Introduction
Truly innovative medicines can reduce both mortality and morbidity, thereby increasing the length and/or the quality-of-life of patientsCitation1,Citation2. However, many of these medicines come at an extra cost compared to the standard of care, and that extra cost is sometimes substantial. At the OECD Ministerial Meeting on “Next Generation of Health Reforms” (January 2017), Ministers underlined that new generation treatments are indeed very costly, with significant budget impact implicationsCitation3. Several authors have expressed these concerns as well, especially pointing to – but not restricting to – orphan medicines and cancer drugsCitation4–6. Others point moreover to the evidentiary uncertainty for some of these treatments, which compromises the justification of the high pricesCitation7.
The Expert Panel on Effective ways of investing in Health recently stated that there is a need for innovative payment models to ensure that innovation “that matters” is produced, that patients have access to innovation, and that health systems are financially sustainableCitation8.
For many years, cost-effectiveness has been applied as a key criterion to assess whether new medicines and other technologies deserve to be reimbursed within healthcare systems, but there is debate on what actually can be considered as cost-effective, which elements must thereby be considered, and how these can guide the pricing and reimbursement of medicines concretelyCitation9–11.
The current conceptual paper proposes an innovative payment model that implies the explicit use of cost-effectiveness, medical need, and budget impact for the assessment and appraisal of the price and reimbursement level of innovative medicines.
Cost plus versus value based pricing
One of the current observations, according to a recent report by the World Health OrganizationCitation12, is that the prices of innovative medicines bear little or no relationship with R&D costs. It has, therefore, been argued that prices should better reflect investments for Research and Development (R&D), a logic which is sometimes referred to as “cost plus pricing”Citation13. The argument is that the starting point for price negotiations should be an agreement among all parties about how much it costs to develop a new medicineCitation14. Although this approach might at first sight seem fair and logic, it raises several issues:
First, it may lead to the wrong incentives, in that the higher the R&D costs, the higher the price that theoretically could be justified.
Second, investment costs for medicines that eventually do not make it to the final stage (i.e. access to patients), because of insufficient effect or due to toxicity, or other reasons, must be amortized and factored into the cost of R&D of the medicines that make it to the market, which may then lead to a perverse situation where a company with many of such failures could justify a higher price for the few products that make it to the market.
Finally, this approach does not sufficiently encourage true innovation. Irrespective of the benefit to patients, reward will be according to R&D costs. Hence, cost plus pricing does not reward value.
A recent cost plus price proposal from Uyl-De Groot and LöwenbergCitation15 tried to address the last issue, but in a rather arbitrary way, and not solving the first two issues.
It seems that a better approach is to start from the principle that decisions on pricing and reimbursement for innovative medicines should account for the added value that they deliver for patients and society, i.e. the so called “value based pricing” approach. This requires first of all a better insight in the meaning of value. Recently, the ISPOR Special Task Force on US Value Assessment Frameworks provided a broad overview of the different possible interpretations of valueCitation16. Neumann et al.Citation16 point to the widespread use of QALYs (quality adjusted life years) as a core element of value, but also observe that this metric has limitations: “QALYs may not always fully capture the health (or well-being) of patients, or incorporate individual or community preferences about the weight to be given to health gain, for example, about disease severity, equity of access, or unmet need”. Hence, better outcomes should not be the sole criterion. For instance, from the original work of Erik NordCitation17, it appears that societal willingness to pay for new treatments depends strongly on the degree of severity or suffering associated with the current situation. This has also been confirmed in more recent work, such as in Shiroiwa et al.Citation18 and Richardson et al.Citation19, where the latter suggest that higher willingness to pay is especially relevant for very severe conditions. Value should, therefore, be defined at least by both disease and treatment related characteristics, which should both be objectivizedCitation20,Citation21.
The principle of value based pricing is then based on the general economic concept that prices of new goods indicate the difference between what currently available goods offer and the value that the new goods can provideCitation22. According to this logic, the higher the added value (in its broader interpretation), the higher the price the innovation deserves. This, however, entails two important questions: (1) How much should society be willing to pay for additional value? And (2) What to do if the added value is subject to evidentiary uncertainty?
