Abstract
Aims: Electroencephalography (EEG) is an established method to evaluate and manage epilepsy; video EEG (VEEG) has significantly improved its diagnostic value. This study compared healthcare costs and diagnostic-related outcomes associated with outpatient vs inpatient VEEG among patients with epilepsy in the US.
Materials and methods: This study used Truven MarketScan Commercial and Medicare Supplemental claims databases. Patients with a VEEG between July 1, 2013 and December 31, 2016 were identified. Index event was the first VEEG claim, which was used to determine inpatient and outpatient cohorts. Continuous health plan enrollment 6 months pre- and 12 months post-index VEEG was required. Primary outcomes were costs during the index event and 12 months post index. A generalized linear model with gamma distribution and a log link was used to estimate adjusted index and post-index costs.
Results: Controlling for baseline differences, epilepsy-related cost of index VEEG was significantly lower for the outpatient ($4,098) vs the inpatient cohort ($13,821; p < 0.0001). The cost differences observed at index were maintained in the post-index period. The 12-month post-index epilepsy-related costs were lower in the outpatient cohort ($6,114 vs $12,733, p < 0.0001). Time from physician referral to index VEEG was significantly shorter in the outpatient cohort (30.6 vs 42.5 days). Patients in the inpatient cohort were also more likely to undergo an additional subsequent follow-up inpatient VEEG (p < 0.0001).
Limitations: Administrative claims data have limitations, including lack of data on clinical presentation, disease severity, and comprehensive health plan information. Generalizability may be limited to a US insured population of patients who met study criteria.
Conclusions: Index VEEG was less costly in an outpatient vs inpatient cohort, and costs were lower during the follow-up period of 12 months, suggesting that outpatient VEEG can be provided to appropriate patients as a less costly option. There were fewer follow-up tests in the outpatient cohort with similar pre- and post-index diagnoses.
Introduction
Epilepsy is a neurological disease characterized by recurring, unprovoked seizures, with diagnosis typically occurring after two such seizuresCitation1. Common etiologies of epilepsy include stroke, infections of the central nervous system, metabolic disturbances, brain tumors, encephalomalacia with gliosis, and cortical venous thrombosisCitation2. In 2015, the Centers for Disease Control and Prevention estimated that 1.2% of the US population had active epilepsy, representing 3.4 million people nationwideCitation3. Approximately one in 26 people will develop epilepsy over the course of their lifetimeCitation4.
Epilepsy is associated with considerable economic and humanistic burden. The total direct cost of epilepsy in the US is estimated to be $15.5 billion annuallyCitation4. A review of studies published since 1995 found that annual per-person, epilepsy-specific costs (in 2013 dollars) ranged from $1,022 to $19,749Citation5. This same review found that these estimates were supported by claims-based studies conducted more recently that used more comparable methods for cost estimation, showing similar annual epilepsy cost estimates that ranged from $8,412–$11,354Citation5. Additionally, studies have consistently shown that annual, epilepsy-specific direct costs are higher for uncontrolled or treatment-refractory epilepsy vs controlled or treatment-responsive epilepsyCitation5–9.
Epilepsy also affects patients’ health-related quality-of-life (HRQoL) (i.e. mental, physical, and social health) and functional ability. A US population-based study found that, compared to adults without epilepsy, adults with epilepsy reported more psychological distress, greater cognitive impairment, greater difficulty engaging in social activities, and reduced HRQoLCitation10. Given its burden, accurate and timely diagnosis of epilepsy is essential for providing appropriate disease management options and preventing significant negative physical, psychological, and socioeconomic consequencesCitation11.
