Abstract
Background: Efficient hemostasis during lumbar surgery (LS) is associated with better perioperative outcomes. Flowable gelatin hemostatic matrix (FGHM) is a new type of absorbable hemostatic agent, which is effective to control bleeding during spinal surgery. This study aimed to assess the impact of FGHM on perioperative outcomes and hospital costs associated with LS.
Methods: This study retrospectively analyzed medical and billing records of patients who underwent LS for spinal degenerative disease in a Chinese tertiary care hospital from 2014 to 2016. The identified patients were further stratified into a FGHM group (n = 108) (using the combination of FGHM and gelatin sponge) and a historical control group (using oxidized cellulose and/or collagen, n = 82) for the adjusted comparisons of the perioperative outcomes using a propensity score matching method. Multiple generalized linear regression was conducted to assess the impact of using FGHM on total hospitalization costs.
Results: Comparisons of 64 propensity score matched pairs showed a significantly lower blood transfusion rate (34.4% vs 64.1%, p = 0.005), lower blood transfusion volume (182.7 ± 312.4 vs 301.3 ± 281.0 mL, p = 0.045), reduced post-surgery drainage tube placement rate (82.8% vs 93.8%, p = 0.046), and shorter post-operative days on antibiotics (6.0 ± 2.6 vs 7.1 ± 2.4 days, p = 0.010) in the FGHM group. Although with a relatively high acquisition price, the use of FGHM for hemostasis in LS did not increase the total hospitalization costs (coefficient = −0.001, p = 0.972).
Conclusions: The use of FGHM in LS improved perioperative outcomes related to hemostatic effects without increasing overall hospital costs in a real-world hospital setting.
Introduction
Spinal fusion procedures, which are conducted for degenerative lumbar disc herniation or lumbar spinal stenosis, could cause significant perioperative blood lossCitation1,Citation2. Excess blood loss increases the need for allogeneic blood transfusion, which could potentially lead to blood-borne disease transmission and the increased mortality riskCitation3. Additionally, significant blood loss during lumbar surgery (LS) is associated with an increased risk of infection and post-operative complications. Thus, efficient hemostasis during lumbar surgery plays a critical role in conservation of allogeneic blood, and the cost saving associated with perioperative outcomesCitation4.
Bleeding of epidural and basivertebral veins during spinal surgery can be managed by varied hemostatic agentCitation5. However, the anatomy of spinal epidural space, characterized by a deep, narrow area outside the dura mater, which contains the spinal cord, greatly limits the utilization of conventional hemostatic techniques in LS. For example, it is difficult to compress the epidural bleeding surface by the oxidized cellulose and collagen spongeCitation6. In addition, swelling of the sponge-type hemostatic agent could lead to neurological compression, and several products are contraindicated for that reasonCitation7. Flowable gelatin hemostatic matrix (FGHM), mixing gelatin granules and saline, can conform and reach the narrow surgical fields by the flexible applicatorCitation8. FGHM has been shown to be safe and effective on stopping epidural bleedingCitation9. SURGIFLO was the first FGHM launched in China in 2015. However, the real-world clinical and economic impact of FGHM for hemostasis in LS remained unclear in the Chinese hospital setting.
Materials and methods
This retrospective cohort study was designed to assess the impact of FGHM on perioperative outcomes and hospital cost associated with LS. The study protocol was reviewed and approved by the research ethics board of the tertiary care hospital where this study was conducted.
Study patients
The study screened the hospital admission diagnosis for lumbar disc herniation or lumbar spinal stenosis from January 2014 to December 2016. The electronic medical records were reviewed to identify patients who underwent lumbar fusion surgeries. FGHM has been commercially available in the study hospital since January 2015. To control the selection bias associated with using FGHM for surgery with higher bleeding risk, a historical control (HC) group was created by including lumbar surgery patients treated with traditional TAH, including oxidized cellulose gauze, collagen sponge, and gelatin sponge, in 2014. The FGHM group was created by including patients received FGHM for hemostasis in lumbar surgery performed in 2015 and 2016. To control the confounding effects associated with surgeon’s skills, this study only included the patients who were operated on by the same surgeon. Patients who underwent repeated LS or other irrelevant operations in the same hospital episode were excluded.
