 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims: To estimate annual cost per response (CPR) in the US and number needed to treat (NNT) among patients receiving guselkumab or adalimumab treatment for moderate-to-severe plaque psoriasis (PsO).
Materials and methods: Results from VOYAGE 1, a double-blind, placebo-controlled, head-to-head, 48-week study of guselkumab compared with adalimumab in patients with moderate-to-severe PsO were used to estimate annual CPR for Psoriasis Area and Severity Index (PASI) 75, 90, and 100 responses. Drug dosing followed US label recommendations and drug costs were based on US annual wholesale acquisition costs. Number needed to treat (NNT) and annual CPR analyses were estimated, and week 48 response rates were assumed to be maintained for both the induction and maintenance years.
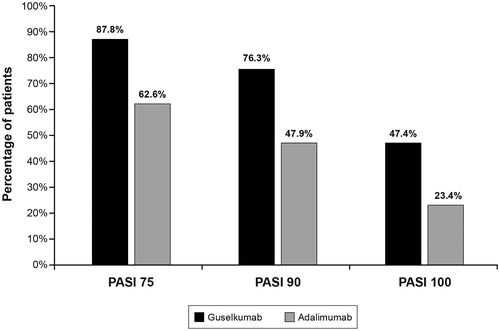
Results: Week-48 PASI 90 response rates were 76.3% for guselkumab and 47.9% for adalimumab. The CPR for PASI 90 in the induction year for guselkumab was $113,861 vs $151,226 for adalimumab. Both drugs had lower CPRs for PASI 90 in the maintenance year: $85,395 for guselkumab and $140,424 for adalimumab for adalimumab. The NNT for a PASI 90 response was 1.3 for guselkumab and 2.1 for adalimumab; CPRs and NNT were also lower for guselkumab than for adalimumab for PASI 75 and PASI 100 for both induction and maintenance years.
Limitations and conclusions: In this analysis, extrapolating 48-week results from a single head-to-head study, guselkumab was more cost-effective with lower NNT than adalimumab in both the induction and maintenance years for PASI 75, PASI 90, and PASI 100 responses.
Introduction
Moderate-to-severe plaque psoriasis (PsO) is a chronic, relapsing, inflammatory immune-mediated skin disease with a significant prevalence in the US. The prevalence of PsO in adults older than 20 years of age in the US is ∼3.2%, or more than 7.4 million people, as estimated by Rachakonda et al.Citation1, using data from the National Health and Nutrition Examination Survey (NHANES). Moderate-to-severe PsO can be treated with either conventional systemic or biologic treatments. The percentage of adults with PsO who are eligible for systemic treatment with conventional drugs or biologics is ∼ 18.2%Citation2.
Currently, biologic treatment has been shown to be more efficacious for treatment of moderate-to-severe PsO than treatment with methotrexate and other conventional agentsCitation3,Citation4. In addition, several more recently approved biologic drugs with different modes of action (e.g. the interleukin-23 blocker, guselkumab, the humanized interleukin-17A antagonist secukinumab, and the humanized interleukin-17A antagonist ixekizumab) have been shown in clinical trials to have efficacy superior to that of older biologics approved for moderate-to-severe PsO (e.g. the tumor necrosis factor (TNF) alpha blockers etanercept and adalimumab, and a human interleukin-12 and -23 antagonist, ustekinumab)Citation3. For example, guselkumab has been shown in two head-to-head phase 3 clinical trials to be superior to adalimumab; and to have superior efficacy in a trial randomizing those with an inadequate response to ustekinumab to either continue ustekinumab or switch to guselkumabCitation5–7.
