Abstract
Objective: The COMBI-AD trial demonstrated the efficacy and safety of dabrafenib and trametinib in combination vs placebo as adjuvant treatment of patients with BRAF V600E/K mutation-positive resected Stage IIIA (lymph node metastasis >1 mm), IIIB, or IIIC melanoma. This analysis evaluated the cost-effectiveness of dabrafenib and trametinib vs observation from a US healthcare payer perspective.
Methods: This evaluation employed a non-homogeneous, semi-Markov, cohort model with health states for relapse-free survival (RFS), post-locoregional recurrence (LR), post-distant recurrence (DR) receiving first-line treatment, and post-DR receiving second-line treatment. A 50-year modeling time horizon was used. Transition probabilities were estimated based on individual patient data (IPD) from the COMBI-AD trial. Health-state utilities were estimated using EuroQol (EQ-5D) index values from COMBI-AD and published sources. Direct medical costs associated with treatment of melanoma were considered, including costs of BRAF mutation testing, medication and administration costs for adjuvant and metastatic treatments, costs of treating recurrence, and costs of adverse events. Costs and quality-adjusted life-years (QALYs) were discounted at 3.0% annually.
Results: Compared with observation, adjuvant dabrafenib and trametinib was estimated to result in a gain of 2.15 QALYs at an incremental cost of $74,518. The incremental cost-effectiveness ratio (ICER) was estimated to be $34,689 per QALY. In deterministic sensitivity analyses, the ICER was sensitive to the cost of dabrafenib and trametinib and the distribution used for projecting RFS beyond the end of follow-up in the COMBI-AD trial. At a cost-effectiveness threshold of $100,000 per QALY, the probability that dabrafenib and trametinib is cost-effective was estimated to be 92%.
Conclusions: Given generally-accepted cost-effectiveness threshold values in the US, dabrafenib plus trametinib is likely to be a cost-effective adjuvant therapy for patients with BRAF mutation positive melanoma. These results may be useful for policy-makers in their deliberations regarding reimbursement and access to this treatment.
Introduction
Melanoma is a malignant tumor arising from melanocytes, typically in the skinCitation1. It was estimated that there would be ∼91,000 new cases of melanoma and 9,300 melanoma related deaths in the US in 2018Citation2. The annual incidence rate of melanoma has increased by over 50% in the past 20 yearsCitation2. Although ∼92% of people diagnosed with melanoma skin cancer in the US survive for 5 years or more after their diagnosisCitation2, the prognosis is highly dependent on disease stage at diagnosis, with an historical 5-year survival from the American Joint Committee on Cancer Staging (AJCC) melanoma registry of ∼40% for those diagnosed with Stage IIIC and less than 20% for those with Stage IV diseaseCitation3.
The availability of immune checkpoint inhibitors and therapies targeting the MAPK pathway, which have been shown to improve progression-free survival (PFS) and overall survival (OS) in patients with unresectable or metastatic melanoma, has revolutionized the treatment of metastatic melanoma in recent yearsCitation4,Citation5. Given the improved outcomes observed with these treatments in the metastatic setting, their use as adjuvant therapy is being evaluated in numerous Phase III trials.
Dabrafenib (Tafinlar, Novartis, East Hanover, NJ, USA) and trametinib (Mekinist, Novartis, East Hanover, NJ, USA) are small-molecule targeted inhibitors of BRAF and MEK kinase, respectively. The efficacy and safety of dabrafenib and trametinib in combination compared with BRAF inhibitor monotherapy as first-line treatment in patients with BRAFV600 mutation-positive unresectable or metastatic melanoma was demonstrated in the COMBI-v and COMBI-d trialsCitation6,Citation7. COMBI-AD is a randomized double-blind, placebo-controlled, phase 3 trial of dabrafenib and trametinib in combination for up to 12 months in patients with completely resected, high-risk stage III melanoma (based on AJCC 7th edition staging), with BRAF V600E or V600K mutation. In the initial analysis of COMBI-AD based on a data cut-off of June 30, 2017 with a median follow-up of 2.8 years, the estimated 3-year probability of relapse-free survival (RFS) was 58% in the dabrafenib and trametinib group and 39% in the placebo group (hazard ratio [HR] = 0.47; 95% confidence interval [CI] = 0.39–0.58; p < 0.001)Citation8,Citation9. The 3-year probability of OS was 86% in the dabrafenib and trametinib group and 77% in the placebo group (HR = 0.57; 95% CI = 0.42–0.79; p = 0.0006), but this level of improvement did not cross the pre-specified interim analysis boundary of p = 0.000019Citation8,Citation9. Based on the initial results of COMBI-AD, dabrafenib and trametinib in combination was approved in the US as adjuvant treatment of patients with melanoma with BRAF V600E or V600K mutations, as detected by an FDA-approved test, and involvement of lymph node(s), following complete resection. Results of an updated analysis of RFS and distant metastases free survival (DMFS) based on a April 30, 2018 data cut-off with a median follow-up of 3.7 years and a maximum follow-up of ∼5 years confirmed the RFS benefit with dabrafenib and trametinibCitation10.