A specific epilepsy syndrome diagnosis is based on the patient’s clinical history, seizure semiology, neurologic status, magnetic resonance imaging (MRI) of the brain, and electroencephalography (EEG) findings. Routine EEG is a long-established method for the evaluation and management of epilepsyCitation12. EEG records electrical activity of the brain over a short period, with or without seizure activation procedures. Although there is strong evidence that routine EEG with unequivocal epileptic activity can help establish an epilepsy diagnosis after the first seizure, the yield of that first EEG in identifying such activity is limited due to relatively low sensitivity of the testCitation13. The use of more than one EEG recordingCitation14, longer monitoring timeCitation15, and use of video during long-term EEGCitation16 significantly improve the diagnostic value of EEG. Continuous recording of the EEG with time-locked video (VEEG) over 1 or more days combines the diagnostic advantages of all these techniques and has increased the ability to diagnose epilepsy and capture a “typical” event in patients.
VEEG services can be provided in inpatient and outpatient settings, with continuous, intermittent, or no monitoring while recording is in progress. Recent advances in technology allow a patient to undergo VEEG studies in the convenience of their home, and disease-area experts suggest that the cost may be considerably lower without compromising diagnostic accuracyCitation12.
Although the advantages and disadvantages of outpatient vs inpatient VEEG have been discussed previouslyCitation12, there are limited real-world data on the actual costs and outcomes associated with inpatient vs outpatient VEEG to healthcare payers. Accordingly, the objective of this study was to compare the costs of healthcare and diagnostic-related outcomes associated with outpatient vs inpatient VEEG services among patients with epilepsy or epilepsy-like conditions.
Methods
Data source
Patients were identified from two IBM Truven Health MarketScan databases. The Truven Health MarketScan Commercial Claims and Encounters database is a collection of paid inpatient, outpatient, and pharmaceutical claims generated by nearly 51 million employees and their dependents per year with employer-sponsored insurance and enrolled in a variety of fee-for-service and managed care plans for the 50 states, Puerto Rico, and US territories. These data include medical claims for enrollees in over 150 different health plans. The Truven MarketScan Medicare Supplemental and Coordination of Benefits database consists of inpatient, outpatient, and prescription claims for Medicare-eligible retirees and their dependents with employer-sponsored supplemental insurance, and has the same structure as the Commercial Claims and Encounters database. The Medicare Supplemental and Coordination of Benefits database consists of claims from 5 million enrollees per year.
Study design
A retrospective cohort study design was used to investigate the differences in outcomes among patients receiving outpatient or inpatient VEEG services. Patients with evidence of an outpatient or inpatient VEEG between July 1, 2013 and December 31, 2016 were identified. Current Procedural Terminology code 95951 (monitoring for localization of cerebral seizure focus by cable or radio, 16 or more channel telemetry, combined EEG and video recording and interpretation, each 24 h) was used to identify all VEEGs. The index VEEG event was defined as the duration of the inpatient admission for patients receiving their first VEEG monitoring in the inpatient setting (“Inpatient” cohort), and as the time between the first and last day of continuous outpatient VEEG monitoring for patients receiving their first VEEG in the outpatient setting (“Outpatient” cohort). All patients were required to be continuously enrolled in a commercial or Medicare supplemental medical and pharmacy benefit plan in the 6-month period prior to and the 12-month period after their index VEEG event. The 6-month pre-index period was used to establish baseline characteristics of patients in both cohorts, whereas outcomes were measured in the 12-month post-index period.
Study population
To be included in the study, patients were required to be aged ≥3 years at the index date and have ≥1 medical claim with a primary diagnosis of epilepsy or epilepsy-like conditions (Supplementary Appendix A) in the 12-month post-index period. To assist in minimizing selection bias between the cohorts, patients receiving VEEG in the inpatient setting were excluded from the study if the length of inpatient stay was more than 7 days. Because the length of stay for the index VEEG hospitalization was limited to less than 7 days in the inpatient setting, the same restriction was imposed on the duration of outpatient VEEG services. Patients were excluded if they had any of the following services during the index event: anesthesia, surgery, emergency room (ER), critical care, emergency ambulance, end-stage renal disease management, care in the post-acute setting, or non-cranial radiology. Patients were further evaluated for diagnoses leading up to the VEEG event. We excluded patients who had pre-index diagnosis codes for coma, anoxic brain damage, unspecified conduction disorder, and unspecified cerebrovascular disease.
