Abstract
Background: Thoracoscopic lobectomy for lung cancer is a complex procedure where endoscopic staplers play a critical role in transecting the lung parenchyme, vasculature, and bronchus. This retrospective study was performed to investigate the economic benefits of powered and tissue-specific endoscopic staplers such as gripping surface technology (GST) and powered vascular stapler (PVS) compared to standard staplers.
Methods: Two hundred and seventy-five patients who received a thoracoscopic lobectomy between 2008 and 2016 were included. Group 1 (n = 117) consisted of patients who received the operation with manual endoscopic staplers, whereas Group 2 (n = 158) consisted of patients who received the operation with GST and PVS.
Results: Patient demographics and clinical characteristics were comparable, except smoking history, pulmonary function, and pleural adhesion. All patients received the operation successfully without mortalities and broncho-pleural fistula. Operation time and blood loss were higher in Group 1. Pleurodesis was performed less in Group 2 than in Group 1 (18.0% vs 3.8%, p < 0.0001). Group 2 had statistically significant lower adjusted hospital costs (Korean Won, 14,610,162 ± 4,386,628 vs 12,876,111 ± 5,010,878, p < 0.0001), lower adjusted hemostasis related costs (198,996 ± 110,253 vs 175,291 ± 191,003, p = 0.0101); lower cartridge related adjusted costs (1,105,091 ± 489,838 vs 839,011 ± 307,894, p < 0.0001) compared to Group 1. As well, Group 2 showed ∼12% lower adjusted total hospital costs compared to Group 1. Multivariable analysis revealed that Group 1 was related to increased hospital costs.
Conclusions: This study showed that thoracoscopic lobectomy with powered and tissue-specific endoscopic staplers were associated with better clinical outcomes and reduced adjusted hospital costs when compared in Korean real-world settings.
Introduction
A video-assisted thoracoscopic surgery (VATS) approach for non-small cell lung cancer (NSCLC) has been introduced as a minimally invasive technique and is being increasingly considered a standard of care for early NSCLC. According to previous studies, VATS lobectomy in NSCLC is associated with lower complication ratesCitation1–3, less post-operative pain, improved quality-of-life, and similar hospital costsCitation4–6 when compared with open thoracotomy.
VATS pulmonary resections are complex procedures, and endoscopic staplers play a critical role since conventional surgical techniques such as suture or tie are difficult to perform during VATS. VATS pulmonary resections are complex procedures in a constricted thoracic space, where endoscopic staplers enable a superior method for tissue and vessel transection compared to clamps, cut and tie approach in open thoracotomy. Endoscopic staplers are used for the division and transection of hilar or segmental vascular and bronchial structures, and division of fissures and lung parenchymeCitation7. These staplers have simplified the operative procedure and reduced surgeon skill-driven variability. However, endoscopic staplers are also associated with post-operative complications, including air leaks and bleeding from staple lines, and broncho-pleural fistula at the bronchial stump.
To overcome these challenges, innovation in endoscopic stapling technology has advanced with the development of powered staplers with battery-operated stapling and cutting functions, improving stability and enabling more precise stapling relative to non-powered (manual) stapling. Furthermore, there are tissue-specific endoscopic staplers such as the Echelon Flex™ Powered Vascular Stapler (PVS; Ethicon, Cincinnati, OH) for pulmonary vessel transections, and the Echelon Flex™ GST System (GST; Ethicon, Cincinnati, OH) for reducing lung tissue slippage during stapling in thoracic surgery. These newer staplers were associated with improved operative outcome in prior studiesCitation8–10. We hypothesized that advanced endoscopic staplers GST and PVS could potentially reduce post-operative complications, improve clinical outcomes, and consequently reduce VATS pulmonary resection costs. Therefore, the purpose of this retrospective study was to compare clinical and economic outcomes associated with the use of manual endoscopic staplers and the more recently launched powered, tissue-specific endoscopic staplers during VATS lobectomy for NSCLC based on institutional data in Korea.
Methods
Study data and patient selection
The study protocol was approved by the Institutional Review Board with a waiver for informed consent owing to the retrospective nature of the analysis (IRB No. 4-2017-0780). Two hundred and seventy-five patients who received VATS lobectomy at a single institution in Seoul, Korea between 2008 and 2016 were included in the analysis. All patients were diagnosed with lung cancer and were older than 19 years of age. Thoracotomy conversions from planned VATS lobectomy were included in the analysis to represent the full spectrum of outcomes and costs for the cohort. All procedures were similar for the operating surgeons regarding the dissection of hilar and mediastinal dissection and stapling technique. The choice of endoscopic stapler was made based on the operating surgeon’s preference. To focus the analysis on assessing the value of innovation on the same platform, only Ethicon-manufactured (Cincinnati, OH) endoscopic staplers and cartridges (or reloads) were included in this study.