Surgical technique
This study identified two procedures, PLIF and minimally invasive surgeries of transforaminal lumbar interbody fusion (MIS-TLIF), under the same general anesthesia approach for LS in the studied patients. FGHM (SURGIFLO, Ethicon Inc, Johnson & Johnson, Somerville, NJ) was used for hemostasis after laminectomy/facetectomy, resection of posterior longitudinal ligament and ligamentum flavum, and after resection of hyperplasia on the centrum brim. Gelatin sponge and oxidized cellulose or collagen were cut into appropriately sized pieces and applied against the bleeding sites during both procedures. After the articular process fusion was completed, the exposed surface of spinal dura was covered by FGHM or absorbable sponge/gauze.
Outcome measures
The surgical records were reviewed to determine operation time and intraoperative blood loss. The recorded intraoperative blood loss was based on the weight of soaked ribbon gauzes and the volume in suction canister subtracted irrigation fluid. The total drainage volume was calculated by summing the recorded drainage volumes during the placement of the drainage tube. Hospital admission date, operation date, and hospital discharge date were used to calculate the total and postoperative lengths of stay (LOS). Number of interbody fusion cage and screws, utilization of hemostatic agent, number of packs of allogeneic transfusion, and the hospital costs were obtained from the billing records.
Statistical data analysis
The patient baseline characteristics of the two study groups (FGHM vs HC) were compared for any significant differences using Student t-test for normally distributed continuous variables, Mann-Whitney U-test for continuous variables with skewed distribution, or Fisher exact test for categorical variables. The identified patient characteristics with significant differences between the two study groups were included as independent variables in the multiple logistic regression model to develop the formula to calculate propensity score for each included patient regarding the chance of receiving FGHM. A greedy approach was used to identify the best matching pairs for FGHM vs HC with the least difference in propensity scores of the pair and meeting the criteria that the propensity score difference between the matched pair was less than 0.001. The propensity score matched pairs were compared for operation time, post-surgery hospital stay length, blood loss, and blood transfusion volume using paired Student t-test. McNemar’s test was used to compare the dichotomous outcomes, including the blood transfusion rate and post-surgery drainage tube placement rate, between the two propensity score matched groups. Wilcoxon’s signed rank test was used to compare the hospital costs associated with LS between the two matched groups. Multiple generalized linear regression analyses with the adjustment for patient baseline characteristics were conducted to confirm the comparison of the two propensity score matched groups regarding total hospital. Furthermore, this study performed a supplement analysis to compare FGHM vs HC in the included patients who underwent PLIF (PLIF sub-group), the procedure accounting for 90.5% of the included patients, for the measured perioperative outcomes. Statistical software SAS 9.2 was used to conduct the data analysis described above, and the statistical significance in these data analyses was defined as a two-sided p-value less than 0.05.
Results
The study initially identified 379 patients who were operated on by the same surgeon for LS from 2014–2016. Eighty-two of the 115 initially identified patients in 2014 were included to create the HC group; 108 out of the 264 initially identified patients in 2015 and 2016 were included to create the FGHM group. The patients in the FGHM group received a combination of FGHM and gelatin sponge for hemostasis associated with LS. The hemostatic agents utilized in the HC group included a combination of oxidized cellulose and gelatin sponge for seven patients (9%), a combination of collagen and gelatin sponge for 46 patients (56%), a combination of oxidized cellulose, collagen, and gelatin sponge for 23 patients (28%), and gelatin sponge only for six patients (7%).
Creating the propensity score matched groups
The FGHM group and HC group had comparable patient baseline characteristics except the distributions of surgery procedures (MIS-TLIF: 13.9% vs 3.7%, p = 0.023; PLIF: 86.1% vs 96.3%, p = 0.023), residence location (regional city: 40.7% vs 24.4%, p = 0.021; rural area: 13.9% vs 31.7%, p = 0.004), and employment status (unemployed: 14.8% vs 4.9%, p = 0.032; peasant: 11.1% vs 24.4%, p = 0.019). The propensity score methods created 64 matched pairs with fully balanced patient baseline characteristics for FGHM vs HC. Of the matched patients, 4.7% of patients received MIS-PLIF procedure. The comparisons of patient baseline characteristics associated with FGHM and HC before and after propensity score match are summarized in .
Table 1. Summary of patient baseline characteristics (before and after propensity score match and PLIF sub-group).