Study measures
Baseline characteristics
The study cohorts were characterized using baseline demographic and clinical characteristics, and pre-index, epilepsy-related and all-cause healthcare costs and resource utilization (specifically the presence of an epilepsy-related inpatient visit or ER visit, and evidence of EEG (Supplementary Appendix D), brain computerized tomography [CT], or [MRI] [Supplementary Appendix E]). Demographic characteristics estimated were age at index, gender, geographic region, insurance plan type (commercial or Medicare), and index year.
Clinical characteristics included the Deyo Charlson Comorbidity Index (DCCI), which predicts 1-year mortality rate in patients with multiple comorbidities (Supplementary Appendix B). The DCCI was calculated for each patient using the Quan modification of the DCCI during the 6-month baseline periodCitation17. The DCCI encompasses 19 medical conditions weighted 1–6, with total scores ranging from 0–37Citation18. The Quan adaptation allows for use of both International Classification of Diseases (ICD), Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes to calculate DCCI scores. Psychiatric comorbid conditions were also characterized. Binary indicators for the following psychiatric comorbidities of epilepsy were assessed: major depressive disorder, other depression, bipolar disorder, anxiety, schizophrenia, and insomnia (Supplementary Appendix C).
Healthcare costs
The primary outcomes were total cost associated with the index VEEG event and the corresponding 12-month post-index epilepsy-related costs. Costs were defined as the actual reimbursement amount by health plans plus any patient cost-sharing. Costs were stratified into medical and prescription costs. Medical costs were further broken down by setting of care: inpatient or outpatient (ER, ambulance service, radiology, physician office, and other outpatient/ancillary services). Epilepsy-related costs were defined as the costs of medical claims with a primary diagnosis of epilepsy or epilepsy-like conditions (note: discharge diagnosis was used for inpatient claims) and the costs of pharmacy claims for epilepsy treatments. All-cause healthcare costs were also examined and were defined as the cost of all prescription and medical claims, regardless of diagnosis. All costs were adjusted to 2017 US dollars using the medical component of the Consumer Price Index.
Diagnostic-related outcomes
To compare processes and outcomes of care across the service settings, we evaluated several metrics. First, the time to the index VEEG was calculated as the time between the last neurologist specialty visit in the pre-index period and the start of the index event. For patients without a specialist visit in the pre-index period, time to the index VEEG was calculated as the last visit for an epilepsy or epilepsy-like condition in the pre-index period and the start of the index event. We also evaluated the need for additional diagnostic testing after the index VEEG. The post-index diagnostic testing was defined as any additional inpatient or outpatient VEEG, long-term EEG, routine EEG, CT scan, or MRI scan in the 12 months following the index VEEG. Time to additional diagnostic testing was the time between the end of the index event and the first occurrence of an additional diagnostic test.
Changes to the patient’s epilepsy designation were evaluated by assessing ICD diagnoses codes immediately prior to the index event and the first diagnoses codes following the index VEEG. Patients were defined as: (1) having the same diagnosis if the same number and type of diagnoses were present prior to and post-index; (2) having a more refined diagnosis if the number of diagnoses in the post-index period were reduced, but two or more diagnoses codes were present in the pre-index period; (3) having a less-refined diagnosis if the number of diagnoses in the post-index period were increased; and (4) a change in diagnosis if the number and type of diagnoses in the period were completely different than the ones in the pre-index period.
