Abstract
Objective: Cervical cancer is a huge public health issue in Morocco which represents the second most frequent and fatal cancer among women. Countries that have not yet introduced the HPV vaccine could benefit greatly, but before implementation it is necessary to perform country-specific economic assessments that include current screening practices.
Methods: A Markov model was developed to simulate the natural history of HPV and cervical cancer so as to calculate the long-term health benefits and costs of HPV vaccination and current screening by visual inspection with acetic acid (VIA). Starting from a previous transition probability matrix used for a model from Spain, the present model was calibrated to cervical cancer incidence from Morocco. Cost survey data was used to estimate the cost of screening and clinical procedures from the public healthcare perspective. Incremental cost-effectiveness ratios were calculated as 2018US$ per additional year of life saved (YLS) and both costs and health outcomes were discounted at 3%.
Results: The expected reduction in lifetime risk of cervical cancer for current screening would be 14% at a cost of US$551/YLS compared with no intervention, assuming VIA every 3 years in women aged 30–49 at 10% coverage. HPV vaccination of pre-adolescent girls at 70% coverage would reduce the lifetime risk of cervical cancer by 62% at a cost of US$1,150/YLS, compared with no intervention. When implementing HPV vaccination in combination with current screening, vaccination would be dominated, and the combined strategy would provide a 69% reduction at a cost of US$2,843/YLS, compared with screening alone. Current screening would be dominated by vaccination when screening coverage is higher than 15%, whereas the combined strategy rapidly exceeds US$4,000/YLS.
Conclusions: HPV vaccination could be highly effective and cost-effective in Morocco. Current screening would be good value for money compared with no intervention, but scaling-up screening coverage would make it inefficient compared with vaccination.
Introduction
Cost-effectiveness analyses are increasingly required in the healthcare decision-making process for the purpose of informing about which new products to include in national public healthcare systems or optimizing those that already existCitation1. Mathematical models are commonly used in these analyses to simulate diseases and the long-term health outcomes and economic impact of different interventions that cannot be explored through experimental studies. The ultimate goal is to determine a set of strategies that result in good value for money.
Cervical cancer is the third most common cancer among women worldwide, and this health issue mostly occurs (∼85%) in low- and middle-income countries, accounting for nearly 12% of all female-related cancersCitation2. In Morocco, cervical cancer ranks second among women with an age-standardized incidence rate of 17.2 per 100,000 women and an age-standardized mortality rate of 12.6 in 2018. There are currently more than 200 virus strains of human papillomavirus (HPV) classified according to the degree of carcinogenesis risk, of which at least 13 are cancer-causingCitation3. HPV types 16 and 18 are the most virulent and are responsible for 70% of cervical cancer cases globally, as well as a varying proportion of other cancer and non-cancer diseasesCitation4,Citation5.
Screening activities have showed that early detection can reduce the morbidity and mortality associated with cervical cancerCitation6. Several screening tests with different performances in terms of sensitivity and specificity are currently available, such as the Pap smear, visual inspection with acetic acid (VIA) and HPV DNA. In Morocco, opportunistic cervical cancer screening based on VIA every 3 years in women aged 30–49 was launched in 2010Citation7,Citation8. However, the coverage rate at the national level is only ∼6–10% of the targeted women, with ∼80–90% of all cervical cancer cases being diagnosed at an advanced stageCitation7,Citation9,Citation10. This low coverage rate might be explained by the lack of mass communication and public awareness campaigns about the cervical cancer screening programCitation7.
Three prophylactic vaccines against HPV infections are currently available and have proved to be highly safe and effective for the prevention of HPV vaccine-type infection and associated diseasesCitation11. The bivalent vaccine protects against HPV types 16 and 18; the quadrivalent vaccine protects against HPV types 6, 11, 16, and 18; and the nonavalent vaccine targets the same HPV types as the quadrivalent vaccine as well as types 31, 33, 45, 52, and 58. The World Health Organization (WHO) points out that developing countries with difficulties in implementing screening programs might reap an important benefit from HPV vaccination as a primary prevention strategy for cervical cancer diseaseCitation12,Citation13.