In the US, healthcare stakeholders making formulary and benefit design decisions seek information about the comparative cost-effectiveness and annual drug treatment costs for different biologics for moderate-to-severe PsO. Efficacy in PsO clinical trials is typically based on percentage improvement from baseline in the Psoriasis Area and Severity Index (PASI) scoreCitation8. The PASI is a combined measure of the area affected by PsO and the severity of signs in those areas. Clinical improvement is commonly measured using PASI 75 (75% improvement or greater from baseline), PASI 90 (90% improvement or greater from baseline), and PASI 100 (100% improvement from baseline) responses. Achieving PASI 90 or PASI 100 response has been shown to be associated with higher patient quality-of-life than achieving a PASI 75 (or lower) responseCitation9,Citation10. Dividing biologic cost by response rates for these PASI response thresholds generates a cost per responder (CPR) for each drug and provides a relative measure of cost-effectiveness. Estimates of the number of people needed to be treated (NNT) to achieve one additional responder for each PASI response level provides a measure of the relative clinical efficacy of treatment with different drugs.
In this study, CPR and NNT analyses were performed using data from the head-to-head VOYAGE 1 trial of guselkumab compared with adalimumab using different measures of response at week 48 (PASI 75, PASI 90, and PASI 100) extrapolated to 52-weeks and estimating induction year or maintenance year biologic costsCitation5. To assess the robustness of these results, scenario analyses were also performed estimating the CPR and NNT for both biologics adjusted by the placebo response rates at 16 weeks.
Methods
The VOYAGE 1 study was a double-blind, head-to-head, 48-week study comparing guselkumab with adalimumab in patients ≥18 years of age with moderate-to-severe PsO and a baseline PASI score ≥12 (mean PASI score in trial = 22)Citation5. The trial was registered on clinicaltrials.gov (identifier: NCT02207231), was conducted with approval from formal ethics review committees at each study site, and patients signed written informed consent before study initiationCitation5. The study also included a 16-week placebo-controlled period, with crossover of the placebo group to guselkumab at week 16 for the remaining 48 weeks. The co-primary endpoints for the study were the proportion of patients achieving an Investigator’s Global AssessmentCitation11 score of cleared/minimal (0/1) and the proportion of patients with a PASI 90 or greater response at week 16. Secondary endpoints included the proportions of patients with PASI 75, PASI 90, and PASI 100 responses at week 48.
Annual drug costs were estimated based on the dosing regimens used in the VOYAGE 1 study, which were aligned with dosing approved by the US Food and Drug Administration (FDA) for both products. For guselkumab, the induction year dosing regimen was 100 mg at weeks 0 and 4 and then every 8 weeks through week 52 for a total of eight doses. The maintenance year dosing regimen was 100 mg every 8 weeks for a total of six doses. For adalimumab, the induction year dosing regimen was 80 mg at week 0, 40 mg at week 1, and 40 mg every 2 weeks through week 52 for a total of 28 doses. The maintenance year dosing regimen was 40 mg every other week through week 52, for a total of 26 doses.
Wholesale acquisition costs (WAC) for the two drugs were taken from the Red Book, March 19, 2019Citation12. presents the induction year and maintenance year dosing and pricing inputs used in the CPR analysis.
Table 1. Dosing and unit prices and annual drug costs in the United States for guselkumab and adalimumab: induction and maintenance years.
Psoriasis is a chronic disease and it is helpful to understand cost-effectiveness estimates over longer timeframes. In this analysis, CPR was estimated for both the induction year and the maintenance year, and it was assumed that efficacy outcomes observed at week 48 from the VOYAGE 1 study were maintained through week 52 in the induction year analysis and through week 104 in the maintenance year analysis. Efficacy of guselkumab was maintained at 100 weeks in the VOYAGE 1 open label extension, however, efficacy data is not available for adalimumab at 100 weeks from that studyCitation13. Long-term efficacy of adalimumab has been previously reported in the REVEAL trialCitation14. The CPR was estimated for PASI 75, PASI 90, and PASI 100 responses as the ratio between annual drug costs and percentage of patients achieving each PASI response outcome. Thus, the following equation was used to estimate the CPR for each PASI response level:
The NNT to obtain one patient with a PASI 75, PASI 90, and PASI 100 response at 48 weeks was also estimated for each drug using the following equations:
NNT 75 = 1 ÷ percentage of patients with PASI 75 response;
NNT 90 = 1 ÷ percentage of patients with PASI 90 response; and
NNT 100 = 1 ÷ percentage of patients with PASI 100 response.
