Abstract
Aims: Effective postsurgical analgesia hastens recovery, reduces hospital length of stay (LOS), and decreases hospitalization costs for total hip arthroplasty (THA). Improving these outcomes is critical for value-based surgical bundled payment programs such as the Medicare Comprehensive Care for Joint Replacement and similar programs for commercial insurance providers. This study compared clinical outcomes and hospitalization costs for patients undergoing THA with and without liposomal bupivacaine (LB).
Materials and methods: This retrospective, comparative cohort study used data from the Premier Healthcare Database from the 10 hospitals with highest use of LB for THA from January 2011 through April 2017. A cohort undergoing THA with LB at those hospitals was compared with a propensity-score matched cohort at those hospitals who had THA without LB. Descriptive, univariate, and multivariable analyses compared post-surgical inpatient opioid consumption, hospital LOS, discharge status, same-hospital readmissions, and total hospitalization costs. Analyses were performed using the Pearson Chi-square test (categorical variables) and Wilcoxon or Student t-test (continuous variables).
Results: For patients with Medicare (with LB, n = 3622; without LB, n = 3610) and commercial insurance (with LB, n = 2648; without LB, n = 2709), use of LB was associated with lower post-surgical inpatient opioid consumption (105 and 81 mg, respectively; p < 0.0001), a 0.7-day shorter LOS (p < 0.0001), a 1.6–1.7-fold increased likelihood of home discharge (p < 0.0001), and no increase in readmissions (p ≥ 0.103). Total hospitalization costs were $561 lower with LB in the Medicare population (p < 0.0001) and $41 higher with LB in the commercial population (p = 0.7697).
Limitations: Hospitalization costs were estimated from the hospital chargemaster. Findings from these 10 hospitals may not represent other US hospitals.
Conclusions: At select hospitals, THA with LB was associated with reduced post-surgical inpatient opioid consumption, shorter hospital LOS, increased likelihood of home discharge, and lower hospitalization costs. Post-surgical pain management with LB may help hospitals in value-based bundled payment programs.
Introduction
Between 2013 and 2015, an estimated one in five American adults reported a diagnosis of arthritis, the most common form of which is osteoarthritisCitation1. The prevalence of osteoarthritis is predicted to increase demand for total hip arthroplasty (THA), with an estimated 572,000 annual procedures being conducted in the US by 2030Citation2, and a majority of these procedures being performed in patients <65 years oldCitation3. Although THA is effective in relieving pain and improving function, it exerts a considerable economic burdenCitation4. In 2011, average hospital charges for THA were $55,226, and average Medicare reimbursement was $12,400Citation5. To improve the quality of care for THA and provide incentives to hospitals for improved coordination of care, the Centers for Medicare & Medicaid Services (CMS) implemented the Comprehensive Care for Joint Replacement (CJR) program in 2016Citation6. With CJR, participating hospitals receive a fixed payment from CMS for all healthcare services related to THA from the time of admission until 90 days after dischargeCitation6. Commercial health insurance providers (e.g. United Health) have implemented similar surgical bundled payment programs.
In surgical bundled payment programs, hospitals must strive to reduce costs while improving outcomesCitation7. For THA, hospital LOS, discharge status, and readmissions are substantial drivers of costsCitation8,Citation9, and inadequate management of post-surgical pain can negatively impact these outcomesCitation10–12. Although opioids are commonly used in post-surgical analgesiaCitation13,Citation14, it is important to consider their potential adverse effectsCitation15–17. A retrospective study of patients undergoing THA or total knee arthroplasty (TKA) reported that opioid-related adverse events (ORAEs) were the most commonly reported complicationsCitation18. ORAEs are associated with longer LOSCitation15,Citation18,Citation19, increased likelihood of discharge to post-acute care facilitiesCitation18, and higher total hospitalization costsCitation15,Citation19. To reduce the consumption of opioids and decrease the risk of ORAEs, clinical practice guidelines for THA recommend multimodal analgesia combining a variety of medications and techniques, including local anesthesia at the surgical site and non-opioid systemic analgesicsCitation13.