Patients were excluded from this study if both powered and non-powered staplers were used together during the operation in view of potential challenges with the interpretability of observed associations. All lung parenchyma, hilar vasculature and bronchus were divided by endoscopic staplers.
Endoscopic staplers
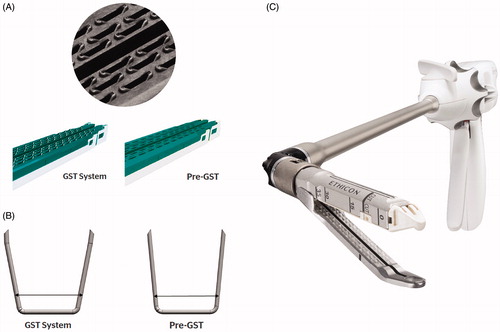
The endoscopic staplers included in this analysis were categorized as Group 1: manual staplers with a standard Echelon cartridge; and Group 2: powered and tissue-specific endoscopic stapler (PVS, GST). Characteristics of the advanced powered staplers are presented in . In Group 1, patients underwent surgery with manual endoscopic staplers (Echelon Flex 45/60). In Group 2, patients were operated on using powered, tissue-specific endoscopic staplers (powered Echelon Flex GST system 45/60, and Echelon Flex powered vascular stapler).
Figure 1. Comparison of conventional staplers and tissue-specific staplers. (A) Gripping surface technology (GST) system cartridge. The gripping surface technology utilizes proprietary pocket extensions to stabilize tissue and hold it in place, as well as support and guide staple legs toward anvil pockets during firing. (B) Re-designed bent staple tips with asymmetrical legs in GST staplers. (C) Powered vascular staplers (PVS) system with narrow anvil and curved tip.
Patient and operative characteristics
All data extracted from the electronic medical records database of the research institution were managed by a dedicated data manager (Byun). Patient characteristics included gender, age, body mass index, smoking history, pulmonary function, location of tumor, tumor history, tumor stage, and pre-operative comorbidities, including hypertension, diabetes, liver cirrhosis, pulmonary disease, cardiovascular disease, chronic kidney disease, and cerebrovascular disease. Pleural adhesion was defined as the intraoperative finding of pleural adhesion and symphysis regardless of its extent and characteristics. Operative characteristics included data on thoracotomy conversion, intraoperative blood loss, hemostasis material usage, intra-operative events like thoracotomy conversion, hypotension, arrhythmia and desaturation, and number of stapler cartridges used. The pathologic stages were calculated based on the American Joint Committee on Cancer (AJCC) 7th edition guidelinesCitation11. The prolonged air leakage was defined when the air leakage was sustained for more than 7 days. The pleurodesis was done by surgeon’s clinical decisions, and usually done when air leakage was prolonged more than 3 or 4 days after operation.
Clinical and economic outcomes
Clinical outcomes included operation time, length of chest tube stay, prolonged air leakage (sustained air leakage for more than 7 days), pleurodesis, intraoperative bleeding, and transfusion. Economic outcomes and healthcare resource use were evaluated during the index admission and included hospital length of stay, total hospital costs, 30-day surgery-related readmission, total hemostat material cost, as well as number and cost of stapler cartridges used. Among the economic outcomes, total hospital costs were analyzed as a primary endpoint. Other economic outcomes and clinical outcomes are analyzed as secondary endpoints. Costs were documented and calculated in South Korean Won (KRW) and also documented as US dollars. All costs were adjusted using conversion factors based on the Resource-based Relative Value scale (RBRVS) in the Korea national health insurance system. Conversion factor was is cost per RBRVS. Total hospital costs for multiple years were adjusted to the reference year (2016), using the following formula:
Adjusted total hospital cost of specific year = Total hospital cost of specific year/(conversion factor of specific year × conversion factor of reference year).