Value for money: issues with the threshold
Value does not necessarily mean “value for money”. Price and reimbursement levels of medicines should reflect an acceptable value for money from a (public) payer’s perspective. This means that, in the interpretation of cost-effectiveness results, it is important to apply thresholds: the maximum amount of money a payer or a society is willing to pay for gaining QALYs needs to be made explicit. According to Danzon et al.Citation23, each payer should adopt a decision rule about what is good value for money given their budget and, therefore, consistent use of a cost-per-quality-adjusted life-year threshold will ensure the maximum health gain for the budget. Several attempts have been made to establish such a threshold. The well-known example is the UK with a threshold of £30,000 for a QALYCitation9. Yet, in reality, interventions that at first sight appear to be cost-effective are not funded, while others with a seemingly very disadvantageous Incremental Cost-Effectiveness Ratio (ICER) are financed in full. The classic example of the first of these situations is the use of Viagra as a remedy for erectile dysfunction. Viagra was shown to have a cost-effectiveness of ±€5,000 per QALY, but there are very few countries where this drug is reimbursed by the health system. Stolk et al. refer to the perception by payers of this treatment as an “unnecessary luxury”, and not responding to a clear medical need. Having sex in advanced stages of life is perceived to be a personal choice, related more to lifestyle than to health problems. Viagra is, thus, considered to be a lifestyle drug, not eligible for public fundingCitation24. In contrast, orphan drugs with an ICER of €150,000 per QALY or more are regularly reimbursedCitation25.
Stolk et al.Citation26 have suggested that the willingness to pay for a QALY should formally depend on the severity of a condition for the patient. They refer to an “acceptable level of health” as a societal reference point. If people fall far beneath that acceptable level of health, they must be helped as a matter of priority and more should be paid for gaining a QALY. The opposite is also true. People with a health problem who are nonetheless above the acceptable level of health essentially have a “luxury” or “comfort” problem and, therefore, cannot necessarily rely on reimbursement of their treatment by the health system. Svensson et al.Citation11 argued recently along the same lines from a Swedish perspective.
Following this way of thinking, in 2015, the ZorgInstituut Nederland (ZIN = Dutch Health Care Institute) introduced a new and progressive approach, based on the premise that the limits of our societal willingness to pay for the gain of a QALY are not determined by a single value, but are dependent on the severity or the health burden of the health problem concerned. This is illustrated in Citation27.
Figure 1. The societal threshold value in relation to the health burden of the disease (adapted fromCitation27).
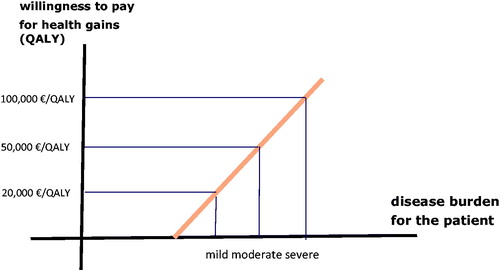
With severe conditions, policy-makers are more willing to pay to gain a QALY than with mild or moderately severe conditions. This, of course, means that the key question then becomes: how does one measure the health burden of a disease?
To solve this problem, the ZIN works with what is called the concept of “proportional shortfall”, which was first introduced by Stolk et al.Citation26 as long ago as 2004. The basic idea is to calculate a ratio or proportion between the number of QALYs a patient would lose as a result of the condition, assuming it is not treated, and the normal number of QALYs that person would have enjoyed if he had never become ill.
An example will serve to clarify. Imagine that a patient is diagnosed with cancer at the age of 50. Let us further imagine that epidemiological research has shown that, if the cancer would not be treated, the patient could expect to experience an average of just five QALYs. Without the illness, he might expect to experience 25 QALYs. In other words, the patient would lose 20 of the 25 QALYs in his “reserve”. The proportional shortfall is, therefore, (25 – 5)/25 = 20/25 = 80%. Real examples are provided in Lindemark et al.Citation28.
The greater the proportional shortfall of an illness, the more it is perceived as being “severe” and the more society should be willing to pay for the gain of QALYs. Recently, Reckers-Droog et al.Citation29 suggested that further investigation into refining proportional shortfall – or exploration of another approach – appears warranted for operationalizing the equity-efficiency trade-off.