On this basis, health policy decision-makers in countries like Morocco need to know the cost-effectiveness of HPV vaccination to decide the best way to allocate their scarce healthcare resources. Hence, the aim of this project is to assess the cost-effectiveness of implementing an HPV vaccination program in Morocco and the current screening with VIA at different frequencies, both strategies separately, and in combination.
Methods
The model
A Markov model was developed to simulate the natural history of HPV infection and cervical cancer in order to calculate the long-term health benefits and costs of different cervical cancer preventive strategies. As shown in Supplementary Figure S1, the static model includes 12 boxes that represent mutually exclusive health states (healthy, HPV infection, cervical intraepithelial lesions (CIN) grade 1–3, International Federation of Gynecology and Obstetrics (FIGO) cervical cancer stages, survival, cervical cancer mortality, and mortality from other causes). Women move from one health state to another in 1-year intervals according to some transition probabilities. All women start the model simulations as healthy and can move to the HPV-infected state by acquiring the infection with some probability. Women who have acquired HPV infection could develop CIN, and those with CIN 1 or with CIN 2/3 could progress, regress, or stay in the same state. Once in the cancer state, a woman may not regress to other health states, and instead progresses through the four FIGO stages. Women may die from cervical cancer in the cancer stages, or may die from other non-cervical cancer causes in every health state and every cycle. The model was developed with Microsoft Excel 2010.
Calibrating the model
For our initial calibration step, a transition probability matrix from a previous validated model for Spain was used to reproduce cervical cancer incidence from MoroccoCitation14,Citation15. To ensure that the model fits the epidemiological data from Morocco, a manual calibration process was performed to reduce uncertainty in transition probabilities selected from various studies. The model was calibrated to reproduce age-specific cervical cancer incidence prior to launching the screening program in 2010 (Supplementary Figure S2).
Strategies considered
Three cervical cancer preventive strategies were included in the base-case analysis: current screening in Morocco of women aged between 30 and 49 years with a VIA test every 3 years at 10% coverage rate; HPV 16/18 vaccination of pre-adolescent girls at 14 years old with two doses at 70% coverage rate assuming 90% efficacy and lifelong immunity; and combined vaccination and screening.
Demographic and epidemiological data
The distribution of the Moroccan female population by age group was extracted from the United Nations’ population prospects for 20151Citation6. Annual transition probabilities depicting the natural history of cervical cancer and HPV infection were also extracted from published epidemiologic articles under the assumption that the mechanism of cervical carcinogenesis from initial infection is universal and that it does not differ greatly between countriesCitation17–20. Epidemiological data on cervical cancer incidence and mortality by age were derived from the cancer registries of the Grand Casablanca Region and from the ICO-HPV Information CenterCitation21,Citation22. The sensitivity and specificity of VIA were derived from a systematic review conducted to compare the test accuracy of the HPV DNA test, cytology, and VIACitation23. The baseline assumptions on screening and vaccination are shown in .
Table 1. Baseline assumptions and direct medical costs indexed at year 2018.
Economic data
Since it is important to use country-specific information but there is a lack of data on costs related to cervical cancer screening procedures in Morocco, a survey was carried out to estimate the direct medical costs of screening, diagnosis, and treatment of pre-cancerous lesions using loop electrosurgical excision procedure (LEEP) and treatment of cervical cancer from the public healthcare perspective in 2015 Moroccan dirhams. The data collection and cost calculation methods are both included in the Supplementary Appendix. All costs were converted to 2018 US dollars (US$) using GDP deflators and average annual exchange ratesCitation24 ().
The lower price reported for HPV vaccine was offered by Merck to the Global Alliance for Vaccines and Immunizations (GAVI) for the poorest countries (US$4.50 per dose), but Morocco is not eligible for GAVI supportCitation25. The negotiated price by the Revolving Fund of the Pan American Health Organization (PAHO) for low- and middle-income countries in Latin America is ∼US$10–US$15 per doseCitation26. Based on this, we assumed an initial vaccine cost of US$10 per dose and a US$5 administration cost.
For the purpose of identifying those strategies that appear to be relatively good value for money, usually a cost-effectiveness threshold is defined. However, there is no universal criterion and there is no consensus on how it should be derivedCitation27. For illustrative purposes, the approach of the Commission on Macroeconomics and Health was used, since there is no specific local threshold defined for MoroccoCitation28,Citation29. This heuristic suggests that an intervention should be considered highly cost-effective if the ICER is less than the country’s per capita gross domestic product (GDP) and cost-effective if the ICER is less than three times the per capita GDP. The estimated per capita GDP in Morocco for the year 2018 is US$2,86024.
