repair vs replacement in severe ischemic MR patients, similarly found no mortality benefit at 2 years, but did find an increase in cardiovascular admissions for the repair group. These mixed findings suggest a greater diversity of treatment options is necessary to address patients’ severe FMR, either with or without ischemic heart disease.
Transcatheter technologies, including endovascular or percutaneous approaches for MV repair, have the potential to improve clinical outcomes and reduce healthcare burden for high-risk surgical patients seeking alternatives to surgery. The recent COAPT studyCitation22 demonstrated lower rates of heart failure hospitalization and all-cause mortality for heart failure patients with moderate-to-severe or severe FMR treated with a novel transcatheter mitral valve repair and guideline-directed medical therapy (GDMT) vs GDMT alone. The study also showed a significantly improved quality of life and functional capacity within 24 months of follow-up. Benefits were shown to be consistent across sub-groups, including those with or without ischemic cardiomyopathy. The control population in the COAPT study also highlighted the poor prognosis of patients receiving the current standard of care – over 80% were hospitalized and almost half died within 2 years, despite the use of GDMT. Among survivors, quality-of-life and functional capacity measures were significantly worsened 2 years from baseline. These results illustrate not only the promise of transcatheter mitral valve repair, but the inadequacies of current medical therapies and the need for additional innovation to increase access to care for this under-treated FMR population.
We found that inpatient hospitalizations, all cause hospital days, and healthcare expenditures for patients who progressed to clinically significant FMR were higher compared to those who did not. On average, the progression cohort had one more all-cause inpatient hospitalization and 11 more all-cause inpatient hospital-days per year than the non-progression cohort. The greater utilization likely contributed to the large cost-differential observed between the two cohorts. Cost-differences were more than 3.5-times higher for the disease progression cohort vs the non-progression cohort, independent of IHD, suggesting that costs associated with FMR are more dependent on the progression itself and not the presence of ischemic FMR. However, because patients with ischemic FMR progressed to clinically significant FMR faster than their non-ischemic counterparts, it can be expected that they will consequently become more expensive to treat. One study found that treatment of severe MR patients with transcatheter mitral valve repair reduced Medicare costs 1 year after intervention compared to pre-MV repairCitation23. Combined with the high clinical utility and economic costs associated with FMR progression found in our study, these results suggest the utility of less invasive interventions to increase access to care which could improve both survival and functional outcomes and, thereby, reduce the overall healthcare burden of severe FMR patients.
Strengths and limitations
This study was novel as the clinical and economic burden of the disease progression from non-clinically significant FMR to clinically significant FMR has not yet been evaluated. Determining FMR disease progression provides a unique opportunity to look at clinical outcomes for patients that start out “healthy”. Many retrospective analyses to date look at sicker populations (e.g. post-myocardial infarction) that may be susceptible to a worse prognosis given their underlying comorbidities. Additional retrospective analyses and prospective randomized trials are needed to explore the relationships between ischemic FMR, disease progression, MV surgery, and mortality. Further economic evaluations will prove beneficial, especially given the high prevalence and economic burden of MR in the western worldCitation2,Citation5,Citation9. Also, an evaluation of the effect of novel interventions for patients with non-clinically significant, ischemic FMR who also have AF, PHTN, or sECHO may show significant clinical outcomes.
Inherent limitations exist with any retrospective database-analysis. For example, coding errors, like the under-coding of non-billable events, are possible. We recognize that we used proxy variables for the progression of MR and did not have actual imaging results. The lack of echocardiography to grade the severity of MR limits the ability to draw firm conclusions on our results. Another weakness of this database is that the cause of death is not provided. Therefore, it is unknown whether death was related to the disease. Due to this limitation, the disease progression model was run twice: once with death as part of the outcome, and once with death as part of the censoring. Results were similar for both composite endpoints. Additionally, because FMR is a secondary manifestation to one or more underlying conditions, some uncertainty exists around whether FMR alone or a patient’s primary cardiomyopathy was the driving force behind any evaluated clinical outcomes.
Conclusions
Progression to clinically-significant FMR, assessed by proxy variables, carries a substantial clinical and economic burden in this retrospective database analysis of Medicare patients. Those who progressed to clinically-significant FMR were older on average and had higher rates of HF-related comorbidities, including chronic pulmonary disease, diabetes, hypertension, and peripheral vascular disease.
Prognosis was poor, regardless of IHDCitation5,Citation24, but patients with ischemic FMR had higher rates of AF, PHTN, sECHO, and were at higher risk for disease progression compared to patients without IHD. Progression to clinically significant FMR is associated with cost-differences over 3.5-times higher, independent of the presence of IHD.
Transparency
Declaration of funding
This study was sponsored by Edwards Lifesciences.
Declaration of financial/other relationships
PAM, HSM, DPC, and CMB have consulting relationships with Edwards Lifesciences. PV, SM, and JV are employees of and CG is a consultant to Edwards Lifesciences, the study sponsor. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
References
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011.
- Nishimura RA, Vahanian A, Eleid MF, et al. Mitral valve disease-current management and future challenges. Lancet. 2016;387:1324–1334.
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol. 2015;65:1231–1248.
- Hussaini A, Kar S. Percutaneous mitral valve repair: potential in heart failure management. Curr Heart Fail Rep. 2010;7:22–26.
- de Marchena E, Badiye A, Robalino G, et al. Respective prevalence of the different carpentier classes of mitral regurgitation: a stepping stone for future therapeutic research and development. J Card Surg. 2011;26:385–392.
- Benjamin MM, Smith RL, Grayburn PA. Ischemic and functional mitral regurgitation in heart failure: natural history and treatment. Curr Cardiol Rep. 2014;16:517.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791.
- Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol. 2008;52:319–326.
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32.
- Moore M, Chen J, Mallow PJ, et al. The direct health-care burden of valvular heart disease: evidence from US national survey data. CEOR. 2016;8:613–627.
- National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities. Hyattsville (MD): National Center for Health Statistics; 2016.
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272.
- Kajimoto K, Minami Y, Otsubo S. Ischemic or nonischemic functional mitral regurgitation and outcomes in patients with acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol. 2017;120:809–816.
- Fattouch K, Guccione F, Sampognaro R, et al. POINT: efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278–285.
- Smith PK, Puskas JD, Ascheim DD, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188.
- Michler RE, Smith PK, Parides MK, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2016;374:1932–1941.
- Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201.
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 2016;374:344–353.
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318.
- Vemulapalli S, Lippmann SJ, Krucoff M, et al. Cardiovascular events and hospital resource utilization pre- and post-transcatheter mitral valve repair in high-surgical risk patients. Am Heart J. 2017;189:146–157.
- Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675–1680.