Another possibility to establish the severity of an illness is to conduct a multi-criteria decision analysis (MCDA). What should count for most when assessing the severity of a condition? The impact on quality-of-life? The likelihood of dying? Other factors? MCDAs make it possible to evaluate these different considerations by giving them a weighting, which, when taken together, determine the perceived level of severity. For instance, in an MCDA conducted in Belgium to compare medical need in different diseases, the researchers looked into the impact of the diseases on life expectancy and quality-of-life, on the age of patients, and the level of discomfort of the current treatmentCitation30. Obviously, the drawback of MCDA is that the outcomes are determined by the selection of criteria. Recent initiatives such as the transparent value framework make use of this approach, although both disease and treatment characteristics are combined in that exerciseCitation31. In the concept of modulated thresholds in function of disease severity, MCDA should contribute to better quantify only that severity. For an overview of other MCDA exercises in this field, we refer to Wahlster et al.Citation32.
Value informed, affordable prices
The above model is not yet complete. Decision-makers typically also take into consideration the budget impact and affordability for the healthcare system. Indeed, even if a treatment is cost-effective, it does not mean automatically that it is affordableCitation33. This is undoubtedly a matter of opportunity cost. Putting too much money in one basket, i.e. one disease, takes away the opportunity to help other patients. A well-known recent example are the second generation direct anti-viral therapies for Hepatitis C: although very effective and even cost effective, their impact on medicines budgets in several jurisdictions would have been huge if all eligible patients were treatedCitation34. Budget impact analyses are, therefore, required to assess the extent to which the healthcare system can afford to pay for the innovation. In this scenario, the possible offsets elsewhere in the system are to be taken into account as wellCitation35.
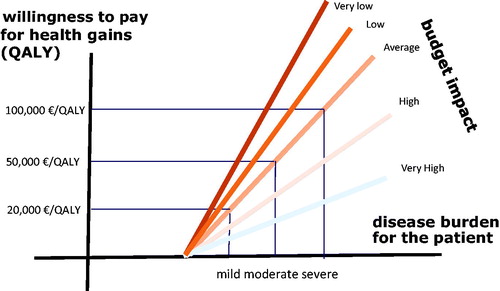
Towse and MauskopfCitation36 discuss several ways to account for budget impact in decisions on pricing and reimbursement. One of these is to adjust the cost-effectiveness threshold in function of the budget impact. A higher budget impact requires a lower threshold and a lower budget impact can permit to work with an increased threshold. Applying this, and in combination with the above, this means that societal thresholds for willingness to pay for health gains need to be modulated depending on the disease burden as well as on the budget impact of the innovative medicine. Hence, for a treatment in an area with a high burden, and with a low budget impact, the societal willingness to pay for additional health outcomes should be higherCitation37,Citation38. The opposite is true for a treatment in a disease with low burden and a high budget impact. Hence, when healthcare payers communicate explicitly about the societal thresholds within a value based pricing logic, and how they are modulated in function of disease burden and budget impact, it should be possible to reward value and at the same time account for affordability. This approach can be called “value informed, affordable pricing” (“VIA pricing”) and may become a practical approach to achieve pricing and reimbursement levels in line with societal values and preferences.
depicts this concept. We can again see the threshold line that was drawn in , but now a number of additional lines have been added to reflect budgetary implications.
When the impact of an intervention on the budget is low or very low, as is the case with ultra-rare conditions, the threshold value can be increased. In 2017, for example, the British National Health Service announced that it was willing to set a threshold value of £300,000 per QALY for “exceptional” cases of this kind. But the same effect also works in the opposite direction: interventions with a high or very high budget impact can lead to the threshold being lowered. This implies a need to rely on the experiences of the past to determine precisely what constitutes “high” or “low” budget impact.
As an example, a new intervention for the treatment of a disease with a moderate health burden costs 35,000 euros per QALY. At first sight, this seems to be acceptable: it is well under the “standard” or average threshold value of 50,000 euros. However, if the budget impact of the intervention is high, this brings the threshold value down to just say 25,000 euros per QALY (figure is illustrative). This means that the intervention is not affordable, unless its price can be reduced. In cases of high budget impact, the company offering the new medicine is more likely to be willing to reduce the price, based on the principle that their turnover will remain high and that it is better to have a large market at a reduced price than no market at all.