Intraoperative blood loss and blood transfusion
The matched FGHM group was associated with significantly lower total transfusion rate (34.4% vs 64.1%, p = 0.005) and also allogeneic transfusion rate (25.0% vs 54.7%, p = 0.004) than the matched HC group. The matched FGHM group was associated with a significantly lower total blood transfusion volume (182.7 ± 312.4 mL vs 301.3 ± 281.0 mL, p = 0.045) and allogeneic transfusion volume (150.0 ± 294.8 mL vs 267.0 ± 290.4 mL, p = 0.043) than the matched HC group. The reduced blood transfusion rate in the FGHM group was also observed in PLIF sub-comparison.
Operation time, drainage, utilization of antibiotics, and hospital stay length after operation
Comparison of the two propensity score matched groups showed a significantly lower rate of drainage tube placement (82.8% vs 93.8%, p = 0.046) and shorter duration of antibiotics treatment after operation (6.0 ± 2.6 days vs 7.1 ± 2.4 days, p = .010) in the FGHM group. However, the comparisons of hospital stay length after surgery associated with the two matched groups didn’t identify statistical significance (5.9 ± 1.9 days vs 6.5 ± 2.1 days, p = 0.093). The operation time, the duration of drainage tube placement, and total drainage output associated with the two matched groups were comparable. The comparison of perioperative outcomes associated with the two propensity score matched groups are summarized in .
Table 2. The summary of the measured surgical outcomes and total cost (before propensity score match and PLIF sub-group).
Table 3. The summary of the measured surgical outcomes and total cost (after propensity score match).
Hospital costs
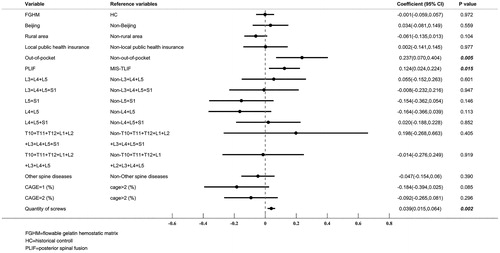
The matched FGHM group was associated with significantly higher acquisition costs of hemostatic agents than the matched HC group (median = ¥3,145 vs ¥431, p < 0.001, ¥1 = US$0.15 as of June 28, 2018). However, the total hospital costs associated with the two propensity score matched groups were very comparable (median = ¥87,707 vs ¥84,735, p = 0.949). The multiple generalized linear regression further confirmed the comparable total hospital costs (coefficient = −0.001, p = 0.972) associated with FGHM and HC groups after the adjustment for the patient baseline characteristics. Of the adjusted patient characteristics, health resources paid by out-of-pocket, PLIF procedure, and number of screws implanted were key factors associated with higher total hospital costs. The results of this multiple generalized linear regression analysis are illustrated in .
PLIF sub-group analysis
Comparison of the health outcomes associated with FGHM and HC in the PLIF sub-group showed similar results to the previous comparison of the two propensity score matched groups for FGHM vs HC ( and ). The FGHM was associated with a significantly lower rate and volume of blood transfusion, and drainage tube placement rate.
Discussion
This study demonstrated the impact of a flowable hemostatic matrix on perioperative outcomes and hospital cost in a Chinese real-world setting. The observed reduction of both blood transfusion rate and volume associated with the FGHM group relative to the HC group was likely to be the result of better hemostasis effects associated with the combination of FGHM and gelatin sponge relative to the combination of the conventional hemostatic agents that included oxidized cellulose, gelatin sponge, and/or collagen. The autologous blood collection technique is commonly applied during spinal surgery in China. Therefore, allogeneic blood transfusion could represent the utilization of blood resource better. The observed significant reductions of allogeneic intraoperative blood transfusion rate and volume further confirmed the better hemostasis effects associated with the combination FGHM and gelatin sponge.
Blood transfusion is an important indicator of perioperative outcome for LS. The less blood loss and lower allogenic transfusion could decrease the risks of anemia and infections, as well as alleviate the pressure on hospital blood supply. Many previous studies reported the association between better hemostasis and decreased blood transfusion. For example, topically applied tranexamic acid or collagen has been shown to significantly decrease blood transfusion rate through improved hemostasis in posterior lumbar fusion surgery with degenerative diseaseCitation10. Another randomized control trial observed that better hemostasis associated with gelatin sponge relative to bipolar cautery resulted in a reduced allogeneic blood transfusion rateCitation11.