While COMBI-AD provided evidence sufficient for the regulatory approval of dabrafenib and trametinib as adjuvant treatment of melanoma, healthcare payers require information on the cost-effectiveness of new pharmaceutical products. To address this need, we evaluated the cost-effectiveness of dabrafenib and trametinib as adjuvant treatment of patients with melanoma from the perspective of a US healthcare payer.
Methods
Overview
This study evaluated the cost-effectiveness of dabrafenib and trametinib as adjuvant treatment for BRAF V600E/K mutation-positive Stage IIIA, IIIB, or IIIC melanoma after complete surgical resection based on results of the COMBI-AD trial (NCT01682083). The comparator of interest was observation, as represented by the placebo arm of COMBI-AD. The evaluation was conducted from a US healthcare payer perspective. A 50-year time horizon was employed. This approximates a lifetime projection consistent with recommended good practices for cost-effectiveness analysisCitation11,Citation12; as patients entering the model were assumed to be 50 years of age, consistent with the mean age in COMBI-ADCitation8, model outcomes were projected until patients were 100 years of age, at which point virtually all patients are projected to be dead. Cost-effectiveness was evaluated using a non-homogeneous, semi-Markov, cohort model, with states defined on recurrence, prior treatment, and death. Non-homogeneous models include time-dependent transition probabilities (e.g. age-specific mortality). Outcomes of interest included expected lifetime costs of melanoma treatment, overall life expectancy expressed as life-years (LYs), and quality-adjusted life-years (QALYs). The incremental cost-effectiveness ratio (ICER) was calculated as the ratio of incremental costs (dabrafenib and trametinib vs observation) to incremental QALYs. Future costs and benefits were discounted at 3.0% annually. All costs were adjusted to 2017 dollars, as necessary, using the consumer price index for medical careCitation13.
Model description
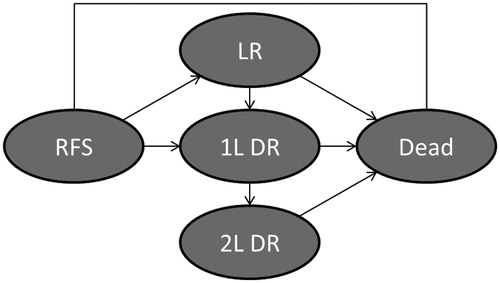
Cost-effectiveness was evaluated using a non-homogeneous, semi-Markov, cohort model with a 6-month cycle duration and consisting of five distinct health states: (1) RFS state; (2) post-locoregional recurrence (LR) state; (3) post-distant recurrence (DR) first-line treatment (1 L DR) state; (4) post DR second-line treatment (2 L DR) state; and (5) dead state (). Patients enter the model in the RFS state, wherein they are assumed to be at risk of LR, DR, or death. Patients with LR who undergo successful re-resection are assumed to enter the LR state, wherein they are assumed to be at risk of DR or death. Patients with LR who are not successfully resected and patients who experience DR transition to the 1 L DR state, wherein they are assumed to be at risk of progression to second-line treatment or death. Those progressing to second-line treatment are assumed to enter the 2 L DR state and are assumed to be at risk for death. Probabilities of events are assumed to vary by time since entering a given health state to reflect the natural history of melanoma and over modeling time to account for the increased risk of death from non-melanoma causes as patients grow older. Costs are conditioned on the health state and the amount of time spent in the state. Utility values are assumed to decline with age based on declines in age-matched general population norm utility values. The model was programmed in Microsoft Excel.