Liposomal bupivacaine (LB; EXPAREL® [bupivacaine liposome injectable suspension], Pacira BioSciences, Inc., Parsippany, NJ) is a non-opioid local anesthetic indicated for single-dose infiltration into the surgical site to provide post-surgical analgesiaCitation20. For hip arthroplasty, LB should be infiltrated throughout the surgical site using multiple, small-volume injections targeting nociceptors in muscles, connective tissues, subcutaneous tissues, and other tissues in different depths and layersCitation21. Unlike conventional local anesthetics such as bupivacaine HCl and ropivacaine HCl, LB is formulated to provide prolonged release of bupivacaine at the infiltration site, with analgesia for up to 72 hCitation22. Previous studies have reported that local infiltration with LB improves post-surgical analgesia compared with traditional bupivacaine HCl and other standard regimens for THACitation23–26, and decreases LOSCitation24,Citation25,Citation27,Citation28, which may reduce hospitalization costsCitation24.
The primary objective of this study was to examine real-world evidence to determine the impact of LB on post-surgical inpatient opioid consumption, hospital LOS, discharge status, readmissions, and total hospitalization costs in patients undergoing THA.
Methods
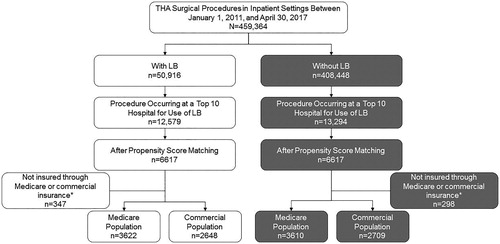
This retrospective, comparative cohort study assessed data obtained from the Premier Healthcare Database (PHD; Premier, Inc., Charlotte, NC), which contains chargemaster data for >600 hospitals that together represent one in five hospitalizations in the US. The analysis included patients who underwent primary THA (International Classification of Diseases, Ninth Revision [ICD-9] or ICD-10 procedure codes; see Supplementary Table S1 for codes) in an inpatient setting with Medicare or commercial insurance as the primary third-party payer. The study group included patients at the 10 hospitals with the highest total use of LB for THA between January 1, 2011, and April 30, 2017, while the comparator group included propensity-scored matched patients from the same hospitals who underwent THA and did not receive LB ().
Figure 1. Patient selection (inclusion/exclusion criteria). LB, liposomal bupivacaine; THA, total hip arthroplasty. *Patients insured through Medicaid or other sources were not included in the analyses due to the small sample size.
Use of LB was identified using 15-digit standard charge codes (e.g. 250250111850000) and charge code descriptions (e.g. Bupivacaine, EXPAREL VL 13.3 mg/mL [1.3%] 20 mL). Propensity score matching was used to match patients within each of the 10 selected hospitals (e.g. LB patients in hospital 1 were matched to non-LB patients in hospital 1) based on age, sex, race, payer type, and Deyo-Charlson Comorbidity Index (DCCI) score using one-to-one nearest neighbor matching. This approach was taken to maximize the population size available within each hospital to select controls and minimize heterogeneity when comparing clinical outcomes and costs across different hospitals.
Patient demographics and clinical characteristics; hospital characteristics; total post-surgical inpatient opioid consumption (in oral morphine equivalent dose; see Supplementary Table S2 for conversions); hospital LOS; proportion of patients discharged home; 30-, 60-, and 90-day same-hospital readmissions; and total hospitalization costs were assessed. Opioid use was identified using the PHD charge variable clin_sum_desc = Analgesic, narcotic. Total hospitalization costs, including both fixed and variable costs, were estimated from charges using charge-to-cost ratios available in the PHD for each recorded item in the hospital chargemaster.
Continuous variables were summarized using mean and SD, and between-group differences were analyzed using the Wilcoxon test or Student t-test (based on normality of the data). Categorical variables were summarized using frequency distributions and percentages, and between-group differences were analyzed using the Pearson Chi-square test. Outliers were removed if they were >3 SD from the meanCitation29. Univariate and multivariable analyses were used to compare outcomes for patients treated with and without LB. Generalized linear mixed models (GLMMs) with log transformation were applied to costs with gamma distribution controlling for demographic and clinical variables. GLMMs with logit link and binary distribution were used for home discharge, with adjustment for variables such as age, sex, race, and DCCI score. Because these analyses involved the collection of existing, deidentified patient data, this study did not meet human subject research criteria and was exempt from institutional review board approval.