The use of FGHM could address some of the gaps from conventional hemostatic agents, which are unable to fully meet the hemostatic needs associated with LS. For example, the physical nature of bipolar cautery makes it challenging to reach bleeding sites between centrum during discectomy, and it also could cause thermal injury to the adjacent vessel and neural structureCitation12. Gelatine sponge can expand ∼ 300-fold after becoming saturated with bloodCitation13, thereby causing spinal cord compressionCitation14. Gelatin sponge is useful for hemostasis associated with the upper-side of spinal dura but not for the ventral surface of spinal dura mater. Oxidized cellulose could increase the risk of passing through the intervertebral foramen, potentially increasing the risk for spinal cord compressionCitation15. Thus, FGHM could be a better treatment option that enables surgeons to use an applicator for precise placement, e.g. in the contours of bleeding surfaces around chipped boneCitation16. Clinical trials have proven the superiority of FGHM for hemostasis over conventional hemostatic agents. A randomized trial observed a significantly higher success rate of hemostasis within 3 min for FGHM vs a gelatin-based hemostatic agent in spinal surgery (97% vs 71%)Citation17. Another randomized trial also showed the reduced blood transfusion associated with the combination of FGHM and thrombin in spinal fusion for idiopathic scoliosis. According to the multiple linear regression analysis in this study, FGHM could reduce total blood loss by 177 mLCitation18. Although, in this study, FGHM showed better hemostasis effects, caution is needed for the potential risk of fragment mixtures of gelatin and blood in the blood scavenging systemCitation19.
The reduced post-surgery drainage tube placement rate associated with FGHM might be the result of less blood loss and tissue damageCitation20. The post-surgery drainage tube placement could be a source of post-operative anemiaCitation1,Citation21. According to the existing association between drainage tube placement and the risks of epidural hematoma and post-operation epidural fibrosisCitation22, the lower drainage tube placement rate implies that the risks of epidural hematoma and post-operation epidural fibrosis could be lower for patients receiving FGHM. Furthermore, the reduced post-surgery drainage tube placement might contribute to the significantly shorter duration of antibiotic treatment by reducing the risk of infection and facilitating patient recoveryCitation23,Citation24. This study observed a comparable operation time between the two study groups. Because FGHM is mainly applied to the spinal epidural space for the control bleeding and the duration of surgery procedure related to spinal epidural space is relatively short in the whole surgery process, the impact of hemostasis effects associated with spinal epidural space on the operation time of LS is a minimum.
Effective intraoperative hemostasis affects medical costs as well. The observed comparable total hospital costs associated with the two study groups suggests that the increased purchasing costs of FGHM could be offset by the saved hospital costs associated with improved perioperative outcomes. The lower blood transfusion rate means the cost saving on allogeneic blood, laboratory test, and transfusion consumable materials for patients. Also, the lower blood transfusion rate implies the release of blood resource pressure for the hospital. The reduced risk of post-surgery drainage tube placement may shorten the length of post-operation hospital stay length, leading to cost saving as well.
The study has a few limitations. To control the bias and confounding effects, this study used relatively strict inclusion and exclusion criteria, which could reduce the generalizability of the study results. Thus, future studies are still needed to confirm the improved hemostasis associated with FGHM in LS operated by other surgeons or in other hospital settings. Our study didn’t identify any surgery-related complications or adverse events associated with the included patients likely because of the high rank of the study hospital, which provided the highly skilled surgeon and high standard operation care. Thus, the impact of hemostatic effects associated with FGHM on the complications or adverse events associated with LS was unlikely to be observed and detected. Even though our study observed shorter duration of antibiotic treatments in the FGHM group, it is hard to conclude lower risk of infection associated with use of FGHM because of confounding effects of prophylactic treatment and clinical practices. Additionally, the observed impact of FGHM on the hospital costs necessitates validation in future studies, as the clinical practice and the patient affordability across Chinese hospitals are highly varied.
Conclusion
In summary, this study demonstrated the superiority of FGHM relative to conventional hemostatic agents regarding the measured outcomes indicating hemostasis in lumbar surgery. The comparable total hospital costs of two groups suggested that the increased purchasing costs of FGHM could be offset by the saved hospital costs for blood transfusion, laboratory tests, and post-surgery care. The limited generalizability indicates the need for future studies to confirm this study’s findings in patients who underwent LS in other hospital settings.