Model estimation
Transition probabilities
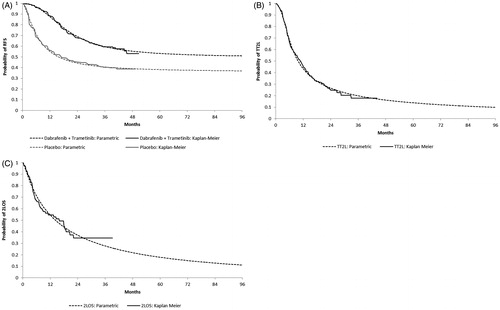
Transition probabilities for RFS, as well as first-line progression-free survival (1LPFS) and time from second-line treatment to death (2LOS) for patients with DR, were based on parametric survival distributions fit to IPD from COMBI-AD using Flexsurv, an R package for fully-parametric modelling of survival dataCitation14 (R version 3.3.3). The following parametric distributions were considered: exponential, Weibull, log-logistic, lognormal, Gompertz, gamma, generalized F, and restricted cubic spline (RCS) distributions. Distributions were selected based on fit statistics (Bayesian Information Criterion [BIC]), visual inspection of fit to Kaplan-Meier plots, hazard functions, time dependent hazard ratios, diagnostic plots for treatment effects, and clinical plausibility. In these analyses, patients with second primary malignancy without concurrent LR or DR events were censored, as such events are unlikely to result in significant costs or effects on health-related quality-of-life (HRQoL). Parametric distributions and Kaplan-Meier curves for RFS, 1LPFS, and 2LOS are shown in .
For RFS, a log-logistic unrestricted cure distribution was selected for the base-case (with restricted distributions, only one parameter of the distribution may differ by treatment arm; with unrestricted distributions, all parameters may differ). This distribution has the best statistical and visual fit, hazards that are consistent with the observed hazards in the COMBI-AD trial, does not yield implausible projections with the survival curves for the two arms crossing, and yields long-term projections of benefit that are intermediate between the other plausible distributions. The projected hazard rate for dabrafenib and trametinib is estimated to be slightly higher than that for observation at the end of follow-up based on the log-logistic unrestricted cure model.
The validity of model projections based on the distribution derived from COMBI-AD was assessed by comparing the projection for the observation arm with long-term RFS data for patients receiving placebo in the EORTC 18071 trial of ipilimumab vs placebo in Stage III melanoma. The EORTC 18071 trial is the most recent trial of adjuvant therapy for Stage III melanoma that includes a placebo arm and for which long-term data are available. The distribution of patients by AJCC stage at diagnosis was generally similar in EORT 18071 vs COMBI- AD (21% vs 18% stage IIIA, 38% vs 41% stage IIIB, and 41% vs 40% stage IIIC, respectively)Citation8,Citation15. Model projections of RFS at 7 years for observation are higher than the reported RFS for placebo in the EORTC 18071 trial (∼35% vs 25%). However, the Kaplan-Meier RFS for placebo in COMBI-AD is higher than that for placebo in EORTC 18071 beyond ∼2 years. Also, while the RFS curve for placebo in EORTC 18071 continues to decline after 2 years, the corresponding curve for placebo in COMBI-AD clearly flattens out at 3 years. The source of these differences is uncertain but might be due to improvements in diagnosis over the period separating the start dates of the two studies that would result in proportionally more subjects in EORTC 18071 that have micro-metastatic disease than in COMBI-AD, which could explain their seemingly worse prognosis. In light of the potential differences, the long-term projections of RFS based on data from COMBI-AD were considered reasonable and used in the base-case and RFS from EORTC 18071 were used in a sensitivity analysis.
Because the COMBI-AD protocol did not require that patients were followed for subsequent DR after LR, it was infeasible to estimate probabilities of DR or death after LR using data from COMBI-AD. Consultation with clinical experts, as well as epidemiological studies, suggested that patients who experience LR would be at increased risk of relapse compared with those in RFS not experiencing LRCitation16. It was, therefore, assumed that the distribution of RFS events would be similar for patients with prior LR, but that the probability of such events would be increased proportionately among patients in the LR state vs the RFS state. It was further assumed that patients would be at greatest risk of recurrence in the first 12 months after experiencing LR and would continue to be at increased risk until ∼5 years, after which the risk would be the same as that for patients without LR. The HR for this increased risk in the first 12 months following LR (HR = 4.47) and the HR for months 13–58 (HR = 1.69) were calculated as the average HR during these periods for second vs first recurrence in a surveillance study of patients with early stage melanomaCitation16.