Scenario analyses are presented for CPR and NNT for guselkumab and adalimumab after adjusting the response rates at 48 weeks for both drugs by the placebo response rates at 16 weeks.
Results
Base-case analyses
The PASI 90 response rates at week 16 were 73.3% for guselkumab, 2.9% for placebo, and 49.0% for adalimumab. The PASI 75, PASI 90, and PASI 100 response rates observed in the study at 48 weeks are presented in .
Figure 1. Percentage of patients reaching PASI 75, 90, and 100 response at 48 weeks in the VOYAGE 1 study (The PASI was used to measure three levels of PsO symptom improvement from baseline: PASI 75 (≥75% improvement), PASI 90 (≥90% improvement), and PASI 100 (100% improvement)). Source: Blauvelt et al.Citation5
Abbreviations. PASI, Psoriasis Area and Severity Index; PsO, psoriasis.
The annual total WAC drug costs for the induction year were $86,876 for guselkumab and $72,437 for adalimumab; the annual total WAC drug costs for the maintenance year were $65,157 for guselkumab and $67,263 for adalimumab. These costs were estimated using the input values for dosing and US prices described in the Methods section () and assuming 100% adherence and persistence with the FDA-labeled regimen.
The CPR results are presented in (induction year) and (maintenance year). The CPR values are lower for guselkumab than for adalimumab based on PASI 75, PASI 90, and PASI 100 response rates for both the induction and maintenance years. For example, the PASI 90 CPR value for guselkumab for the induction year is $113,861, compared with $151,226 for adalimumab, and the PASI 90 CPR value for guselkumab for the maintenance year is $85,395, compared with $140,424 for adalimumab. NNT values were calculated using 48-week response rates, and are assumed to be the same for both the induction and maintenance years (). The NNTs for guselkumab were lower than those for adalimumab for all PASI measures, consistent with the superior efficacy of guselkumab in the trial.
Figure 2. United States cost per responder for three PASI response levels induction year (Using wholesale acquisition cost as of March 19, 2019). Source: Thompson’s Online Micromedex Red BookCitation12. Abbreviation. PASI, Psoriasis Area and Severity Index.
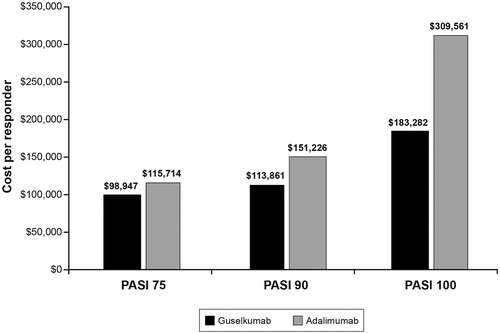
Figure 3. Cost per responder for three PASI response levels: maintenance year (Using wholesale acquisition cost as of March 19, 2019). Source: Thompson’s Online Micromedex Red BookCitation12. Abbreviation. PASI, Psoriasis Area and Severity Index.
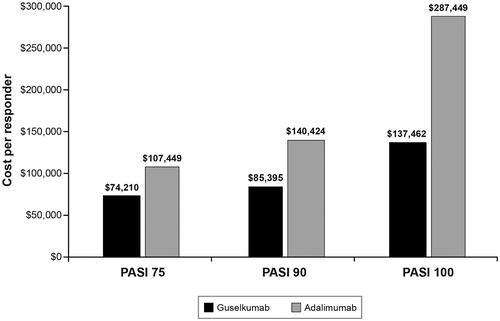
Table 2. Cost per responder in the US and number needed to treat base-case and scenario analyses.Table Footnotea
Scenario analysis
The results of placebo-adjusted scenario analyses estimating the CPR and NNT for guselkumab and adalimumab after subtracting the placebo response at 16 weeks (5.7% for PASI 75, 2.9% for PASI 90, and 0.6% for PASI 100) are very similar to the base-case results ().