Outcomes, measurements, and cost-effectiveness analysis
For each strategy, the model predicts the life expectancy (LE) and the lifetime cost per woman. The incremental cost-effectiveness ratio (ICER) was the measurement used to perform the cost-effectiveness analysis, defined as the difference in cost between two interventions, divided by the difference in health (LE in our case). Therefore, the ICER represents the incremental cost associated with one additional year of life saved (YLS). Both cost and health outcomes were discounted at an annual rate of 3%. To identify which parameters and assumptions are the most influential in the results, one-way and two-way sensitivity analyses were carried out for the vaccination’s coverage and cost, as well as for the coverage, sensitivity, and cost of the VIA test.
Results
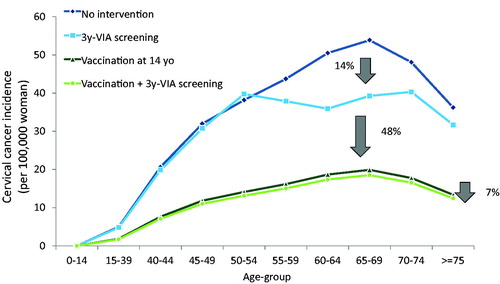
The expected reduction in lifetime risk of cervical cancer for current screening is 14% compared with no intervention, assuming VIA every 3 years in women aged 30–49 at 10% coverage (). Pre-adolescent vaccination alone has an important impact on lifetime risk of cervical cancer, reducing it by 63% compared with no intervention and by 48% compared with VIA every 3 years. An additional reduction of 7% is also obtained when combining vaccination with the existing screening program compared with vaccination alone.
A cost-effectiveness analysis was performed, comparing the current screening in Morocco using VIA every 3 years with different frequencies of screening. It was found that, as the frequency increases, both the effectiveness and the costs rise, and screening with VIA at any frequency would be dominated by VIA once-in-a-lifetime that would be cost-saving compared with no intervention (). Vaccination alone at a 70% coverage rate would cost US$1,150 per YLS compared with no intervention (), well below the per capita GDP threshold. The current screening program with VIA in Morocco (10% coverage to women aged 30–49 years) would cost US$551 per YLS compared with no intervention (). When implementing HPV vaccination in combination with existing screening, vaccination alone would be dominated, and the combined strategy has an incremental cost-effectiveness ratio of US$2,843 per YLS compared with screening alone, which would be considered cost-effective based on the GDP per capita threshold (). When the screening coverage rate is higher than 15%, vaccination is no longer dominated, instead screening alone is dominated and the ICER for the combined strategy increases rapidly, exceeding US $4,000/YLS above 20% ( and Supplementary Table S4).
Table 2. Discounted life expectancy (LE), discounted total lifetime cost per woman, and incremental cost-effectiveness ratio for evaluated strategies.
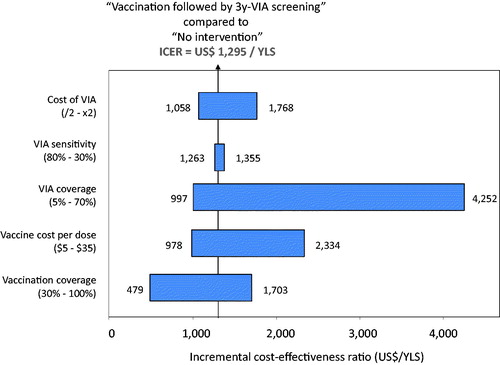
The one-way sensitivity analysis shows that the cost-effectiveness of adding vaccination to the existing screening program remains relatively stable on varying the cost per dose up to current tender prices in high-resource countries (∼US$35 per dose) and vaccination coverage (up to 100%); in neither case does the ICER exceed the per capita GDP. The combined strategy is also stable on varying the sensitivity of the VIA test and the cost of VIA, whereas it is sensitive to the increase in coverage of the VIA test ()Citation30. Indeed, when the coverage of VIA goes from 5% to 100%, the ICER jumps from US$997 per YLS to US$5,702 per YLS compared with no intervention, exceeding the per capita GDP threshold at a 50% coverage rate unless the cost of the VIA test drops by one third (Supplementary Figure S3). At coverage of 75%, the combined strategy exceeds the per capita GDP threshold unless the cost of the VIA test drops by one half. At the current price, the ICER for the combined strategy is below three times the per capita GDP, even at 100% coverage. The slight variation on the sensitivity of the VIA test is due to the low coverage rate of 10% assumed for the current existing screening and the small population covered (from 30 to 49), which excludes most of the female population at risk for cervical cancer from screening.