Statistical analyses
All observed data are presented as means and standard deviations for continuous variables and as frequencies and percentages for categorical variables. Groups 1 and 2 were compared by the Wilcoxon rank-sum test or independent t-test for continuous variables according to the normality of the data and by the Chi-square test or Fisher’s exact test for categorical variables. To compare outcomes between the two groups, potential confounder variables such as patient demographics, clinical characteristics, and operative characteristics were adjusted using the multivariable linear regression model. First, the significant variables in bivariable hypothesis tests and clinically significant variables were entered into a multivariable linear regression model. Next, variables having large p-values in the multivariable linear regression model were deleted. Complete cases were applied in the regression model because the proportion of missing observations was 1.45%. We checked the appropriateness of the regression model including linearity, homogeneity, independence of error terms, and normality by residual analysisCitation12. We also confirmed that there is no multicollinearity problem. Since secondary endpoints provide exploratory natures, multiplicity adjustment was not considered. The sample size in this study provide over 80% of power to detect the moderate Cohen’s effect size (0.5) with a two-tailed α of 0.05. A two-sided p-value of 0.05 was considered to indicate a statistically significant difference for all analyses. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Patient and operative characteristics
Among the 275 patients, 117 belonged in Group 1 (manual) and 158 belonged in Group 2. Patient demographics and clinical characteristics are summarized in . Groups 1 and 2 had a mean (standard deviation) age of 61.8 ± 10.5 years and 63.7 ± 9.9 years; and included 59.0% and 50.0% male patients, respectively. The two groups presented a similar prevalence rate of almost all comorbidities. The groups differed in the proportion of patients with smoking history and diffusing the capacity for carbon monoxide. However, the mean of pack-years of smoking was similar between the two groups.
Table 1. Patient demographics and clinical characteristics.
Operative characteristics are summarized in . The incidence of pleural adhesion which was found during the operation was higher in Group 1 than in Group 2 (29.1% vs 4.4%, p < 0.0001). The mean operation time was also longer in Group 1 (189.9 ± 77.5 min vs 137.1 ± 49.9 min, p < 0.0001) compared to that in Group 2. Intraoperative blood loss (182.4 ± 328.7 mL vs79.8 ± 110.0 mL, p = 0.0004), and related usage of hemostatic material per case (0.91 vs 1.34, p < 0.0001) were also higher in Group 1 compared to Group 2. Group 2 reported a lower mean number of stapler cartridges used per procedure (5.2 vs 6.2, p = 0.0170) compared to Group 1. There was no broncho-pleural fistula or operative moralities recorded in either group. During hospitalization, significantly less pleurodesis was necessary among patients in Group 2 compared to those in Group 1 (18.0% vs 3.8%, p < 0.0001). And, finally, patients in Group 2 had a shorter average length of hospital stay, and lower incidence of prolonged air leaks compared those in Group 1, although both point estimates marginally missed statistical significance.
Table 2. Comparison of operative outcomes between two groups.
Economic outcomes
The economic outcomes are summarized in . Group 2 had statistically significant lower adjusted (to base year of 2016) hospital costs (KRW 14,610,162 ± 4,386,628 [$12,281.58 ± 3,687.17] vs KRW 12,876,111 ± 5,010,878 [$10,822.99 ± 4,211.88], p < 0.0001), lower adjusted hemostasis related costs (KRW 198,996 ± 110,253 [$167.28 ± 92.68] vs KRW 175,291 ± 191,003 [$147.35 ± 160.56], p = 0.0101); lower cartridge related adjusted costs (KRW 1,105,091 ± 489,838 [$928.88 ± 411.70] vs KRW 839,011 ± 307,894 [$705.17 ± 258.78], p < 0.0001), when compared to Group 1. The adjusted mean total hospital cost in Group 1 was ∼12% than that of Group 2 (), which indicates significant savings potential for the institution and healthcare system.
Table 3. Comparison of economic outcomes between two groups.
Results of multivariable analyses for predictors of total hospital costs
Multivariable analysis revealed statistically significant factors associated with blood loss and adjusted total hospital costs (). Multivariable regression demonstrated that, when controlled for variability in other characteristics belonging to Group 2 was associated with a lower adjusted total hospital cost compared to that of Group 1. In addition, lower body mass index (< 18.5 kg/m2), presence of pulmonary disease, and the location of the lesion (right lower lobe and left upper lobe) were associated with increased adjusted total costs.
Table 4. Multivariable regression for predictors of adjusted total hospital cost.