Results
Hospital matching and patient characteristics
A total of 459,364 THAs were performed at all hospitals in the PHD during the study period; LB was used in 50,916 (11.1%) of these procedures (). The 10 hospitals with the highest use of LB for primary inpatient THA had 12,579 such procedures, representing 24.7% of the total LB used in the PHD for THA. Hospital characteristics are provided in Supplementary Table S3. All 10 hospitals included in the analysis were urban, and most were large community hospitals in the South census region. These institutions began using LB in THA procedures between 2012 and 2014. At several hospitals, use in THA was high (>90%) throughout the analysis period, whereas at others it was more moderate and/or variable over time (Supplementary Figure S1).
After propensity score matching and excluding patients not insured through Medicare or commercial insurance, the study population included a total of 12,589 patients, with 7,232 Medicare patients (3,622 with LB and 3,610 without LB) and 5,357 commercial insurance patients (2,648 with LB and 2,709 without LB). Patient demographics and clinical characteristics are provided in . At baseline, mean age was slightly but significantly lower in Medicare patients treated with LB vs those treated without LB (p = 0.04; ). Admission source and admission type differed significantly between treatment cohorts in the Medicare (p < 0.001, both) and commercial insurance populations (source, p = 0.038; type, p = 0.008; ). All other demographics were similar between the LB and non-LB groups.
Table 1. Patient demographics and clinical characteristics.*
Outcomes
In univariate analyses, mean post-surgical inpatient opioid consumption was significantly lower for patients with LB vs without LB in both the Medicare (244.2 mg MED vs 319.9 mg MED; p < 0.001) and commercial insurance (255.0 mg MED vs 355.2 mg MED; p < 0.001) populations (). In multivariable analyses that controlled for age (≥65 vs <65 years), sex, race, and DCCI score, mean inpatient opioid use was 105 mg MED lower with LB in Medicare patients and 81 mg MED lower with LB in the commercial insurance population (p < 0.0001, both; ). When analyzed for each post-operative day (i.e. post-operative day 1, 2, 3, 4), inpatient opioid use was significantly lower with LB for both the Medicare population and commercial insurance population on days 1–3 (each p < 0.001; Supplementary Table S4). The most common inpatient opioids noted in the PHD chargemaster for THA were oral oxycodone 5 mg (14% of total), intravenous fentanyl 0.5 mg/mL (12% of total), and oral tramadol 50 mg (9% of total).
Table 2. Univariate analyses of total opioid use, hospital LOS, discharge status, and total hospitalization costs.
Table 3. Multivariable analysis of total opioid consumption.
In univariate analyses, patients with LB vs without LB had a significantly shorter mean LOS in both the Medicare (2.4 vs 2.9 days; p < 0.001) and commercial insurance (1.9 vs 2.4 days; p < 0.001) populations (). In multivariable analyses controlling for age (≥65 vs <65 years), sex, race, and DCCI score, mean LOS with LB was 0.7 days shorter in both the Medicare and commercial insurance populations (p < 0.0001, both; ).
Table 4. Multivariable analysis of hospital LOS, home discharge status, and total hospitalization costs.
In univariate analyses, proportions of patients by discharge status significantly differed with vs without LB in both the Medicare (p < 0.001) and commercial insurance (p < 0.001) populations (). In multivariable analyses controlling for age (≥65 vs <65 years), sex, race, and DCCI score, the likelihood of home discharge with LB vs without LB was 1.66-times higher for the Medicare population (p < 0.0001) and 1.57-times higher for the commercial insurance population (p < 0.0001, ). In univariate analyses, there were no significant differences with and without LB in same-hospital 30-, 60-, or 90-day readmissions in either the Medicare or commercial insurance populations ().
In univariate analyses, total hospitalizations costs with LB were $261 lower for the Medicare population (p = 0.031) and $160 higher for the commercial insurance population (p = 0.169; ). In multivariable analyses controlling for age (≥65 vs <65 years), sex, race, and DCCI score, total hospitalization costs with LB were $561 lower in the Medicare population (p < 0.0001) and $41 higher in the commercial insurance population (p = 0.7697; ). Total hospitalization costs by chargemaster category (e.g. supplies, operating room, room and board) are shown in Supplementary Table S5. In both the Medicare and commercial insurance populations, pharmacy costs were higher with LB, but costs in other categories were generally lower with LB.