Discussion
The results of the base-case and scenario analyses indicate that the CPR and NNT are consistently more favorable for guselkumab than for adalimumab, indicating that guselkumab is a more cost-effective biologic drug choice. Although adalimumab is less costly than guselkumab based upon WAC in the induction year, it is more costly based on WAC in the second year of treatment, assuming a full year of treatment and 100% adherence to the US FDA labels for both biologic drugs.
In a recent study, Wu et al.Citation15 estimated annual CPR in the US for several biologic drugs indicated for the treatment of moderate-to-severe psoriasis. The annual CPR for adalimumab was estimated to be $82,655 for PASI 75, $119,178 for PASI 90, and $284,702 for PASI 100. These estimates are in 2017 dollars and assume a hypothetical drug discount over list price of 20%. The number of doses of adalimumab per year is not presented, and guselkumab was not included in this study. The estimates of PASI response for adalimumab by Wu et al.Citation15 are similar to those in the VOYAGE 1 study, but the CPRs for adalimumab are all lower than our estimates for both the induction and maintenance years, primarily because of the 20% drug discount, which was not included in our estimates.
Three US studies have presented CPR estimates for induction in PsOCitation16–18. All of these estimated CPRs are based on efficacy and drug cost data from only 12–16-week studies. Thus, results for adalimumab from these studies are not directly comparable with those presented in our study due to differences in time horizon. Guselkumab was not included in these studies.
Limitations of our study include that the response rates were derived from a single head-to-head 48-week clinical trial. While similar differences in response rates between guselkumab and adalimumab were shown in the VOYAGE 2 studyCitation6 at 24 weeks, only the VOYAGE 1 trialCitation5 included both guselkumab and adalimumab at their recommended dosing for a full 48 weeks. Our analysis also assumed that the response rates for both guselkumab and adalimumab at 48 weeks would be maintained at both 52 weeks and 104 weeks if treatment was continued as recommended in the product labels. This assumption could have an impact on our results if maintenance of response over time differs between therapies. However, Griffiths et al.Citation13 reported that the proportion of patients in response was maintained from 48-weeks to 100-weeks in patients taking guselkumab in the VOYAGE 1 study. In addition, the REVEAL responder analysis reported that adalimumab patients that achieved a PASI 75 response at weeks 16 and 33 generally maintained response through week 100Citation14. Our analysis did not account for other costs associated with biologics, such as those related to administration, monitoring, or adverse events. While this is a limitation worth noting, administration and monitoring requirements are similar for guselkumab and adalimumab, and rates and types of adverse events were generally comparable between the guselkumab and adalimumab groups through week 48 in the VOYAGE 1 trialCitation5. We also did not include medical care or other indirect costs associated with PsO that might change with treatment. WAC costs in the US were used in this model, and results may vary depending on payer-specific costs for each therapy. Lastly, in the base-case analysis, we did not adjust the 48-week response rates for guselkumab and adalimumab by the placebo response at 16 weeks. However, in the scenario analysis, we showed that the base-case results were not sensitive to this adjustment.
The strengths of our study include the CPR comparison of two biologics using efficacy based on PASI 75, PASI 90, and PASI 100 response rates from a 48-week head-to-head study. Given that PsO is a chronic condition, use of annual costs and 48-week outcomes to estimate the CPR are more illustrative of the use of biologics than monthly or short-term costs and outcomesCitation16–18.