Discussion
In this retrospective analysis of hospital data, we found that adjusted total hospital costs, hemostatic material, and stapler cartridges costs among lung cancer patients who underwent VATS lobectomy were lower in the powered stapler group (Group 2) than in the manual stapler group (Group 1). The use of Echelon powered staplers (PVS, GST) was associated with ∼12% lower observed adjusted total hospital costs compared to manual staplers. The powered stapler group also demonstrated better operative outcomes in terms of operation time, intraoperative blood loss, usage of hemostatic materials, and number of stapler cartridges. As Group 1 included more patients with pleural adhesion, multivariable adjustment of such difference was attempted using regression analysis. Even after such adjustment, the use of powered and tissue-specific endoscopic staplers (Group 2) was associated with a lower adjusted total hospital cost (). As such, this study suggests that the use of advanced powered and tissue-specific stapling systems (GST and PVS) were not only associated with improved clinical outcomes, but were also associated with lower hospital costs compared with using manual staplers.
The benefits of using powered and tissue-specific staplers suggested by the findings of this study are supported by other evidence presented elsewhere. Powered staplers were developed to increase stability and enable more precise stapling relative to non-powered (manual) staplingCitation8. Ethicon powered staplers were found to reduce movement at the distal tip by 88% compared to manually-fired devices, thus potentially causing less trauma to adjacent tissue during thick transectionsCitation8. Tissue-specific endoscopic staplers were designed specifically to transect various thickness of intrathoracic tissues: GST for lung parenchyma and bronchus, and PVS for pulmonary vessels.
Compared to earlier generation endoscopic staplers, the GST system has been accredited with several advantages, such as the fact that gripping surface technology utilizes proprietary pocket extensions to stabilize the tissue and hold it in place, as well as supports and guides staple legs toward anvil pockets during firing. Re-designed bent staple tips with asymmetrical legs also help mitigate any remaining tissue slippage during firing. As a result, the GST system has shown exceptional staple line integrity across the broadest range of tissue thicknesses with each cartridge/reload. The PVS 35-mm articulating endocutter, on the other hand, has been designed to enable more precise placement of the staple line on fragile pulmonary vessels using a narrower anvil than the standard six-row staplers. Due to its narrower design, the need for dissection around the pulmonary vasculature for stapling is reducedCitation10. It seems likely that the improved clinical and economic outcomes associated with the use of powered and tissue-specific staplers (Group 2) might have been derived from the combination of both the powered delivery mechanism and the tissue effects of the cartridge/reload.
An interesting and significant finding in this study has been the average number of stapler cartridges used per procedure—which was less in Group 2 compared to that in Group 1. Since there was no modification in operative techniques and patient management policies across the groups, this finding might have been related to the design characteristics of the relevant endoscopic staplers and cartridges. In particular, the gripping surface technology in the GST system utilizes proprietary pocket extensions to stabilize the tissue and hold it in place—which thereby reduces tissue slippage and the need for additional cartridge to cover tissue pushed out of the cutline during stapler firing.
Especially in thoracic surgery, stapler failures such as slippage or malformation of the staple line in lung tissue can cause intraoperative bleeding, air leaks from the stapling line, and the need for additional cartridges—all leading to an increase of the total operation time and costs. Use of advanced powered and tissue-specific endoscopic stapling systems may help reduce such unfavorable outcomes, as suggested by the findings of this study. The incidence of prolonged air leaks was marginally lower in Group 2 compared to Group 1, although the difference was not statistically significant. Although the stapler might have been associated with the finding in some part, this result should be interpreted with caution because the incidence of pleural adhesion was higher in Group 1, which may have confounded the better observed results in Group 2. However, for findings related to intraoperative bleeding, the statistically significant more favorable results for Group 2—both in terms of volume of bleeding and hemostat material use, points to potential benefits of the precision and access of the PVS system and the resulting reduced need for hilar structure dissection, despite other factors such as pleural adhesion being in play.
These results are consistent with previous experimental and clinical studies which have reported the benefits of powered and tissue-specific endoscopic staplers. In terms of powered staplers, previous real-world database studies have reported that powered endoscopic staplers had better clinical outcomes and cost effectiveness in the field of bariatric surgery and pulmonary resection demonstrated by lower bleeding/hemostasis related complications, shorter hospital length of stay and lower hospital costs compared to manual endoscopic staplersCitation8,Citation9. The consistency between findings of this study and results of previously published peer-reviewed literature offers additional face validity and global reliability of this analysis. In addition, pre-clinical studies involving the GST stapling system have demonstrated significantly greater mucosal capture in gastrointestinal tissue—leading to lesser potential for leaks compared to competing manual staplers in a porcine modelCitation13. Also, a recent study using an innovative model of physiologic lung breathing demonstrated that a uniform staple line delivered with a powered GST stapler had lesser number and volume of air leaks compared to a graduated staple line delivered by a manual stapler on porcine lungs—both under ventilator, as well as under physiologic breathing conditionsCitation14.