Discussion
Our results show that scaling up coverage of screening with VIA more than 15% in Morocco is not cost-effective, whereas HPV 16/18 vaccination for pre-adolescent girls at age 14 with a coverage rate of 70% is highly cost-effective and could reduce cervical cancer by 63%. Provided the cost per vaccinated girl is US$10 per dose, we have found that vaccination alone costs US$1,150 per YLS, well below the 2018 per capita GDP (US$2,860). Even when the vaccine cost per dose is increased up to current tender prices in high-resource countries, pre-adolescent HPV vaccination would be considered good value for money. The existing screening program with VIA every 3 years for women aged between 30 and 49 years at 10% coverage costs US$551 compared with no intervention. However, this would be considered dominant compared with vaccination at US$10 per dose and 70% coverage rate when coverage of VIA is higher than 15%. The high cost of screening and subsequent follow-up and treatment together with an inefficient screening with VIA and low coverage in a small fraction of women at risk of cervical cancer makes screening alone unattractive in Morocco compared to HPV vaccination at reasonably low prices. Vaccination combined with existing screening practices in Morocco costs US$2,843 per YLS, which is less than Morocco’s per capita GDP. The higher the screening coverage, the greater the ICER, exceeding the per capita GDP threshold at a 50% coverage rate unless the cost of the VIA test drops by one third.
To our knowledge, our study is the first cost-effectiveness analysis calibrated to the Moroccan context that takes into account the existing screening program. Only a simple Excel-based model was used to project the population-level health and economic impact of preadolescent HPV vaccination in 20 countries, including MoroccoCitation31. However, this model was not calibrated to Morocco and did not include screening strategies. The authors conclude that HPV vaccination would be cost-saving at a cost of $5 per dose, cost-effective at a cost of $26.75 per dose, and would exceed the cost-effectiveness threshold at a cost of $54.25 per dose. The calibration procedure is a crucial aspect for the model’s performance, especially when it involves policy decisionsCitation15. Several cost-effectiveness analyses in low- and middle-income countries have examined the value of HPV vaccination in combination with VIACitation32–36. All of these studies are in line with our results, considering HPV vaccination to be cost-effective in resource-poor settings if the vaccine cost decreases significantly in relation to the current out-of-pocket price. In such circumstances, the best cervical preventive option would be a combined strategy of VIA screening with HPV vaccination.
The WHO recommends introducing the HPV vaccines into national immunization programs and using VIA in resource-constrained settingsCitation11,Citation37. However, if HPV testing is feasible, the WHO suggests a strategy of screening with an HPV test over a strategy of screening with VIA. This is increasingly achievable, since new methods of HPV testing for low-resource settings that require low staff qualifications are being developed, alongside alternative sample collection methods such as cervical specimen self-samplingCitation38. Furthermore, based on estimates of the manufacturing costs of HPV vaccines, the price for the GAVI alliance for introducing the HPV vaccine in the poorest countries could be around $0.50 per dose; much lower than the current price of $4.5039. The same study states that prices for lower- and middle-income countries could also be lowered.
Although there have been more studies on cervical cancer prevention in Africa in recent years, there are still several countries with little to no research ever conducted in this areaCitation40. Given the substantial morbidity and mortality caused by this cancer in this setting, more research is needed to inform about which feasible, sustainable strategies can maximize women’s health. Our analysis is the first attempt to assess the epidemiologic and economic impact of HPV vaccination and existing screening practices in Morocco. The ultimate aim is to provide information in the long-term on value for money for cervical cancer prevention strategies in Morocco to help decision-makers who have to work on prioritization of public health investment.