Statistical analyses
Standard summary measures (means and standard deviations) were used to present continuous measures, and frequencies and percentages were used to summarize categorical measures. Baseline demographic and clinical characteristics were presented for the population as a whole and stratified by VEEG site of care. Statistical differences in demographic and clinical characteristics across the different cohorts were evaluated using Chi-square tests or Fisher’s exact tests for categorical measures and t-tests or Wilcoxon rank sums tests (if distribution is non-normal) for continuous measures. Given the large sample size of the study, standardized differences were also provided to assess differences in baseline characteristics, with a value of ≥0.1 indicating a meaningful effect size between the two cohorts.
For cost comparisons, a generalized linear model with a log link and a gamma distribution was used to assess differences in all-cause and epilepsy-related costs between VEEG patients in the outpatient and inpatient settings, controlling for patients’ demographic and clinical characteristics, as provided above. Time to the index VEEG was adjusted for differences in patients’ baseline characteristics using a generalized linear model with a log link and a negative binomial distribution. Differences in the likelihood of additional diagnostic testing were evaluated using Cox proportional hazards model, which takes into the account the presence and timing of the diagnostic tests. All statistical tests were conducted using SAS 9.4 statistical software and were based on a 2-sided hypothesis of no difference between cohorts at a significance level of 0.05.
Results
Study population
Study inclusion and exclusion criteria and associated patient flow are described in . The starting population included 48,176 patients with VEEG services during the identification period from July 1, 2013 to December 31, 2016. Of these, a total of 13,958 patients (n = 5,271 in the inpatient VEEG cohort and n = 8,687 in the outpatient VEEG cohort) met all the inclusion criteria, without meeting any exclusion criteria. Baseline demographics and clinical characteristics of these patients are presented in . The sample was 57.9% female, mean age was 29.9 years, and most (93.0%) patients were commercially insured. The largest proportion of patients was located in the South geographic region (41.9%) and the smallest proportion was located in the West geographic region (10.2%). Based on standardized differences, meaningful differences between cohorts were observed for age category, payer type, geographic region, and index year.
Table 1. Attrition table for inpatient and outpatient video EEG.
Table 2. Demographic and baseline clinical characteristics.
VEEG length of testing varies depending on certain clinical features, including how often a person usually has seizures, type of seizures, and the reason for monitoring. Testing may take a few days to a week or more. In our study, the average length of VEEG service for the overall sample was 1.5 days (±1.6). The duration of testing in the outpatient cohort was significantly lower (0.8 days) compared to the inpatient cohort (2.7 days). The index VEEG was completed in less than a day for over 50% of outpatient VEEG patients. In contrast, 74% of inpatient VEEG patients were in the hospital for at least 2 days for their index VEEG. These findings are consistent with previous US data; for example, a recent retrospective cohort study by Syed et al.Citation19 examined diagnostic VEEG monitoring among ∼10,000 patients in the US and found a median of 4 days for length of adult inpatient VEEG and 1 day for pediatric inpatient VEEG.
The mean DCCI was 0.60 in the overall sample, indicating a low level of comorbidity across all patients. However, patients in the outpatient cohort were slightly more likely to have comorbid conditions, with a larger percentage of patients with a diagnosis of one or more DCCI conditions. Psychiatric comorbidities were balanced between groups.
Cost outcomes
Epilepsy-related healthcare costs in all medical settings and pharmacy costs were significantly lower in the outpatient VEEG cohort (all p < 0.0001, ). Even after controlling for baseline differences, the epilepsy-related cost of the index diagnostic event was significantly lower for the outpatient VEEG cohort ($4,098) compared to the inpatient VEEG cohort ($13,821), p < 0.0001 (). The 12-month post-index, epilepsy-related healthcare costs were also statistically lower in the outpatient VEEG cohort ($6,114) than the inpatient cohort ($12,733), p < 0.0001 (). The cost differences were most notable for inpatient costs, other outpatient costs, and pharmacy costs. When evaluating 12-month all-cause costs between the cohorts (), costs for the outpatient VEEG cohort were again significantly lower than the inpatient cohort after controlling for baseline differences ($25,824 vs $39,203; p < 0.0001).