The exact order of magnitude of the different thresholds could be determined by a trade-off exercise among decision-makers between cost per QALY, disease severity, and budget impact. Again, MCDAs that can contribute to better understand the relative weight of these metrics are recommended to make progress in this field. For an extensive overview of the possibilities of MCDA with this regard I refer to Phelps et al.Citation39. Another field of research is the conduct of regression analyses based on observed reimbursement decisions. A good example of the latter is the study conducted by Charakopou et al.Citation40 in 2015 in Scotland, where it was found that the Odds ratio for reimbursement in case of an ICER below £30,000 per QALY was 2.96 (1.64–5.36), whereas the Odds ratio for reimbursement in case of a Budget impact in year 5 below £500,000 was 1.46 (0.94–2.26). Finetuning the methods of such exercises is increasingly important in the years ahead. Of course, specific characteristics of each country, such as ability to pay, epidemiological data, and cultural and societal values play a prominent role here.
In conclusion, we could turn value based pricing into value informed, affordable pricing by explicitly modulating thresholds of societal willingness to pay, thereby accounting for disease severity and budget impact. A research agenda for better estimating disease severity and quantifying the trade-off between cost-effectiveness and budget impact is required.
This if of course just one part of a bigger picture that should also investigate how to deal with evidence gaps at the time of submission, contracting between pharma and (multiple) countries and making the switch from paying for a product towards paying for a service, the latter stipulated by the expert panel on investing in healthcareCitation8.
Transparency
Declaration of funding
This paper was not funded.
Declaration of financial/other interests
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. A peer reviewer on this manuscript has disclosed that they consider themselves an academic competitor to the author of this paper. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
None reported.
References
- Council of the European Union. Council Conclusions on Innovation for the Benefit of Patients. Official Journal of the European Union. December 2014.
- Annemans L, Cleemput I, Hulstaert F, et al. Valorising and creating access to innovative medicines in the European Union. Front Pharmacol. 2011;2:Article 57.
- Ministerial Statement ‘The next generation of health reforms’. OECD Health Ministerial Meeting. 17 January 2017. Available from: http://www.oecd.org/health/ministerial/ministerial-statement-2017.pdf [Accessed 15 April 2019].
- Luzzatto L, Hyry HI, Schieppati A, et al. Outrageous prices of orphan drugs: a call for collaboration. Lancet. 2018;392:791–794.
- Howard DH, Bach PB, Berndt ER, et al. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29:139–162.
- Prasad V, De Jesús K, Mailankody S, et al. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14:381–389.
- Cohen D. Cancer drugs: high price, uncertain value. BMJ. 2017;359:j4543.
- Expert Panel on Effective Ways of Investing in Health (EXPH). Opinion on innovative payment models for high-cost innovative medicines. Brussels: European Commission; January 2018.
- Neumann PJ, Bliss SK, Chambers JD. Therapies for advanced cancers pose a special challenge for health technology assessment organizations in many countries. Health Aff. 2012;31:700–708.
- Santos AS, Guerra-Junior AA, Godman B, et al. Cost-effectiveness thresholds: methods for setting and examples from around the world. Expert Rev Pharmacoecon Outcomes Res. 2018;18:277–288.
- Svensson M, Nilsson FOL, Arnberg K, et al. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics. 2015;33:1229–1236.
- Pricing of cancer medicines and its impacts. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO.
- Dholakia UM. When cost-plus pricing is a good idea. Harvard Business Review. July 12, 2018. 4 p.
- Ghinea N, Lipworth W, Kerridge I. Propaganda or the cost of innovation? Challenging the high price of new drugs. BMJ. 2016;352:i1284.
- Uyl-De Groot C, Löwenberg B. Sustainability and affordability of cancer drugs: a novel pricing model. Nat Rev Clin Oncol. 2018;15:405–406.
- Neumann PJ, Willke RJ, Garrison LP, et al. A health economics approach to US value assessment frameworks—introduction: an ISPOR special task force report [1]. Value in Health. 2018;21:119–123.
- Nord E. Concerns for the worse off: fair innings versus severity. Soc Sci Med. 2005;60:257–263.
