Abstract
Aims: Adalimumab, infliximab, and ustekinumab have been approved for patients with moderate-to-severe Crohn’s disease in Japan. This study compared the relative efficacy and cost-effectiveness of adalimumab, infliximab, and ustekinumab in patients with Crohn’s disease based on data from randomized controlled trials.
Methods: Data were extracted from four phase 3 clinical trials: CHARM, NCT00445432, ACCENT I, and IM-UNITI. A network meta-analysis (NMA) compared 1-year clinical remission rates in patients who responded to treatment during an induction phase. Remission was defined as a Crohn’s Disease Activity Index score <150. The number needed to treat (NNT) was defined as the inverse of the risk reduction (compared with placebo) estimated from the NMA among initial responders. Cost per incremental remitter was calculated based on the projected per patient drug cost (2018 Japanese Yen [¥]) and the NNT.
Results: Among initial responders, the remission rates were 45.2%, 31.9%, 27.4%, 24.1%, and 15.6% for adalimumab 40 mg every other week (EOW), infliximab 5 mg/kg every 8 weeks, ustekinumab 90 mg every 8 weeks, ustekinumab 90 mg every 12 weeks, and placebo, respectively. The NNT was the lowest for adalimumab 40 mg EOW. Compared with adalimumab, the incremental cost per remitter was numerically higher for infliximab (¥5,375,470) and statistically higher for ustekinumab 90 mg every 8 weeks and ustekinumab 90 mg every 12 weeks (¥42,788,597 and ¥41,495,543, respectively).
Limitations: Indirect comparisons are limited by the availability of suitable clinical evidence and there may be residual heterogeneity that could not be adjusted for.
Conclusion: Adalimumab was associated with a numerically lower cost per remitter compared with infliximab and a statistically lower cost per remitter compared with ustekinumab in patients with moderate-to-severe Crohn’s disease in Japan.
Introduction
Crohn’s disease (CD) is a chronic, inflammatory bowel disease that primarily affects the small intestine and colon. The disease is typically characterized by recurring abdominal pain, diarrhea, fever, and weight loss, and is associated with significant morbidity, medical therapy, and/or surgery, and slightly increased mortalityCitation1. The prevalence of CD in Japan is increasing, and currently affects ∼0.03% of the adult Japanese population, or ∼43,000 peopleCitation2.
About a decade ago, medical treatment for patients with CD was limited to 5-aminosalicylate agents, including sulfasalazine and mesalamine; corticosteroids; immunomodulating agents; and elemental diets. In addition, up to 57% of patients with CD required at least one surgical resection of the intestineCitation1. More recently, biologic therapies have become available for the treatment of patients with moderate-to-severe CD in Japan, including two tumor necrosis factor inhibitors (adalimumab and infliximab) and an interleukin 12/23 inhibitor (ustekinumab). These biologic therapies have provided increased efficacy compared with non-biologic conventional therapy, and have become the standard of care for patients with severe or steroid-resistant diseaseCitation3. However, while biologic therapies provide increased efficacy, they generally are associated with higher costs.
Given the disease chronicity, long disease duration, frequent early adult onset, and the need for hospitalization or surgery, poorly managed CD can lead to substantial costsCitation4–6. Patients that achieve remission have significantly improved quality-of-life, higher employment rates, and lower hospitalization and surgery ratesCitation7.
Although biologics improved disease management, the cost-effectiveness of these agents is a growing concern in Japan due to budget constraints. The number needed to treat (NNT) and the incremental cost per remitter (CPR) provide simple and dependable measures of comparative effectiveness that have both clinical and economic significance for third-party payers and physiciansCitation8–11. Network meta-analyses (NMA) using the NNT and the incremental CPR have been conducted to indirectly compare a variety of biologic therapies for the treatment of moderate-to-severe CD in the United States (US)Citation12. However, no NMAs have assessed the efficacy and cost-effectiveness of biologic treatments in Japan. Therefore, reliable evidence about the comparative efficacy and cost-effectiveness of biologic therapies is needed to inform both clinical and economic decisions about their use.
The aim of this study was to indirectly compare the relative efficacy and the cost-effectiveness of adalimumab, infliximab, and ustekinumab in Japanese patients with moderate-to-severe CD based on published data from randomized controlled trials using the NNT and the incremental CPR.