This study has several limitations. First, we did not grade the characteristics, amount, and location of pleural adhesion, even though it was anticipated to have been an important factor related to the operative outcomes. Such grading was not possible owing to the retrospective nature of this study which was limited by the amount and granularity of clinical data available. Second, this study was performed in the environment of the Korean Health Insurance System. As such, while the findings provide directional support and global validation of the potential benefits associated with the use GST and PVS staplers, generalization of the results to other countries and healthcare systems need to be made with caution. In fact, hospitalization costs under the Korean Health Insurance System are low (about 10 US dollars per day) compared to many other countries, and so the benefit in total hospital costs associated with Group 2 might have been under-estimated in this study. Third, to enable comparability of the core technology platform, this study involved products from one company (Ethicon) only. As such, directional extension of these findings to other comparable technologies are not workable with this analysis and should be avoided. Powered technology platforms not included in this analysis may not automatically be associated with the benefits visible here, and should be tested independently in future assessments. And, lastly, the retrospective nature of this study presents intrinsic risks of confounding factors that are unobserved and, therefore, not adjusted for. Also, no cause–effect relationship between the outcomes and the use of any stapling technology can be established. Randomized controlled clinical assessments are recommended to document the true causal benefits of advanced staplers.
However, in spite of these limitations, this study is significantly meaningful as the first real-world comparative analysis of manual staplers and the advanced innovation of GST and PVS staplers in thoracoscopic pulmonary resection. Miller et al.Citation9 reported the results of a similar study which compared clinical and economic outcomes associated with the use of powered and manual endoscopic staplers during thoracoscopic pulmonary resection in the US—but the GST system was not specifically identifiable in their results.
In conclusion, this retrospective real-world data analysis provides additional, Asia/Korea-based evidence of the fact that VATS lobectomy procedures performed with advanced powered and tissue-specific staplers may be associated with superior clinical outcomes and lower hospital costs. Prospective clinical studies should be designed to confirm this finding in the future.
Transparency
Declaration of funding
This work was supported by Johnson & Johnson Medical.
Declaration of financial/other interests
DJK and SYP are compensated consultants for Johnson & Johnson Medical, and HJP and JHC are employees of Johnson & Johnson Medical. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgements
None reported.
References
- Roviaro G, Varoli F, Vergani C, et al. State of the art in thoracospic surgery: a personal experience of 2000 videothoracoscopic procedures and an overview of the literature. Surg Endosc. 2002;16:881–892.
- Imperatori A, Rotolo N, Gatti M, et al. Peri-operative complications of Video-Assisted Thoracoscopic Surgery (VATS). Int J Surg. 2008;6:S78–S81.
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg. 2016;49:870–875.
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–844.
- Rodgers-Fischl PM, Martin JT, Saha SP. Video-assisted thoracoscopic versus open lobectomy: costs and outcomes. South Med J. 2017;110:229–233.
- Mafé JJ, Planelles B, Asensio S, et al. Cost and effectiveness of lung lobectomy by video-assisted thoracic surgery for lung cancer. J Thorac Dis. 2017;9:2534
- Özyurtkan MO, Kaba E, Toker A. Technological innovation in video-assisted thoracic surgery. J Vis Surg. 2017;3:20.
- Roy S, Yoo A, Yadalam S, et al. Comparison of economic and clinical outcomes between patients undergoing laparoscopic bariatric surgery with powered versus manual endoscopic surgical staplers. J Med Econ. 2017;20:423–433.
- Miller DL, Roy S, Kassis ES, et al. Impact of powered and tissue-specific endoscopic stapling technology on clinical and economic outcomes of video-assisted thoracic surgery lobectomy procedures: a retrospective, observational study. Adv Ther. 2018;35:707–723.
- Ng CS, Pickens A, Siegel JM, et al. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devices. Eur J Cardiothorac Surg. 2015;49:i73–i78.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714.
- Hickey GL, Dunning J, Seifert B, et al. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg. 2015;48:180–193.
- Thompson SE, Young MT, Lewis MT, et al. Initial assessment of mucosal capture and leak pressure after gastrointestinal stapling in a porcine model. Obes Surg. 2018;28:3446–3453.
- Eckert CE, Harris JL, Wong JB, et al. Preclinical quantification of air leaks in a physiologic lung model: effects of ventilation modality and staple design. MDER. 2018;11:433–442.