- Shiroiwa T, Saito S, Shimozuma K, et al. Societal preferences for interventions with the same efficiency: assessment and application to decision making. Appl Health Econ Health Policy. 2016;14:375–385. Jun
- Richardson J, Iezzi A, Maxwell A. How important is severity for the evaluation of health services: new evidence using the relative social willingness to pay instrument. Eur J Health Econ. 2017;18:671–683.
- Annemans L, Aymé S, Le Cam Y, et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL). Orphanet J Rare Dis. 2017;12:50.
- Lakdawalla DN, Doshi JA, Garrison LP, et al. Defining elements of value in health care—a health economics approach: an ISPOR Special Task Force report [3]. Value Health. 2018;21:131–139.
- Taylor D, Craig T. Value based pricing for NHS medicines: magic bullet, counterfeit treatment or the mixture as before? Health Econ Policy Law. 2009;4:515–526.
- Danzon PM, Drummond MF, Towse A, et al. Objectives, budgets, thresholds, and opportunity costs—a health economics approach: an ISPOR Special Task Force Report [4]. Value in Health. 2018;21:140–145.
- Stolk EA, Brouwer WB, Busschbach JJ. Rationalising rationing: economic and other considerations in the debate about funding of Viagra. Health Pol. 2002;59:53–63. Jan
- Schlander M, Garattini S, Holm S, et al. Incremental cost per quality-adjusted life year gained? The need for alternative methods to evaluate medical interventions for ultra-rare disorders. J Comp Eff Res. 2014;3:399–422.
- Stolk EA, Van Donselaar G, Brouwer WB, et al. Reconciliation of economic concerns and health policy: illustration of an equity adjustment procedure using proportional shortfall. Pharmacoeconomics. 2004;22:1097–1107.
- ZIN. Kosteneffectiviteit in de praktijk (cost-effectiveness in practice) – in Dutch, 26 June 2015.
- Lindemark F, Norheim OF, Johansson KA. Making use of equity sensitive QALYs: a case study on identifying the worse off across diseases. Cost Eff Resour Alloc. 2014;12:16.
- Reckers-Droog VT, van Exel NJA, Brouwer WBF, et al. Looking back and moving forward: on the application of proportional shortfall in healthcare priority setting in the Netherlands. Health Pol. 2018;122:621–629.
- Cleemput I, Devriese S, Kohn L, et al. Incorporating societal preferences in reimbursement decisions – Relative importance of decision criteria according to Belgian citizens. Health Services Research (HSR) Brussels: Belgian Health Care Knowledge Centre (KCE); 2014. KCE Reports 234. D/2014/10.273/91.
- Godman B, Bucsics A, Vella Bonanno P, et al. Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 2018;6:328.
- Wahlster P, Goetghebeur M, Kriza C, et al. Balancing costs and benefits at different stages of medical innovation: a systematic review of Multi-criteria decision analysis (MCDA). BMC Health Serv Res. 2015;15:262.
- Birch S, Gafni A. Information created to evade reality (ICER): things we should not look to for answers. Pharmacoeconomics. 2006;24:1121–1131.
- Brennan T, Shrank W. New expensive treatments for hepatitis C infection. JAMA. 2014;312:593–594.
- Niezen MG, de Bont A, Busschbach JJ, et al. Finding legitimacy for the role of budget impact in drug reimbursement decisions. Int J Technol Assess Health Care. 2009;25:49–55.
- Towse A, Mauskopf JA. Affordability of new technologies: the next frontier. Value Health. 2018;21:249–251.
- Griffits EA, et al. Acceptance of health technology assessment submissions with incremental cost-effectiveness ratios above the cost-effectiveness threshold. Clinicoecon Outcomes Res. 2015;7:463–476.
- Paulden M, Stafinski T, Menon D, et al. Value-based reimbursement decisions for orphan drugs: a scoping review and decision framework. Pharmacoeconomics. 2015; 33:255–269.
- Phelps CE, Lakdawalla DN, Basu A, et al. Approaches to aggregation and decision making—a health economics approach: an ISPOR Special Task Force Report [5]. Value Health. 2018;21:146–154.
- Charakopou M, Majer IM, Raad J de, et al. Which factors enhance positive drug reimbursement recommendation in Scotland? A retrospective analysis 2006–2013. Value Health. 2015;18:284–291.