Transparency
Declaration of funding
The research was funded by Ethicon Inc, Somerville, NJ, USA.
Declaration of financial/other relationships
The authors have no conflicts of interest to disclose. A peer reviewer on this manuscript has disclosed that they are a consultant for Baxter. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
None reported.
References
- Qureshi R, Puvanesarajah V, Jain A, et al. Perioperative management of blood loss in spine surgery. Clin Spine Surg. 2017;30:383–388.
- Mummaneni PV, Dhall SS, Eck JC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. part 11: interbody techniques for lumbar fusion. SPI. 2014;21:67–74.
- Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135–148.
- Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine. 2007;32:537–543.
- Price JS, Tackett S, Patel V. Observational evaluation of outcomes and resource utilization from hemostatic matrices in spine surgery. J Med Econ. 2015;18:777–786.
- Xu D, Ren Z, Chen X, et al. A randomized controlled trial on effects of different hemostatic sponges in posterior spinal fusion surgeries. BMC Surg. 2016;16:80–85.
- Menovsky T, Plazier M, Rasschaert R, et al. Massive swelling of Surgicel® Fibrillar™ hemostat after spinal surgery. Case report and a review of the literature. Minim Invasive Neurosurg. 2011;54:257–259.
- Fuchs M, Iooss P, Srour R, et al. Surgiflo® without thrombin, a credible alternative to Floseal® in spinal surgery. Pharmacien Hospitalier Et Clinicien. 2015;50:289–295.
- Gazzeri R, De BC, Galarza M. Use of a thrombin-gelatin hemostatic matrix (Surgiflo®) in spinal surgery. Surg Tech Int. 2014;25:280–285.
- Xu D, Zhuang Q, Li Z, et al. A randomized controlled trial on the effects of collagen sponge and topical tranexamic acid in posterior spinal fusion surgeries. J Orthop Surg Res. 2017;12:166–172.
- Wu J, Jin Y, Zhang J, et al. Hemostatic techniques following multilevel posterior lumbar spine surgery. Clin Spine Surg. 2014;27:442–446.
- Lu D, Ding WG, Sheng HF, et al. The efficacy and safety of using a bipolar sealer to prevent blood loss in spine surgery: a meta-analysis. Int J Surg. 2017;46:37–46.
- Sabel M, Stummer W. Haemostasis in spine surgery. the use of local agents: Surgicel® and Surgifoam®. Eur Spine J. 2004;13:S97–S101.
- Alander DH, Stauffer ES. Gelfoam-induced acute quadriparesis after cervical decompression and fusion. Spine. 1995;20:970–971.
- Rodbelt AR, Miles JB, Foy PM, et al. Intraspinal oxidised cellulose (Surgicel®) causing delayed paraplegia after thoracotomy – a report of three cases. Ann R Coll Surg Engl. 2002;84:97–99.
- Hu SS. Blood loss in adult spinal surgery. Eur Spine J. 2004;13:S3–S5.
- Renkens KL, Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine. 2001;26:1645–1650.
- Helenius I, Keskinen H, Syvanen J, et al. Gelatine matrix with human thrombin decreases blood loss in adolescents undergoing posterior spinal fusion for idiopathic scoliosis. Spine. 2016;98B:395–401.
- Kumar S, Goyal K, Dubey S, et al. Anaphylactic reaction after autologous blood transfusion: a case report and review of the literature. Asian J Neurosurg. 2015;10:145–147.
- Zou H, Li Z, Sheng H, et al. Intraoperative blood loss, postoperative drainage, and recovery in patients undergoing lumbar spinal surgery. BMC Surg. 2015;15:76.
- Schroeder GD, Kurd MF, Kepler CK, et al. Postoperative epidural hematomas in the lumbar spine. J Spinal Disord Tech. 2015;28:313–318.
- Hasan M, Mehmet E, Sebnem O. Are drains useful for lumbar disc surgery? A prospective, randomized clinical study. Clin Spine Surg. 2006;19:171–177.
- Cho SK, Yi JS, Park MS, et al. Hemostatic techniques reduce hospital stay following multilevel posterior cervical spine surgery. J Bone Joint Surg Am. 2012;94:1952–1958.
- ter Gunne AFP, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine. 2009;34:1422–1428.