Methods
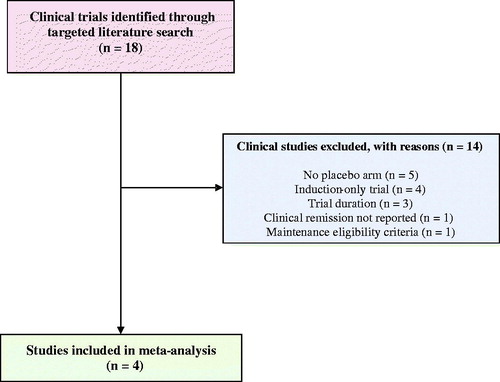
A targeted literature review was conducted to identify clinical trials of biologic treatments for patients with moderate-to-severe CD. The targeted review included searches of clinicaltrials.govCitation13, the University Hospital Medical Information Network (UMIN) clinical trial registryCitation14, the Japan Pharmaceutical Information Center–Clinical Trials Information (JapicCTI)Citation15, the Japan Medical Association–Center for Clinical Trials (JMACCT)Citation16, and the Ichushi-Web database of the Japan Medical Abstracts Society (JAMAS)Citation17. In addition, the targeted literature review leveraged published systematic literature reviews and meta-analyses of moderate-to-severe CD including Stidham et al.Citation18, Hazlewood et al.Citation19, Cholapranee et al.Citation20, and Singh et al.Citation21.
Clinical trials that met the following criteria were included: (1) phase 3 randomized controlled trials; (2) maintenance trials that included an induction-only placebo arm and at least one treatment arm receiving adalimumab, infliximab, or ustekinumab; (3) maintenance trials that required clinical response (decrease in Crohn’s Disease Activity Index [CDAI] score by ≥70 points) in the induction period for maintenance trial eligibility; (4) reported remission rates (CDAI score <150); and (5) studies that followed patients for 40–60 weeks.
Clinical remission rates (defined as a CDAI score <150) after 1-year of treatment were employed to assess the effectiveness of each therapy. Owing to differences in reporting intervals between trials, 1-year remission rates were from week 56 for adalimumab, week 54 for infliximab, and week 52 for ustekinumab.
The NMA were conducted using a fixed-effects binomial modelCitation22 to indirectly compare clinical remission at 1 year in patients who responded to active treatment during an induction phase. Estimated comparative treatment effects were summarized using posterior means and 95% credible intervals (CrI) for all pairwise rate differences vs placebo.
For each treatment, the NNT specifies the number of patients who needed to be treated in order to achieve one additional patient with clinical remission. The NNT was calculated as the inverse of the difference in the estimated remission rates in the maintenance period between active therapy and placebo among initial responders.
The CPR was calculated as the NNT per incremental remitter multiplied by the expected 1-year direct medical costs, which includes drug and drug administration costs per patient. Drug costs per patient were estimated among initial responders with the assumption of 100% compliance to the indicated dosages up to week 52 (). Unit drug costs (2018 Japanese Yen [¥]) were based on the official price of Japan’s National Health Insurance as of April 2018Citation23. Recommended dosing schedules for all treatments were used as follows: adalimumab, 160 mg at week 0, 80 mg at week 2, and 40 mg every other week thereafter; infliximab, 5 mg/kg at weeks 0, 2, and 6, and 5 mg/kg every 8 weeks thereafter; and ustekinumab, 260 mg, 390 mg, and 520 mg via intravenous infusion for patients <55 kg, 55–85 kg, and >85 kg at week 0, respectively, and 90 mg via subcutaneous injection every 8 or 12 weeks thereafter.
Table 1. Total yearly cost per patienta.
When necessary, unit drug costs were prorated in order to obtain total drug costs over 52 weeks. Per-infusion drug costs for infliximab and ustekinumab were calculated based on the average weight of an adult in the Japanese population (60 kg) and included costs due to wastage. Infusion administrations were assumed to cost the same for infliximab and ustekinumab and were obtained from Japan’s National Health Insurance payment information as of April 2018Citation23. For adalimumab, costs include a reimbursement item called “administration and instruction fee for self-injection at home”, which physicians can claim to payers on a monthly basis. This fee is not directly linked to each injection but is triggered by the physician’s office visit related to adalimumab self-injection. In the base case, the maximum possible reimbursement cost was assumed. For infliximab, there were additional fees that could have been applied if the institution met some requirements, including exclusive staff, equipment, and location of service of the infusion. To be conservative, these additional fees were not included in the calculations.