Table 3. Post-index epilepsy-related healthcare costs for VEEG patients, by cohort.
Table 4. Index all-cause and epilepsy-related adjusted costs.
Table 5. Post-index (12-month) all-cause and epilepsy-related adjusted costs.
Diagnostic-related outcomes
Time to index VEEG was statistically significantly shorter among patients having an outpatient VEEG (30.6 days) when compared to patients having an inpatient VEEG (42.5 days), before () and after () adjusting for baseline differences. When taking into account the timing and presence of these additional diagnostic tests, assessed using a Kaplan Meier curve and a Cox proportional hazards model (; ), patients in the outpatient VEEG cohort were at a lower risk of requiring additional diagnostic testing over the 12-month follow-up period (hazard ratio [HR] = 0.832, p < 0.0001). When follow-up tests were needed (), long-term EEG, EEG, MRI, and CT scans were almost exclusively conducted in the outpatient setting in both cohorts (>80% in both cohorts); however, patients in the inpatient VEEG cohort were significantly more likely than patients in the outpatient VEEG cohort to have any follow-up VEEG testing done in the inpatient setting (41.3% and 27.7%, respectively; p < 0.0001).
Figure 1. Kaplan-Meier curve for post-index diagnostic procedure. Post-index diagnostic testing was defined as any additional procedure in the 12 months following the index VEEG. (1) Inpatient or outpatient VEEG (CPT: 95951). (2) Long-term EEG (CPT: 95827, 95950, 95953, 95956). (3) Routine EEG (CPT: 95812, 95813, 95816, 95819, 95822). (4) Brain CT/MRI scan (CPT: 78608, 70450, 70460, 70470, 70551, 70552, 70553). Abbreviations. CPT, Current Procedural Terminology; CT, computed tomography; EEG, electroencephalography; MRI, magnetic resonance imaging; VEEG, video electroencephalography.
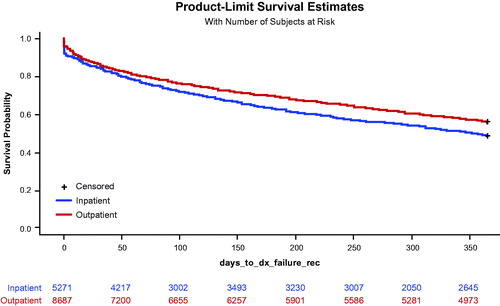
Table 6. Clinical outcomes in VEEG patients, by cohort.
Table 7. Adjusted clinical time outcomes.
Table 8. Cox proportional hazards model for post-index diagnostic procedure.
Although statistically significant differences were observed in the change in diagnosis pre- and post-index overall (), the magnitude appeared to be practically similar across most categories, with statistical differences being primarily driven by the percentage of patients with new diagnoses.
Table 9. Clinical outcomes in VEEG patients, pre- and post-VEEG diagnoses.
Discussion
This study is among the first real-world studies to assess and compare epilepsy-related healthcare resource utilization and economic outcomes among patients who received VEEG in outpatient vs inpatient settings. Using a large commercial claims database and supplemental Medicare database, this study indicated that patients who receive outpatient VEEG had significantly lower index diagnostic costs. Additionally, patients in the outpatient VEEG cohort had lower 12-month epilepsy-related costs post their index diagnostic event. Our findings support previously published economic models that suggest the direct costs of outpatient monitoring were 66% of the direct costs of inpatient VEEGCitation20.
Overall, our findings are important in managing the total cost of care among patients with epilepsy and epilepsy-related conditions from a societal and payer perspective. A recent US study using healthcare claims data from four of the largest insurers between 2013 and 2017 found that among Americans under the age of 65 who were covered by employer-sponsored insurance, spending per person grew 4.2% in 2017Citation21. Although the overall use of healthcare services was mainly consistent over the 2013–2017 period, the year-over-year cost increase was driven primarily by price increases in the services offered. Thus, as the healthcare environment continues to seek opportunities to reduce cost while providing equal or greater quality, the use of outpatient VEEG may be a value-based approach for patients requiring VEEG diagnostic procedures.