Conclusion
In conclusion, since multiple biologic options are available for treatment of moderate-to-severe PsO, it is important to consider the value of each therapy. CPR and NNT analyses are approaches for ranking the cost-effectiveness and clinical efficacy, respectively, of different treatments. In particular, annual CPR estimates presented in this study allow US decision-makers to use cost-effectiveness as a measure of biologic drug value based on a range of PASI response levels. In this study, guselkumab was shown to be a more cost-effective and efficacious option than adalimumab in patients with moderate-to-severe PsO using both CPR and NNT analyses based on PASI 75, PASI 90, and PASI 100 responses in both the induction and maintenance years from the VOYAGE 1 trial.
Transparency
Declaration of funding
Funding for technical writing for this study was provided to RTI-HS, a paid contractor of Janssen Scientific Affairs, LLC.
Declaration of financial/other relationships
AT and EM are employees of Janssen Scientific Affairs and stock holders of Johnson & Johnson, the manufacturers of guselkumab. The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed consulting and receiving grants from both Janssen and Abbvie, the makers of the products under study, as well as a number of other companies that market biologics for psoriasis. Another reviewer has disclosed researching for both Janssen and AbbVie plus giving lectures on behalf of both companies. The reviewers have no other relevant financial relationships or otherwise to disclose.
Previous peer-reviewed presentations
Fall Clinical Dermatology Conference, October 12–15, 2017, Las Vegas, NV (induction year estimates only); American Academy of Dermatology Annual Meeting, February 16–20, 2018, San Diego, CA (induction year estimates only); Academy of Managed Care Pharmacy Annual Meeting, April 23–26, 2018, Boston, MA (induction and maintenance year estimates).
Acknowledgements
Technical writing and editing support was funded by Janssen Scientific Affairs (LLC) and provided by Josephine Mauskopf and John Forbes of RTI Health Solutions (RTI-HS).
References
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516.
- Helmick CG, Lee-Han H, Hirsch SC. Prevalence of psoriasis among adults in the U.S.: 2003–2006 and 2009–2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37–45.
- Cline A, Hill D, Lewallen R, et al. Current status and future prospects for biologic treatments of psoriasis. Expert Rev Clin Immunol. 2016;12:1273–1287.
- Kircik LH, Del Rosso JQ. Anti-TNF agents for the treatment of psoriasis. J Drugs Dermatol. 2009;8:546–559.
- Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active-comparator, controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the Phase III, double-blind placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418–431.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, Phase III NAVIGATE trial. Br J Dermatol. 2018;178:114–123.
- Fredriksson T, Pettersson U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica. 1978;157:238–244.
- Revicki DA, Willian MK, Menter A, et al. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology (Basel). 2008;216:260–270.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to psoriasis area severity index 90 and 100. J Drugs Dermatol. 2015;14:1086–1088.
- Langley RG, Feldman SR, Nyirady J, et al. The 5-point investigator’s global assessment (IGA) scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26:23–31.
- Truven Health Analytics. Red Book. 2019 [cited 2019 Mar 19]. Available from: http://www.micromedexsolutions.com.
- Griffiths CEM, Papp KA, Kimball AB, et al. Long-term efficacy of guselkumab for the treatment of moderate-to-severe psoriasis: results from the Phase 3 VOYAGE 1 trial through two years. J Drugs Dermatol. 2018;17:826–832.
- Gordon K, Papp K, Poulin Y, et al. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from reveal. J Am Acad Dermatol. 2012;66:241–251.
- Wu JJ, Feldman SR, Rastogi S, et al. Comparison of the cost-effectiveness of biologic drugs used for moderate-to-severe psoriasis treatment in the United States. J Dermatolog Treat. 2018;29:769–774.
- Martin S, Feldman SR, Augustin M, et al. Cost per responder analysis of ustekinumab and etanercept for moderate to severe plaque psoriasis. J Dermatolog Treat. 2011;22:138–143.
- D’Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015;72:589–598.
- Al Sawah S, Foster SA, Burge R, et al. Cost per additional responder for ixekizumab and other FDA-approved biologics in moderate-to-severe plaque psoriasis. J Med Econ. 2017;20:1224–1230.