There are several limitations to our approach, including the model’s structure, input data, assumptions, and uncertainty of the parameters. Some of these are inherent in decision-analytic models and were addressed following scientific criteria of good practices or using standard approachesCitation41,Citation42. For example, our model inevitably requires assumptions about the costs of interventions and procedures, the effectiveness of VIA screening and vaccination, and about cervical cancer incidence and HPV prevalence data. As the quality of the results is only as good as the quality of the assumptions and synthesized data, the most reliable information from Morocco was included in the modelCitation43. Subsequently, to ensure the credibility of the results, model predictions on cervical cancer incidence were calibrated to real empirical data from Morocco. In addition, sensitivity analyses were performed which can help appreciate the impact of uncertainties in the key parameters. However, some other limitations are more difficult to address. For example, our model does not capture herd immunity effects, nor does it include the potential benefits of vaccination on other non-cervical HPV-related diseases such as other anogenital cancers or genital warts, and it does not reflect the impact of cross-protection against other non-vaccine HPV types. These limitations may lead the health benefits of HPV vaccination to be under-estimated. Further, as our model is a simple representation of the natural history of HPV infection and cervical cancer and not a detailed model with screening complex strategies that incorporate different visits, all women diagnosed with a CIN lesion are treated and there is no loss-to-follow-up. In order to deal with all these issues, it would be necessary to use more complex approaches such as dynamic microsimulation models. Another limitation is that our analysis is carried out under the favorable assumption of high efficacy against HPV 16/18 with two doses; therefore, any evidence regarding the reduction of the efficacy in two doses, the inclusion of waning immunity or the need for a booster to obtain long-term protection would lead to less attractive results for HPV vaccination. Finally, although the threshold based in the GDP is often used in cost-effectiveness analysis in low- and middle-income countries, recent papers suggest that thresholds representing likely health opportunity costs tend to be below one GDP per capitaCitation44,Citation45. In our analysis, the ICER for vaccination represents an eleventh of the per capita GDP in Morocco, which may indicate that vaccination would be cost-effective with a much lower threshold; whereas the ICER for combined vaccination and existing screening with VIA and low coverage is between one and two times the per capita GDP, which may indicate that it could be displacing more health than it generates.
Conclusions
Based on our results, healthcare policymakers in Morocco should explore the possibility of supporting the implementation of pre-adolescent HPV vaccination for girls. However, they should also consider that introducing HPV vaccine into a national immunization program depends not only on the cost-effectiveness, but also on other important factors such as affordability, sustainable financing, avertable burden, feasibility, and equity. Further analyses with more complex approaches that include other non-cervical HPV-related diseases, vaccines that provide protection against more high-risk HPV types, and other screening tests such as HPV testing as suggested by the WHO are needed in order to reconfirm our results and to help guide the next steps on cervical cancer prevention in Morocco. Finally, a country like Morocco, where there has been problems with screening coverage, should also consider evaluating the cost-effectiveness of HPV self-collection, which has demonstrated to be a powerful tool to increase coverage and has the potential to overcome many of the barriers shown by other screening techniques.
Transparency
Declaration of funding
This work was partially supported by grants from the Instituto de Salud Carlos III through the projects RD12/0036/0056, PI11/02090, PIE16/00049, and PI16/01254 (Co-funded by European Regional Development Fund. ERDF, a way to build Europe). With the support of the Secretariat for Universities and Research of the Department of Business and Knowledge of the Government of Catalonia. Grants to support the activities of research groups (SGR 2017–2019). Grant number 2014SGR756, 2014SGR1077 and 2017SGR1718. The research leading to these results has received funding from the European Union's Seventh Framework Programme (FP7/2007–2013) under grant agreement n° Health-F3-2013-603019 (COHEAHR) and MNSIRSES [FP7-People-2013-IRSES612216]. The research leading to these results has received funding from RecerCaixa [2015ACUP00129]. We thank CERCA Programme/Generalitat de Catalunya for institutional support and Lalla Salma Foundation for the Prevention and Treatment of Cancers for financial and technical support. None of these entities played a role in data collection or analysis, or in the interpretation of the results.