Results
A total of four randomized controlled phase 3 trials were identified in the targeted literature review for the following treatments: adalimumab 40 mg every other week (CHARMCitation24 and NCT00445432Citation25), infliximab 5 mg/kg every 8 weeks (ACCENT I)Citation26, and ustekinumab 90 mg every 8 weeks and every 12 weeks (IM-UNITICitation27; ). A summary of these trials is provided in .
Table 2. Summary of included trialsa,b.
Per the NMA, the clinical remission rates for adalimumab, infliximab, ustekinumab every 8 weeks, ustekinumab every 12 weeks, and placebo were 45.2%, 31.9%, 27.4%, 24.1%, and 15.6%, respectively. All of the biologic therapies were statistically more efficacious than placebo. Additionally, adalimumab was statistically more efficacious than ustekinumab 90 mg every 8 weeks and ustekinumab 90 mg every 12 weeks. No statistical differences in efficacy were found between adalimumab and infliximab or infliximab and ustekinumab. Adalimumab had the lowest NNT among initial responders vs placebo (NNT = 3.39; 95% CrI = 2.31–5.85), followed by infliximab (NNT = 6.17; 95% CrI = 3.01–26.74), ustekinumab 90 mg every 8 weeks (NNT = 8.54; 95% CrI = 4.36–34.01), and ustekinumab 90 mg every 12 weeks (NNT = 11.89; 5.32–175.44; ). The 1-year CPR relative to placebo was ¥6,622,627 (¥4,514,941–¥11,453,888) for adalimumab, ¥12,146,387 (¥5,925,245–¥52,677,647) for infliximab, ¥49,696,687 (¥25,379,337–¥197,941,565) for ustekinumab 90 mg every 8 weeks, and ¥48,405,826 (¥21,665,119–¥714,198,246) for ustekinumab 90 mg every 12 weeks (). Per-patient yearly total costs used in the CPR analysis are presented in . Adalimumab was found to have a numerically lower CPR than infliximab (difference of ¥5,375,470 [–¥2,367,233–¥45,655,360]) and a significantly lower CPR than ustekinumab (differences of ¥42,788,597 [¥18,085,701–¥191,142,693] for ustekinumab every 8 weeks and ¥41,495,543 [¥14,448,032–¥708,223,380] for ustekinumab every 12 weeks).
Table 3. Remission ratea and NNTb.
Table 4. Cost per incremental remitterTable Footnotea.
Discussion
This analysis is the first to compare the relative efficacy and cost-effectiveness of biologic therapies approved for the treatment of patients with moderate-to-severe CD in Japan. With increased emphasis on the evaluation of cost-effectiveness in Japan, this analysis may provide key insights to inform treatment decisions and to assess the economic value of the various biologic treatments for CD. This study found that adalimumab had the lowest NNT and CPR among the four biologic regimens analyzed.
The results for the NMA presented in this paper are similar to the Singh et al.Citation21 NMA which found a large effect size for adalimumab (odds ratio [OR] = 4.42 relative to placebo) and a moderate effect size of infliximab (OR = 2.86), certolizumab pegol (OR = 2.25), ustekinumab (OR = 2.02), and vedolizumab (OR = 2.32) for maintenance of clinical remission in patients who had responded to induction therapy with the same medication. The results are also in line with a previous NMA of moderate-to-severe CD in the US, which found that adalimumab tends to be more efficacious (NNT = 7.30; 3.87–13.45 vs 11.55; 4.53–35.17) and more cost-effective (CPR = $121,863; $64,670–$224,447 vs $174,846; $68,563–$224,447) than infliximabCitation12. The cost-effectiveness results are also in line with a previous NMA from a Japanese perspective on psoriasis, which found adalimumab to be more cost-effective than infliximab and ustekinumabCitation28.
In this analysis, the CPR cost calculation for adalimumab included indirect administration costs. When only direct administration costs were included (i.e., no costs related to adalimumab self-injection), the CPR was ¥6,299,675 (compared with ¥6,622,627 in the base case), and the relative relationship with the other treatments remained unchanged.
Among the three treatments assessed, total drug costs for maintenance treatment with ustekinumab were significantly higher than the other two medications. The increased drug cost of ustekinumab is due to the order in which the indications of drugs received their approval for reimbursement by the National Health Authority. At the initial listing, ustekinumab had carried indications for psoriasis vulgaris and psoriatic arthritis and was priced in comparison with adalimumab (daily drug cost). Upon expansion to include the indication for CD, the per unit price remained unchanged despite a doubling in the required dose for treatment (the relative per dose cost of ustekinumab 90 mg is 14.0-times higher than adalimumab 40 mg in Japan; this relative unit cost is much higher than the 8.5 ratio in the US, the 6.1 ratio in the United Kingdom, and the 2.7 ratio in GermanyCitation29–31). This results in overall higher drug costs for ustekinumab in the treatment of CD when compared with adalimumab and infliximab.