Additional information comparing patient demographics, clinical characteristics, total inpatient opioid use, LOS, discharge location, and total hospitalization costs for patients with and without LB within each of the 10 hospitals included in this study is provided in Supplementary Table S6.
Discussion
This retrospective analysis of administrative hospital data reviewed clinical and economic outcomes for 12,589 patients undergoing THA over a 6-year period at 10 hospitals with the highest use of LB in the PHD. These results suggest that use of LB for post-surgical analgesia after THA was associated with significantly lower post-surgical inpatient opioid use, a shorter hospital LOS, and an increased likelihood of home discharge for both Medicare and commercial insurance populations. In the Medicare population, use of LB was also associated with significantly lower total hospitalization costs by $561. When multiplied by the number of Medicare-insured patients in our analyses, this translates to an estimated total cost savings of $2,031,942 for patients who received LB for THA at these 10 hospitals. In the commercial insurance population, total hospitalization costs were higher by $41 with LB, but this difference was not statistically significant. For commercially insured patients, the estimated total costs increase was $108,568 for patients who received LB for THA at these 10 hospitals. It is worth noting that, while hospital pharmacy costs were higher with LB in both the Medicare and commercial insurance populations, these costs were completely (Medicare population) or partially (commercial insurance population) offset by lower non-pharmacy costs. This distinction is important since products such as LB that may increase costs in one hospital budget (e.g. pharmacy), while reducing costs in other budgets (e.g. supply), may prove challenging to implement in siloed cost containment environments.
Although post-discharge care was not considered in our analysis of hospitalization costs, the cost savings from increased home discharge observed with LB may offset the small increase in hospitalization costs under bundled payment programs that include such care. Post-discharge costs are thought to account for nearly 50% of the total costs for a surgical episode of care related to TJACitation8. A retrospective analysis of the Truven MarketScan Commercial Research Database demonstrated that discharge to a skilled nursing facility (SNF) increased the costs of a 90-day episode of care for primary THA by $4,486Citation8. The decreased frequency of discharge to a SNF with LB could further reduce the total costs of a surgical episode of care for THA.
Collectively, the current results provide real-world evidence that LB can reduce post-surgical inpatient opioid use, with a resulting reduction in hospital LOS and Medicare-associated hospitalization costs after THA, suggesting the potential for improved value of care. Studies have reported that reducing hospital LOS is critical to reducing the costs of THACitation9, and that patients with a shorter hospital LOS after TKA had lower total costs of care in the 2 years after their surgeryCitation30, although it’s unclear if the findings in TKA might also apply to THA.
The results of this large retrospective analysis are consistent with previous studies of LB for THA. In a small (n = 28) controlled cohort study, patients with LB had significantly reduced opioid use during the first 24 h after THA and a reduction in mean LOS by 0.5 days compared with those without LBCitation31. A large (n = 7,704,919) retrospective study of the Premier database that only examined LOS and discharge location also showed an approximate 0.5-day reduction in mean LOS after THA in patients receiving LB, and a significant decrease in the percentage of patients discharged to a SNF or inpatient rehabilitation center (23% vs 30%)Citation27. Another retrospective analysis reported that patients undergoing THA and TKA with LB as part of a multimodal analgesia protocol had hospital supply and medication costs that were $1,246 lower than patients who received multimodal analgesia with bupivacaine HClCitation23.
The strengths of our analyses are the large sample sizes (i.e. over 10,000 patients), extended time frame (i.e. 6-year period), and high internal validity when comparing clinical and economic outcomes for matched patients within the same hospitals. By focusing on the 10 hospitals with the highest use of LB for THA, the sample sizes within each hospital were sufficiently large to use propensity-score matching with several variables, and confounding factors that may exist when comparing such outcomes across different hospitals were reduced. However, the 10 hospitals with the highest use of LB are likely not reflective of the outcomes achieved with LB in the other hospitals in the PHD. For example, the higher use of LB may suggest greater familiarity with the product and an optimized administration technique (e.g. use of multiple, small-volume injections throughout the surgical siteCitation21), which has been reported as important to the outcomes achieved with LBCitation32. Although randomized controlled trials are generally considered the gold standard for determining the efficacy of an intervention, large, real-world observational studies are also valuable in evaluating the effectiveness of a multimodal intervention that may change over timeCitation33.