In our study, cost differences between the two cohorts appeared to be primarily a function of the setting of care. Cost disparities between sites of care have been established in various therapeutic areas, as services and medications delivered in an inpatient setting have been consistently more costly than those delivered in an outpatient setting. For example, Gordon et al.Citation22 found that, among a matched cohort of US oncology patients (breast, colorectal, and lung cancers), total costs of care were significantly lower per patient per month in outpatient community-based clinics relative to hospital-based care, despite patients receiving the exact same chemotherapy regimens ($12,548 vs $20,060 2015 US dollars, respectively). A 2016 article posted on the Health Affairs Blog by Higgins and VeselovskiyCitation23, indicated that differences in the price of services offered across sites of care not only affects the costs to payers, but also the costs incurred by patients. For payers, cost differences ranged between 21% and 258% higher when the same services were offered by hospital-based practices vs outpatient practices.
When evaluating diagnostic-related outcomes, we found that time to index VEEG was significantly shorter among patients having an outpatient VEEG compared to patients having an inpatient VEEG. Although we could not assess reasons for this difference with our study, it may be that access to hospitals, limited epilepsy monitoring units, increased wait times, scheduling, or a patients’ ability to accommodate a hospital stay may play a critical role in obtaining the VEEG services in the inpatient settingCitation4,Citation12.
Our study showed somewhat surprising results when evaluating the need for additional diagnostic testing after the index VEEG event, where patients in the inpatient VEEG cohort were more likely to require additional testing than those in the outpatient VEEG cohort. As highlighted by BenbadisCitation12, inpatient and outpatient VEEG are thought to have similar diagnostic accuracy. However, the inpatient setting tends to be a more controlled and artificial environment, which may reflect limited real-world components needed to accurately assess and diagnose epilepsy conditions under normal conditionsCitation12. Evaluating patients in a real-world scenario may enhance diagnostic assessment and may, therefore, result in fewer additional tests compared to the artificial inpatient environment. One cannot rule out, however, that the patient cohorts are substantially different, even after we attempted to minimize differences between the cohorts, which may point to the differences in diagnostic needs in two patient cohorts.
It is interesting to note that when follow-up testing was conducted, procedures were primarily conducted in an outpatient setting. Thus, the site of care setting of the initial VEEG may reflect a physician’s preferences for the diagnostic setting of care, and may indicate an educational opportunity to discuss the comparable diagnostic accuracy between the approach, as well as the advantages and disadvantages of outpatient VEEG services. Diagnostic comparability, although not directly measured in our study, may be indirectly supported, given that just over 50% of patients in both cohorts had the same diagnosis after the VEEG procedure, and just over 25% had a change in diagnosis, regardless of setting. These findings are consistent with studies that show comparable diagnostic success rates with a lower cost in an outpatient settingCitation15,Citation20,Citation24. Although the clinical details of the VEEG are not available in our study, the rates of diagnostic changes pre- and post-index across both cohorts were practically similar, which may underscore the similar diagnostic aspects of both settings.