Declaration of financial/other relationships
WM, TE, CN, NT, AZ, GA, DM and MD state that they have no competing personal or financial interests in relation to this study. MD-Institutional support: The HPV vaccine trials and epidemiological studies were sponsored by GlaxoSmithKline (GSK), Merck, Roche, and Sanofi Pasteur MSD. A peer reviewer on this manuscript has disclosed being a principal investigator of several HPV screening projects sponsored in part by BD Diagnostics, receiving honoraria from BD Diagnostics, being a principal investigator of EU HORIZON2020 SME contract 666-800, principal investigator of study sponsored in part by Agena Biotech, Genomica SAU, and Life River Biotech. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Ethics approval and consent to participate
This manuscript has been revised for its publication by the Clinical Research Ethics Committee of Bellvitge University Hospital. Verbal informed consent was obtained from all participants in this study. Data of participants were anonymized for the purposes of this analysis. The confidential information of the patients was protected according to national normative data.
Appendix CEA HPV Vaccination Morocco
Download PDF (332.8 KB)Acknowledgements
None reported.
References
- Rawlins MD. Crossing the fourth hurdle. Br J Clin Pharmacol. 2012;73:855–860.
- Ferlay J, Colombet M, Soerjomataram I, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2018 [cited 2018 Oct 15]. Available from: https://gco.iarc.fr/today.
- Papillomavirus Episteme (PaVE). National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID). [cited 2019 May] Available from: https://pave.niaid.nih.gov/#home.
- Clifford GM, Smith JS, Plummer M, et al. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73.
- Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527.
- Vaccarella S, Franceschi S, Engholm G, et al. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111:965–969.
- Selmouni F, Sauvaget C, Belakhel L, et al. Organization and evaluation of a pilot cervical cancer screening program in Morocco. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2016;132:25–28.
- Selmouni F, Belakhel L, Sauvaget C, et al. Evaluation of the national cervical cancer screening program in Morocco: achievements and challenges. J Med Screen. [cited 2019 Jan 16]. DOI:10.1177/0969141318824627.
- Berraho M, Obtel M, Bendahhou K, et al. Sociodemographic factors and delay in the diagnosis of cervical cancer in Morocco. Pan Afr Med J. 2012;12:14.
- Elmajjaoui S, Ismaili N, El Kacemi H, et al. Epidemiology and outcome of cervical cancer in National Institute of Morocco. BMC Womens Health. 2016;16:62.
- WHO. WHO | Human Papillomavirus (HPV) position paper. Geneva: WHO; 2017 [cited 2017, November 4]. Available from: http://www.who.int/immunization/policy/position_papers/hpv/en/.
- Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull WHO. 2007;85:719–726.
- Tsu V, Murray M, Franceschi S. Human papillomavirus vaccination in low-resource countries: lack of evidence to support vaccinating sexually active women. Br J Cancer. 2012;107:1445–1450.
- Georgalis L, de Sanjosé S, Esnaola M, et al. Present and future of cervical cancer prevention in Spain: a cost-effectiveness analysis. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 2016;25:430–439.
- Moriña D, de Sanjosé S, Diaz M. Impact of model calibration on cost-effectiveness analysis of cervical cancer prevention. Sci Rep. 2017;7:17208.
- World Population Prospects-Population Division-United Nations. [cited 2018, April 11]. Available from: https://esa.un.org/unpd/wpp/.
- Myers ER, McCrory DC, Nanda K, et al. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151:1158–1171.
- Torvinen S, Nieminen P, Lehtinen M, et al. Cost effectiveness of prophylactic HPV 16/18 vaccination in Finland: results from a modelling exercise. J Med Econ. 2010;13:284–294.
- Annemans L, Rémy V, Oyee J, et al. Cost-effectiveness evaluation of a quadrivalent human papillomavirus vaccine in Belgium. PharmacoEconomics. 2009;27:231–245.
- Szucs TD, Largeron N, Dedes KJ, et al. Cost-effectiveness analysis of adding a quadrivalent HPV vaccine to the cervical cancer screening programme in Switzerland. Curr Med Res Opin. 2008;24:1473–1483.
- Cancer registries, Morocco. [cited 2018, March 23]. Available from: http://www.irc.ma/en/statistiques/registre-des-cancers/.