In the absence of robust data indicating that 1LPFS would differ depending on treatment received in the adjuvant setting, 1LPFS was assumed to be the same regardless of initial adjuvant treatment. Accordingly, when fitting parametric survival distributions to 1LPFS data from COMBI-AD, data for all patients who experience DR were pooled across randomized treatment arms. Because the occurrence of disease progression following DR was not collected in COMBI-AD, initiation of second-line therapy was employed as a proxy for progression. Accordingly, 1LPFS events were defined as either death or initiation of second-line therapy for DR. Initiation of first- and second-line treatments for DR were determined for each patient with DR by identifying the first post-treatment anti-cancer therapy (PTACT) received after DR. Any PTACT initiated during the 45-day period following receipt of first PTACT was considered to be included in first-line treatment regimen. Receipt of any new PTACT after this period was assumed to constitute the initiation of second-line treatment. 2LOS was calculated as the time from initiation of second-line treatment to death. Survival distributions for 1LPFS and 2LOS were estimated using methods similar to those employed for RFS. For the base-case, the generalized F and lognormal distributions were selected for 1LPFS and 2LOS, respectively.
The probability distributions of RFS events by type were estimated based on IPD from COMBI-AD and were assumed to be dependent on initial adjuvant treatment and constant over time. For patients with LR, all subsequent RFS events were assumed to be DR or death. The distribution of 1LPFS events by type was assumed to be the same across treatment arms and constant over time ().
Table 1. Probabilities and utility values.
General population mortality estimates were based on US lifetablesCitation17. Patients entering the model were assumed to be 50 years old, consistent with the mean age in COMBI-ADCitation8.
Utility values
Health state utility values were estimated using data for EQ-5D-3L assessments from COMBI-AD. Utility values were based on the US value set and were analyzed using generalized estimating equations (GEEs) regression with covariates for baseline EQ-5D utility value and health state at assessment including RFS on-treatment (dabrafenib and trametinib patients only), RFS off-treatment, LR, and DR. RFS off-treatment included assessments for patients randomized to placebo and for those randomized to dabrafenib plus trametinib who had discontinued treatment. GEE regression was conducted using the SAS PROC GENMOD procedure (SAS version 9.4). Predicted mean utility values were calculated using the GEE regression and the mean baseline utility value for both treatment groups combined in order to control for differences in baseline utility values. For patients receiving placebo, the mean utility value for RFS off-treatment was used for all time in the RFS state. For patients receiving dabrafenib and trametinib in combination, the mean utility value for the RFS state was calculated as a weighted average of the RFS on-treatment and RFS off-treatment utilities, with weights based on estimates from COMBI-AD of the proportion of patients in the RFS state who would remain on therapy at the start of each month. Because the predicted mean utility values for patients who are on treatment from COMBI-AD would reflect the impact of adverse events (AEs) on HRQoL, no additional utility decrements were applied for patients experiencing AEs. As over 90% of post-DR assessments in COMBI-AD were performed within 30 days of relapse, EQ-5D data following DR from COMBI-AD was not employed to estimate utility values following DR. Rather, utility values for the 1 L DR and 2 L DR states were estimated based on mean utility values from the COMBI-v trialCitation18 and the Checkmate 066 trialCitation19, respectively. These utility values were assumed to be the same for all treatments. Health state utility values were adjusted for age-related declines in HRQoL using age- and gender-matched US population norms for the EQ-5DCitation20.
Costs
It was assumed that patients with Stage III melanoma would not undergo BRAF testing in the absence of adjuvant therapy with dabrafenib and trametinib. Accordingly, patients receiving dabrafenib and trametinib were assigned the cost of BRAF testing, accounting for the costs of patients who would test negativeCitation21–33, and the costs of retesting for patients with invalid initial test resultsCitation34. Patients receiving observation who experience disease recurrence were assumed to undergo BRAF testing to inform metastatic treatment. The cost of BRAF testing was based on the Center for Medicare and Medicaid Services (CMS) Clinical Diagnostic Laboratory Fee ScheduleCitation35.