Another limitation of the current study is that total hospitalization costs included only items recorded in the chargemaster that were billed by the hospital, without confirmation that an item was actually utilized (e.g. a 20-mL vial of LB may be charged, but the patient may not have received the full 20-mL dose), and did not include other important costs such as physician services (e.g., surgeon, anesthesiologist) that are billed independently. Similarly, the route of administration for medications in the PHD is limited to oral, parenteral, etc., and is unable to distinguish between local infiltration at the surgical site and use in regional anesthesia. The analysis also did not consider the costs of healthcare services after discharge (e.g. SNF admission, readmissions), which could materially impact the total costs of a surgical episode of care over 90 days. It was also limited to inpatient THA procedures and is unable to determine if LB may also be beneficial for the growing interest in outpatient THA. Further, this study did not assess post-discharge opioid use. The PHD also lacks important clinical information such as patient-reported outcomes (e.g. pain, range of motion, ambulation, satisfaction) that are increasingly important to evaluate the value of care provided by hospitals. Finally, in the Medicare population, a skilled nursing patient has a required 3-night stay, which might have reduced the ability to detect differences between the two cohorts.
Conclusions
Under value-based bundled payment programs such as CJR for Medicare and similar programs for commercial insurance carriers, hospitals are striving to reduce their costs while maintaining or improving the quality of care they provide for common surgical procedures such as THA. Optimizing post-surgical analgesia while reducing opioid use is increasingly recommended to achieve these goals. The results from this large, retrospective analysis of hospital administrative data provide real-world evidence that LB use may be associated with decreased opioid use, shorter hospital LOS, and increased likelihood of home discharge in both Medicare and commercial insurance populations.
Transparency
Declaration of funding
This study was funded by Pacira BioSciences, Inc. Pacira BioSciences, Inc. participated in the study conception and design; collection, analysis, and interpretation of the data; and review of the manuscript. The authors were responsible for review and final approval to submit for publication.
Declaration of financial/other interests
CVA is a member of the Health Outcomes and Economics Advisory Board for Pacira BioSciences, Inc. SD and AK were employees of Pacira BioSciences, Inc., at the time of the study. JR is a consultant for Pacira BioSciences, Inc. BTM has nothing to disclose. A peer reviewer on this manuscript has disclosed consulting to Heron Therapeutics, AcelRx Pharma and Neumentum Pharmaceuticals. All companies are developing drugs for acute pain management. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Previous presentations
Data from this article were previously presented at the International Society for Pharmacoeconomics and Outcomes Research 21st Annual Meeting in Washington, DC (May 21–25, 2016).
Supplemental Material
Download MS Word (133.6 KB)Acknowledgements
Editorial support was provided by Krystina Neuman, at C4 MedSolutions, LLC (Yardley, PA), a CHC Group company, and Paul Cavanaugh, an employee of Pacira BioSciences, Inc.
References
- Barbour KE, Helmick CG, Boring M, et al. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66:246–253.
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785.
- Kurtz SM, Lau E, Ong K, et al. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467:2606–2612.
- Nwachukwu BU, Bozic KJ, Schairer WW, et al. Current status of cost utility analyses in total joint arthroplasty: a systematic review. Clin Orthop Relat Res. 2015;473:1815–1827.
- Nwachukwu BU, McCormick F, Provencher MT, et al. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplasty. 2015;30:15–18.
- Centers for Medicare & Medicaid Services. 2019. Comprehensive care for joint replacement model. [cited 2018 June 21]. Available from: https://innovation.cms.gov/initiatives/cjr
- Chen KK, Harty JH, Bosco JA. It is a brave new world: alternative payment models and value creation in total joint arthroplasty. J Arthroplasty. 2017;32:1717–1719.
- Nichols CI, Vose JG. Clinical outcomes and costs within 90 days of primary or revision total joint arthroplasty. J Arthroplasty. 2016;31:1400–1406. e1403.