As noted, inpatient VEEG may offer certain advantages, for example, the ability to reduce medications safely to obtain a seizure, a relatively controlled environment, a better ability to address technical problems with technologists during recording, and easier performance of activation proceduresCitation12. Conversely, disadvantages of inpatient VEEG include the artificial environment of the hospital, access to hospitals/neurologists may be limited or inconvenient for some patients, and the high cost of services rendered in an inpatient settingCitation12. A recent retrospective cohort study by Syed et al.Citation19 examined outcomes of in-home diagnostic video-EEG monitoring among ∼10,000 patients over a 1-year period and found that, although selected outcomes are similar on outpatient and inpatient monitoring, outpatient monitoring for non-urgent and non-surgical epileptic events is a useful method to evaluate paroxysmal events that may be considered when inpatient level-of-care is not medically necessary. In such patients, outpatient monitoring may potentially avoid the high-cost of inpatient monitoring and avoid other inconveniences associated with inpatient VEM. Outpatient monitoring may also benefit patients who report seizures or epileptic events that occur in certain environments or with specific triggers that may not be replicated in the hospital settingCitation19. A German population-based retrospective analysis of health insurance data showed that VEEG is under-used, even in severely drug-refractory patientsCitation25. In that study, annual rates of admission for VEEG averaged from 1.6–3.2%. Although this was a different healthcare system and relies on different treatment guidelines, in such refractory patients, outpatient VEEG might be a useful and timely available option for the same reasons as in the US (e.g. access to epilepsy centers, higher costs of hospitalization).
To date, there has been little attention paid to the cost and healthcare implications of outpatient VEEG as compared to inpatient VEEG. As noted, in situations that are appropriate for outpatient VEEG, it offers the advantages of the regular environment and stress circumstances where epileptic events generally occur, and patients can stay in the privacy and comfort of their own home without having to travel to stay in an EMU (the distances in some cases may be significant). Outpatient VEEG has also been shown to have lower wait times, results supported by this study, and may also result in less time off from work and away from family. For payers, outpatient monitoring offers an economically viable alternative to inpatient monitoring, with evidence that supports comparable diagnostic accuracy.
However, some (less complex) patients (e.g. patients with frequent non-convulsive episodes) may be more clinically suited to cost-effective outpatient monitoring, whereas more complex patients (e.g. patients with severe convulsions or patients with a mixture of different clinical events) are more suited to a comprehensive inpatient evaluation. Also, patients with refractory focal epilepsy who undergo a pre-surgical evaluation are more likely to be admitted for an inpatient study. As such, we recognize that there are indications for both inpatient and outpatient VEEG studies and that neither is necessarily appropriate for all patient populations.
Limitations
Some other study limitations should be noted. The use of administrative claims in a retrospective study is associated with inherent limitations, including lack of data on patients’ clinical presentation, severity of disease, and comprehensive health plan information. Although our study controlled for observable patient demographics and clinical characteristics across the cohorts, residual confounding cannot be ruled out. Thus, the inpatient cohort may be a more severe or compromised population that requires inpatient evaluation, therefore needing additional diagnostic testing, which results in higher costs. In our study, the number of patients with an epilepsy diagnosis (pre-index and at index) was higher in the inpatient cohort; as these are costly patients, this may be driving some of the cost differences observed by setting. It should also be noted that the results of this study are only generalizable to a US insured population of patients who met study inclusion criteria (and did not meet any of the exclusion criteria); and, therefore, may not reflect outcomes outside of this studied population.
Conclusion
Index VEEG procedures were significantly less costly in an outpatient vs inpatient cohort, and epilepsy-related healthcare costs were lower during the follow-up period of 12 months after the index procedure. These findings support limited data and anecdotal clinical reports that outpatient VEEG offers an economic advantage over inpatient VEEG. Additionally, a lower need for additional diagnostic testing in the outpatient VEEG cohort, coupled with the comparable changes in the pre- and post-index diagnoses, was observed for VEEG conducted in the outpatient setting.
Transparency
Declaration of funding
The study was funded by Alliance of Family Companies.
Declaration of financial/other interests
CMB and JS are employees of Alliance Family of Companies. Xcenda received funding to conduct this study. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgements
Ann Cameron, PhD, of Xcenda LLC provided writing assistance, and Binglin Yue, MS, of Xcenda LLC provided statistical assistance.
Supplemental Material - Table
Download MS Word (21.8 KB)Supplemental Material - Appendices
Download MS Word (25.3 KB)References
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482.
- Kaur S, Garg R, Aggarwal S, et al. Adult onset seizures: clinical, etiological, and radiological profile. J Family Med Prim Care. 2018;7:191–197.
- Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active Epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:821–825.
- England MJ, Liverman CT, Schultz AM, Strawbridge LM, editors. Epilepsy across the spectrum: promoting health and understanding. Washington (DC); National Academies Press; 2012.
- Begley CE, Durgin TL. The direct cost of epilepsy in the United States: a systematic review of estimates. Epilepsia. 2015;56:1376–1387.
- Chen SY, Wu N, Boulanger L, et al. Antiepileptic drug treatment patterns and economic burden of commercially-insured patients with refractory epilepsy with partial onset seizures in the United States. J Med Econ. 2013;16:240–248.
- Cramer JA, Wang ZJ, Chang E, et al. Healthcare utilization and costs in adults with stable and uncontrolled epilepsy. Epilepsy Behav. 2014;31:356–362.
- Cramer JA, Wang ZJ, Chang E, et al. Healthcare utilization and costs in children with stable and uncontrolled epilepsy. Epilepsy Behav. 2014;32:135–141.
- Manjunath R, Paradis PE, Parise H, et al. Burden of uncontrolled epilepsy in patients requiring an emergency room visit or hospitalization. Neurology. 2012;79:1908–1916.
- Kobau R, Cui W, Kadima N, et al. Tracking psychosocial health in adults with epilepsy-estimates from the 2010 National Health Interview Survey. Epilepsy Behav. 2014;41:66–73.
- Smith D, Defalla BA, Chadwick DW. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. QJM. 1999;92:15–23.
- Benbadis SR. What type of EEG (or EEG-video) does your patient need? Expert Rev Neurother. 2015;15:461–464.
- Baldin E, Hauser WA, Buchhalter JR, et al. Yield of epileptiform electroencephalogram abnormalities in incident unprovoked seizures: a population-based study. Epilepsia. 2014;55:1389–1398.
- Salinsky M, Kanter R, Dasheiff RM. Effectiveness of multiple EEGs in supporting the diagnosis of epilepsy: an operational curve. Epilepsia. 1987;28:331–334.
- Modur PN, Rigdon B. Diagnostic yield of sequential routine EEG and extended outpatient video-EEG monitoring. Clin Neurophysiol. 2008;119:190–196.
- Goodwin E, Kandler RH, Alix JJ. The value of home video with ambulatory EEG: a prospective service review. Seizure. 2014;23:480–482.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Syed TU, LaFrance WC Jr, Loddenkemper T, et al. Outcome of ambulatory video-EEG monitoring in a ∼10,000 patient nationwide cohort. Seizure. 2019;66:104–111. Mar
- Brunnhuber F, Amin D, Nguyen Y, et al. Development, evaluation and implementation of video-EEG telemetry at home. Seizure. 2014;23:338–343.
- Health Care Cost Institute [Internet]. 2017 Health care cost and utilization report; 2019 [cited 2019 Apr 11]. Available from: https://www.healthcostinstitute.org/research/annual-reports/entry/2017-health-care-cost-and-utilization-report.
- Gordon L, Blazer M, Saundankar V, et al. Community setting versus a hospital setting: a matched-claims analysis of patients with breast, colorectal, and lung cancers. J Oncol Pract. 2018;14:e279–e238.
- Higgins A, Veselovskiy G. Health Affairs [Internet]. Does the site of care change the cost of care? Health Affairs Blog; 2016 [cited 2019 Apr 12]. Available from: https://www.healthaffairs.org/do/10.1377/hblog20160602.055132/full/.
- Foley CM, Legido A, Miles DK, et al. Diagnostic value of pediatric outpatient video-EEG. Pediatr Neurol. 1995;12:120–124.
- Strzelczyk A, Griebel C, Lux W, et al. The burden of severely drug-refractory epilepsy: a comparative longitudinal evaluation of mortality, morbidity, resource use, and cost using German health insurance data. Front Neurol. 2017;8:712.