Medication costs for adjuvant and metastatic treatments were based on wholesale acquisition cost (WAC) pricesCitation36. Administration costs were from the CMS Physician Fee ScheduleCitation37. Assumed dosages were from pivotal trials and approved prescribing information. Patients were assumed to receive treatment until the maximum duration of therapy (12 months for dabrafenib and trametinib), discontinuation, relapse (adjuvant therapy), progression (first-line therapy), or death (second-line treatment), whichever occurred first. Relative dose intensities (RDI) for dabrafenib (0.838) and trametinib (0.905) and quarterly probabilities of discontinuation among patients remaining alive and free of recurrence were from COMBI-AD. Patients with LR were assumed to receive observation only following resection. For patients with DR, the distributions of first- and second-line treatments for metastatic disease were based on data from COMBI-AD, with treatment regimens determined as described above and regimens not currently approved in the US recoded to the most similar treatment available. For patients receiving second-line treatments, the probabilities of discontinuation of such therapy were taken from the CheckMate 037 trial of pembrolizumab after ipilimumab in metastatic melanomaCitation38.
Costs of monitoring and follow-up in the adjuvant and metastatic settings, and the treatment of LR and DR (excluding the costs of medications) were based on results of a retrospective health insurance claims study of melanoma patientsCitation39. Based on NCCN guidelines for follow-up and monitoring of patients with melanoma, costs of follow-up in years 3–5 and 6 or more were estimated to be 50% and 25% of those in years 1–2, respectivelyCitation40. Monthly costs of follow-up and monitoring for patients with DR were calculated by subtracting costs of adjuvant and metastatic regimens and BRAF and other molecular testing (i.e. costs already captured in the model) from the mean per-patient per-month all-cause healthcare cost during an episode of DR. These costs were also adjusted by year, based on NCCN guidelines, as described aboveCitation40.
The model includes the cost of Grade 3–5 AEs with an incidence of 5% or more in COMBI-AD, which included hypertension and pyrexia. Grade 1–2 events were not considered because they are generally self-limited and are, therefore, not likely to be associated with substantial treatment costs. Costs of AEs were based on a health insurance claims study of costs of adverse events for melanoma patientsCitation41.
Terminal care costs were based on a published cost-effectiveness evaluation in the metastatic settingCitation42. All costs are 2017 US dollars, with adjustment based on the medical care consumer price index (CPI), as appropriate ().
Table 2. Costs inputs.
Analyses
LYs and QALYs were calculated by health state. Expected costs were calculated by health state and category of service. One-way deterministic sensitivity analyses were undertaken to explore the impact on the ICER of changes in key model parameters. Scenario analyses were performed in which the model generated results for alternative sets of parameter estimates or assumptions. Probabilistic sensitivity analyses (PSA) were generated by simultaneously sampling from estimated probability distributions of model parameters. For selected parameters derived from COMBI-AD (i.e. parametric survival distributions and distributions of events by type) model inputs were sampled from the joint bootstrap distributions of these parameters derived from bootstrap samples of data from the COMBI-AD trial. The probabilistic ICER was calculated based on the ratio of the mean incremental cost to the mean incremental QALYs. Incremental costs and QALYs from the PSA were plotted on the cost-effectiveness plane, and cost-effectiveness acceptability curves were calculated for each treatment.
Results
Base-case results
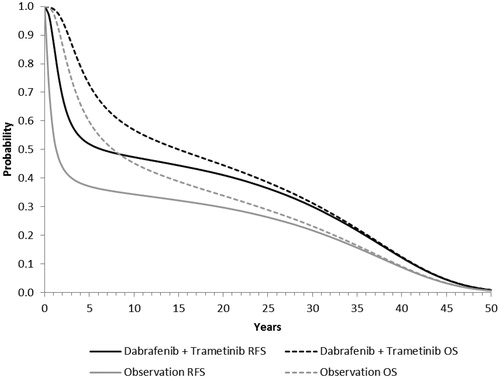
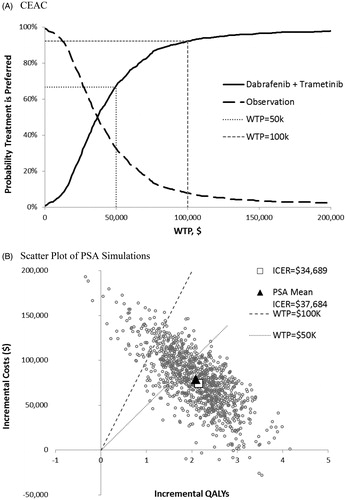
Base-case cost-effectiveness results for dabrafenib and trametinib are reported in . Model projections of RFS and OS for dabrafenib plus trametinib and observation are shown in . Patients receiving dabrafenib and trametinib as adjuvant treatment were estimated to experience 2.34 more total LYs and 2.15 additional QALYs (both discounted) compared with patients receiving observation only. The estimated gain in QALYs was driven largely by an increase in QALYs in the RFS state (2.70 QALYs), which was partly offset by losses in QALY in other states. Expected total melanoma-related healthcare costs were estimated to be $74,518 greater with dabrafenib and trametinib compared with observation. The increase in costs was due to higher costs in the RFS state, offset in part by the savings in costs in other health states. The ICER for dabrafenib and trametinib vs observation was estimated to be $34,689 per QALY gained.