- Molloy IB, Martin BI, Moschetti WE, et al. Effects of the length of stay on the cost of total knee and total hip arthroplasty from 2002 to 2013. J Bone Joint Surg Am. 2017;99:402–407.
- Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop (Belle Mead NJ). 2015;44:397–405.
- Clement RC, Derman PB, Graham DS, et al. Risk factors, causes, and the economic implications of unplanned readmissions following total hip arthroplasty. J Arthroplasty. 2013;28:7–10.
- Scuderi GR. The challenges of perioperative pain management in total joint arthroplasty. Am J Orthop (Belle Mead NJ). 2015;44:S2–S4.
- Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157.
- Grant CRK, Checketts MR. Analgesia for primary hip and knee arthroplasty: the role of regional anaesthesia. Cont Ed Anaesth Crit Care Pain. 2008;8:56–61.
- Minkowitz HS, Gruschkus SK, Shah M, et al. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71:1556–1565.
- Oderda GM, Gan TJ, Johnson BH, et al. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27:62–70.
- Ilfeld BM. Continuous peripheral nerve blocks: an update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg. 2017;124:308–335.
- Halawi MJ, Vovos TJ, Green CL, et al. Opioid-based analgesia: impact on total joint arthroplasty. J Arthroplasty. 2015;30:2360–2363.
- Kessler ER, Shah M, Gruschkus SK, et al. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33:383–391.
- EXPAREL® (bupivacaine liposome injectable suspension). Full Prescribing Information, Pacira Pharmaceuticals, Inc., San Diego, CA, 2018.
- Joshi GP, Cushner FD, Barrington JW, et al. Techniques for periarticular infiltration with liposomal bupivacaine for the management of pain after hip and knee arthroplasty: a consensus recommendation. J Surg Orthop Adv. 2015;24:27–35.
- Hu D, Onel E, Singla N, et al. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33:109–115.
- Barrington JW, Olugbode O, Lovald S, et al. Liposomal bupivacaine: a comparative study of more than 1000 total joint arthroplasty cases. Orthop Clin North Am. 2015;46:469–477.
- Asche CV, Ren J, Kim M, et al. Local infiltration for postsurgical analgesia following total hip arthroplasty: a comparison of liposomal bupivacaine to traditional bupivacaine. Curr Med Res Opin. 2017;33:1283–1290.
- Yu SW, Szulc AL, Walton SL, et al. Liposomal bupivacaine as an adjunct to postoperative pain control in total hip arthroplasty. J Arthroplasty. 2016;31:1510–1515.
- Emerson RH, Barrington JW, Olugbode O, et al. Comparison of local infiltration analgesia to bupivacaine wound infiltration as part of a multimodal pain program in total hip replacement. J Surg Orthop Adv. 2015;24:235–241.
- Cherian JJ, Barrington J, Elmallah RK, et al. Liposomal bupivacaine suspension can reduce length of stay and improve discharge status of patients undergoing total hip arthroplasty. Surg Technol Int. 2015;27:235–239.
- McGraw-Tatum MA, Groover MT, George NE, et al. A prospective, randomized trial comparing liposomal bupivacaine vs fascia iliaca compartment block for postoperative pain control in total hip arthroplasty. J Arthroplasty. 2017;32:2181–2185.
- Howell DC, Rogier M, Yzerbyt V, et al. Statistical Methods in Human Sciences. New York: Wadsworth; 1998.
- Lovald ST, Ong KL, Malkani AL, et al. Complications, mortality, and costs for outpatient and short-stay total knee arthroplasty patients in comparison to standard-stay patients. J Arthroplasty. 2014;29:510–515.
- Domb BG, Gupta A, Hammarstedt JE, et al. The effect of liposomal bupivacaine injection during total hip arthroplasty: a controlled cohort study. BMC Musculoskelet Disord. 2014;15:310.
- Mont MA, Beaver WB, Dysart SH, et al. Local infiltration analgesia with liposomal bupivacaine improves pain scores and reduces opioid use after total knee arthroplasty: results of a randomized controlled trial. J Arthroplasty. 2018;33:90–96.
- Frieden TR. Evidence for health decision making - beyond 1randomized, controlled trials. N Engl J Med. 2017;377:465–475.