- Bruni L, Albero G, Serrano B, et al. [Internet]. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Morocco. Summary Report 10 December 2018. [cited 2018 Apr]. Available from: https://www.hpvcentre.net.
- Mustafa RA, Santesso N, Khatib R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstet. 2016;132:259–265.
- The World Bank [Internet]. World Development Indicators, WDI online version. [cited 2019 May] Available from: https://databank.worldbank.org/data/reports.aspx?source=world-development-indicators.
- GAVI alliance. Millions of girls in developing countries to be protected against cervical cancer thanks to new HPV vaccine deals. [cited 2017, December 15]. Available from: http://www.gavi.org/library/news/press-releases/2013/hpv-price-announcement/.
- Levin CE, Van Minh H, Odaga J, et al. Delivery cost of human papillomavirus vaccination of young adolescent girls in Peru, Uganda and Viet Nam. Bull WHO. 2013;91:585–592.
- Kvizhinadze G, Wilson N, Nair N, et al. How much might a society spend on life-saving interventions at different ages while remaining cost-effective? A case study in a country with detailed data. Popul. Health Metr. 2015;13:15.
- Bertram MY, Lauer JA, De Joncheere K, et al. Cost-effectiveness thresholds: pros and cons. Bull WHO. 2016;94:925–930.
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull WHO. 2015;93:118–124.
- Diaz M, Moriña D, Rodríguez-Salés V, et al. Moving towards an organized cervical cancer screening: costs and impact. Eur J Public Health. 2018;28:1132–1138.
- Kim J, Sharma M, O’Shea M, et al. Model-based impact and cost-effectiveness of cervical cancer prevention in the Extended Middle East and North Africa (EMENA). Vaccine. 2013;31:G65–G77.
- Diaz M, Kim JJ, Albero G, et al. Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India. Br J Cancer. 2008;99:230–238.
- Goldie SJ, Diaz M, Kim S-Y, et al. Mathematical models of cervical cancer prevention in the Asia Pacific region. Vaccine. 2008;26:M17–29.
- Praditsitthikorn N, Teerawattananon Y, Tantivess S, et al. Economic evaluation of policy options for prevention and control of cervical cancer in Thailand. PharmacoEconomics. 2011;29:781–806.
- Guerrero AM, Genuino AJ, Santillan M, et al. A cost-utility analysis of cervical cancer screening and human papillomavirus vaccination in the Philippines. BMC Public Health. 2015;15:730.
- Chanthavilay P, Reinharz D, Mayxay M, et al. The economic evaluation of human papillomavirus vaccination strategies against cervical cancer in women in Lao PDR: a mathematical modelling approach. BMC Health Serv Res. 2016;16:418.
- WHO. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. Geneva: World Health Organization; 2013.
- Toliman PJ, Kaldor JM, Tabrizi SN, et al. Innovative approaches to cervical cancer screening in low- and middle-income countries. Climacteric. 2018;21:235–238.
- Clendinen C, Zhang Y, Warburton RN, et al. Manufacturing costs of HPV vaccines for developing countries. Vaccine. 2016;34:5984–5989.
- Finocchario-Kessler S, Wexler C, Maloba M, et al. Cervical cancer prevention and treatment research in Africa: a systematic review from a public health perspective. BMC Womens Health. 2016;16:29.
- McGhan WF, Al M, Doshi JA, et al. The ISPOR good practices for quality improvement of cost-effectiveness research task force report. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2009;12:1086–1099.
- Trikalinos TA, Siebert U, Lau J. Decision-analytic modeling to evaluate benefits and harms of medical tests—uses and limitations. Med Decis Making. 2009;29:E22–E29.
- Griebsch I. Economic evaluation in health care: merging theory with practice. New York: Oxford University Press; 2001.
- Ochalek J, Lomas J, Claxton K. Cost per DALY averted thresholds for low- and middle-income countries - Research Database, The University of York. 2015 [cited 2019, April 29]. Available from: https://pure.york.ac.uk/portal/en/publications/cost-per-daly-averted-thresholds-for-low-and-middleincome-countries(12487fa5-e63f-4ac3-9fa4-03b2795065eb).html.
- Shillcutt SD, Walker DG, Goodman CA, et al. Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. PharmacoEconomics. 2009;27:903–917.