Table 3. Base-case results.
Sensitivity analyses
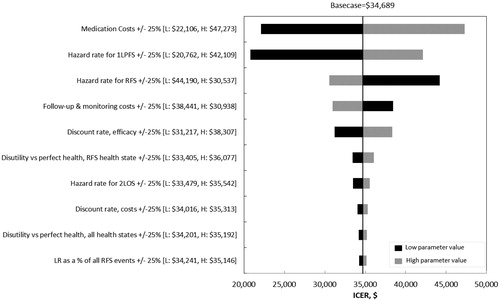
Results of the deterministic sensitivity analyses are shown in . The ICER was most sensitive to changes in medication costs, the hazard rate for 2LPFS, and follow-up and monitoring costs. Scenario analyses indicated that use of hazard rates based on EORTC 18071 for RFS after 50 months would result in an ICER of $49,941 (). Use of the gamma unrestricted cure distribution and log-logistic restricted cure distributions for RFS would result in ICERs of $12,458 and $136,308 per QALY gained, respectively. Limiting the timeframe of the model to 10 and 20 years would yield ICERs of $86,964 and $48,025 per QALY gained, respectively.
Table 4. Results of scenario analyses.
The mean ICER from the probabilistic sensitivity analyses was $37,684 (compared with $34,689 in the deterministic analysis). At a cost-effectiveness threshold of $100,000 per QALY, there was a 92% probability that dabrafenib and trametinib in combination is preferred to observation ().
Discussion
In this study, which used a Markov model and data from the COMBI-AD trial and other sources, the cost-effectiveness dabrafenib and trametinib vs observation for BRAF V600E/K mutation-positive Stage IIIA, IIIB, or IIIC melanoma was estimated to be $34,689 per QALY gained, which is well below generally accepted threshold for cost-effectiveness in the USCitation44. Not surprisingly, estimates of cost-effectiveness were sensitive to the costs of medications, including dabrafenib and trametinib. Results were also sensitive to the parametric survival distributions used to project RFS beyond the end of follow-up in the COMBI-AD trial. Because data on RFS are generally unavailable from long-term registries of melanoma patients, such as the American Joint Committee on Cancer (AJCC) registry or SEER, it is difficult to assess the validity of long-term projections of RFS based on COMBI-AD. While projections of RFS at 7 years for observation based on COMBI-AD are greater than corresponding observed RFS in the placebo arm of the EORTC 18071 trial, these differences may be due to differences in patient populations of the two studies. Similarly, it is difficult to validate projections of OS from the model with estimates from AJCC or SEER because the latter reflect OS from the era prior to the widespread use of targeted treatments and immunotherapies for metastatic melanoma. Given this uncertainty, a number of scenario analyses were conducted to assess the robustness of model results to changes in the survival distributions for RFS, 1LPFS, and 2LOS. In none of these scenario analyses did the ICER for dabrafenib and trametinib exceed $150,000 per QALY gained.
Limitations
This analysis is subject to a number of limitations. Because maximum follow-up in the COMBI-AD trial was only 52 months, it was necessary to project RFS, 1LPFS, and 2LOS beyond the end of follow-up using parametric survival distributions fit to data from COMBI-AD. As noted above these projections are associated with substantial uncertainty that may impact estimates of cost-effectiveness. In COMBI-AD, patients with LR were not followed for subsequent recurrence and probabilities of subsequent recurrence events following LR could not be directly estimated from the trial. Instead, these were estimated using data on RFS from COMBI-AD, and data on the relative risk of recurrence for patients with first vs subsequent recurrence from a published study. Because patients with DR were not followed for subsequent disease progression in COMBI-AD, initiation of 2 L treatment for DR, based on PTACT received in COMBI-AD, was used as a proxy for disease progression. As capture of PTACT in COMBI-AD may have been incomplete, the probabilities of 1LPFS events may, therefore, have been under-estimated, which would bias the analysis in favor of dabrafenib and trametinib. Additionally, as follow-up is ongoing in COMBI-AD, survival estimates for 1LPFS and 2LOS are based on patients who experience events earlier rather than later and may be biased to the extent that the former have worse prognosis than the latter. Estimates of the treatment mix following LR, DR, and progression from COMBI-AD may not represent typical clinical practice in the US or any other specific setting. The model employs a 6-month cycle, which may introduce some imprecision despite application of a half-cycle correction. Estimates of costs other than medication and medication administration were based on claims data from 2007–2017 and may not represent current clinical practice in the US. Finally, the cost-effectiveness of dabrafenib and trametinib was not compared with that of nivolumab and pembrolizumab. Although these two medications have recently been approved as adjuvant treatment of melanoma, results for the Keynote-054 trial of pembrolizumab and for the sub-group of patients in the Checkmate 238 trial of nivolumab with Stage IIIB-C melanoma were unavailable at the time this analysis was conducted. Unlike the COMBI-AD trial, the Keynote-054 and Checkmate 238 trials enrolled both BRAF mutant and wild-type patients, and have substantially shorter follow-up (maximum of approximately 24 months in each trial at first analysis)Citation33,Citation45 than the COMBI-AD trial (maximum approximately 50 months)Citation8. These differences make indirect comparisons of efficacy of dabrafenib plus trametinib with immune checkpoint inhibitors subject to potential bias. Future studies should assess the cost-effectiveness of targeted- and immune-therapies when mature data on RFS and OS for both classes of treatments are available.
Conclusions
From a US healthcare payer perspective, the cost-effectiveness for dabrafenib and trametinib vs observation in patients with BRAFV600 mutation-positive Stage IIIA-C melanoma is well below the generally accepted thresholds in the US. Results of this study may be useful for payers making reimbursement decisions regarding dabrafenib and trametinib in this clinical setting.
Transparency
Declaration of funding
Funding for this evaluation was provided by Novartis Pharmaceuticals Corp. East Hanover, NJ.
Declaration of financial/other relationships
TD is a partner at Policy Analysis Inc., a healthcare research consultancy which has received research funding and/or consulting fees from Abbvie, Amgen, Bristol Myers Squibb, EMD Serono, Jazz Pharmaceuticals, Lilly, Merck, Merck Group, Novartis, Pfizer, Sanofi, Seattle Genetics, Takeda, and 21st Century Oncology. MG is currently an employee of Sage Therapeutics Inc. and was previously employed by Policy Analysis Inc. DS and AM are currently employees of Policy Analysis Inc. BN, DM, PG, and RK are currently employees of Novartis Pharmaceuticals Corp. SG was previously employed by Novartis Pharmaceuticals Corp., and owns stock in Provectus Biopharmaceuticals and Mannkind Corporation. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgements
None reported.
References
- Boyle GM. Therapy for metastatic melanoma: an overview and update. Expert Rev Anticancer Ther. 2011;11:725–737.
- Surveillance‚ Epidemiology‚ and End Results Program. Cancer stat facts: melanoma of the skin; 2017 [cited 2017 May 1]. Available from: https://seer.cancer.gov/statfacts/html/melan.html.
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206.
- Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Seminars Oncol. 2015;42:429–435.
- Niezgoda A, Niezgoda P, Czajkowski R. Novel approaches to treatment of advanced melanoma: a review on targeted therapy and immunotherapy. Biomed Res Int. 2015;2015:1.
- Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631–1639.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39.
- Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813.
- Hauschild A, Santinami M, Long GV, et al. COMBI -AD: adjuvant dabrafenib plus trametinib for resected stage III BRAF V600-mutant melanoma. Ann Oncol. 2017;28:v605–v49.
- Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600–mutant stage III melanoma. JCO. 2018;36:3441–3449.
- Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–modeling studies. Value Health. 2003;6:9–17.
- Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258.
- Bureau of Labor Statistics. CPI-All urban consumers. Medical care in US city average, all urban. 2018 [cited 2018 Jan 18]. Available from: https://data.bls.gov/cgi-bin/surveymost?cu.
- Jackson C. Package ‘flexsurv’. Flexible parametric survival and multi-state models; 2016 May 11 [cited 2016 Sep 26]. Available at: https://cran.r-project.org/web/packages/flexsurv/flexsurv.pdf.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855.
- Salama AK, de Rosa N, Scheri RP, et al. Hazard-rate analysis and patterns of recurrence in early stage melanoma: moving towards a rationally designed surveillance strategy. PLoS One. 2013;8:e57665.
- Arias E, Heron M, Xu J. United States life tables, 2014. Natl Vital Stat Rep. 2017;66(4):1–64.
- Grob JJ, Amonkar MM, Karaszewska B, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015;16:1389–1398.
- Paly V, Colby C, Gilloteau I, et al. Predictors of utility over time among patients with treatment-naive advanced melanoma from the phase 3 checkmate 066 trial. Value Health. 2015;18:A474.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420.
- Akman T, Oztop I, Baskin Y, et al. The role of BRAF mutation in patients with high-risk malignant melanoma treated with high-dose adjuvant interferon therapy. Med Oncol. 2015;32:440.
- Barbour AP, Tang YH, Armour N, et al. BRAF mutation status is an independent prognostic factor for resected stage IIIB and IIIC melanoma: implications for melanoma staging and adjuvant therapy. Eur J Cancer. 2014;50:2668–2676.
- Boursault L, Haddad V, Vergier B, et al. Tumor homogeneity between primary and metastatic sites for BRAF status in metastatic melanoma determined by immunohistochemical and molecular testing. PLoS One. 2013;8:e70826.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Johansson CC, Egyhazi S, Masucci G, et al. Prognostic significance of tumor iNOS and COX-2 in stage III malignant cutaneous melanoma. Cancer Immunol Immunother. 2009;58:1085–1094.
- Knol AC, Pandolfino MC, Vallee A, et al. Comparative analysis of BRAF, NRAS and c-KIT mutation status between tumor tissues and autologous tumor cell-lines of stage III/IV melanoma. Exp Dermatol. 2015;24:70–73.
- Mann GJ, Pupo GM, Campain AE, et al. BRAF mutation, NRAS mutation, and the absence of an immune-related expressed gene profile predict poor outcome in patients with stage III melanoma. J Invest Dermatol. 2013;133:509–517.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF V600 mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314–4321.
- Picard M, Pham Dang N, D'Incan M, et al. Is BRAF a prognostic factor in stage III skin melanoma? A retrospective study of 72 patients after positive sentinel lymph node dissection. Br J Dermatol. 2014;171:108–114.
- Rutkowski P, Gos A, Jurkowska M, et al. Molecular alterations in clinical stage III cutaneous melanoma: correlation with clinicopathological features and patient outcome. Oncol Lett. 2014;8:47–54.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753–1757.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359–368.
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus Ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824.
- BioMerieux B. THxID™ BRAF [package insert] 2013. Available from: http://www.biomerieux-usa.com/sites/subsidiary_us/files/braf-package-insert-1.pdf.
- CMS Clinical Diagnostic Laboratory Fee Schedule [database on the Internet]. Center for Medicare and Medicaid Services (CMS); 2017 [cited 2017 Aug 22]. Available from: https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/17CLAB.zip.
- Red Book online® [database on the Internet]. Truven Health Analytics; 2017 [cited 2017 Sep 6].
- CMS Physician Fee Schedule Relative Value File [database on the Internet]. Center for Medicare and Medicaid Services (CMS); 2017 [cited 2017 Aug 22]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/RVU17A.zip.
- Larkin J, Minor D, D'Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in checkmate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36:383–390.
- Tarhini A, Ghate SR, Ionescu-Ittu R, et al. Postsurgical treatment landscape and economic burden of locoregional and distant recurrence in patients with operable nonmetastatic melanoma. Melanoma Res. 2018;28(6):618–628.
- National Comprehensive Cancer Network. NCCN Guidelines Version 1. 2018; 2017 [cited 2017 Oct 12]. Available from: www.nccn.org/professionals/physician.
- Arondekar B, Curkendall S, Monberg M, et al. Economic burden associated with adverse events in patients with metastatic melanoma. J Manag Care Spec Pharm. 2015;21(2):158–164.
- Wang J, Chmielowski B, Pellissier J, et al. Cost-effectiveness of pembrolizumab versus ipilimumab in ipilimumab-naive patients with advanced melanoma in the United States. J Manag Care Spec Pharm. 2017;23:184–194.
- MPI Group. The cost of dispensing study. 2015 [cited 2017 Oct 31]. Available from: https://mopa.memberclicks.net/assets/docs/cost%20of%20dispensing.pdf
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797.
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801.