The difference in CPR among biologics analyzed in this study was very large. However, since CD is considered an intractable disease, patient out-of-pocket expenses are capped by the Designated Intractable Disease Medical Expenses subsidy program at ¥2,500 to ¥30,000 per month based on each patient’s income groupCitation31,Citation32. Furthermore, there is no incentive for physicians to give cost-concerned prescriptions. High-cost drugs are reimbursed based on fee-for-service payment, or bundled cost of each drug treatment when hospitalized by the National Health Insurance System. Therefore, it is possible that physicians tend to give less consideration to the budget impact of each therapy when making treatment decisions.
In light of a shift towards value-based medicine, such considerations will influence therapy choice by physicians and will contribute to a sustainable health system. Additionally, Health Technology Assessment (HTA), including analyses of cost-effectiveness, was introduced in Japan on an experimental basis in 2016, with the outline of the full HTA system expected to be finalized in 2018Citation33. Cost-effectiveness evaluations, in turn, will be utilized to reform the current drug pricing system and re-price drugs. These changes to the healthcare system emphasize the growing importance of informed cost-effective treatment options in Japan. As the primary clinical goal in the treatment of Crohn’s disease is remission and the primary cost drivers are drug costsCitation34, this evaluation purposely utilized the simple and straightforward cost-per-remitter metric to capture the relative value. Future economic evaluation could extend the time-horizon, add additional cost components, and translate remission into quality-adjusted life-years.
It should be noted that there are a number of differences between the IM-UNITI maintenance trial of ustekinumab and the other included trials which may limit generalizability. For example, the IM-UNITI required a higher level of response (decrease in CDAI ≥ 100), had a longer induction period (8 weeks), and enrolled a larger proportion of patients with a history of disease refractory to tumor necrosis factor antagonist treatment (44.8%). In addition, the half-life of ustekinumab is longer than adalimumab and infliximab, which may lead to a carryover effect observed during the maintenance periodCitation35–38. This carryover effect, or other unobserved differences in patient characteristics, across trials could help explain the abnormally high remission rate observed for the placebo treatment arm (35.9%). Because the NMA is conducted on the OR scale relative to the placebo arm, this high placebo remission rate may have resulted in an under-estimated treatment effect for ustekinumab. Thus, results related to ustekinumab should be interpreted with caution.
In addition, indirect trial comparisons are limited by the availability of suitable clinical evidence. In the current study, there were barriers to trial selection, including study design, patient inclusion criteria, and study duration and endpoints, which resulted in the inclusion of only four trials. As a result of the limited number of qualifying trials, a fixed-effects model was used in this analysis (as opposed to the less restrictive random-effects model). Furthermore, there may be residual heterogeneity that could not be adjusted for in the NMA. Finally, the differences in the definition of the 1-year period used for maintenance therapy varied across the clinical trials (week 56 for adalimumab, week 54 for infliximab, and week 52 for ustekinumab), however these small differences are expected to have minimal impact on the results. Future analyses could benefit from the availability of data from head-to-head trials to strengthen the network used in this analysis.
Conclusions
This study evaluated the clinical and economic value of adalimumab, infliximab, and ustekinumab among patients with moderate-to-severe CD in Japan. While all three biologics provided clinical improvements relative to placebo, adalimumab had the lowest NNT and the lowest incremental CPR.
Transparency
Declaration of funding
Funding for this research was provided by AbbVie.
Declaration of financial/other interests
MD, YK, and NU are employees of AbbVie GK. KAB and JC are employees of Analysis Group, Inc., which has received consultancy fees from Abbvie GK. FU has no conflict of interest. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All the authors contributed to study design, data analysis and interpretation, and manuscript development. AbbVie participated in the interpretation of data, review, and approval of the publication.
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Loftus EV Jr., Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16:51–60.
- Asakura K, Nishiwaki Y, Inoue N, et al. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44:659–665.
- Antunes O, Filippi J, Hebuterne X, et al. Treatment algorithms in Crohn’s – up, down or something else? Best Pract Res Clin Gastroenterol. 2014;28:473–483.
- Bodger K. Cost of illness of Crohn’s disease. Pharmacoeconomics. 2002;20:639–652.
- Yu AP, Cabanilla LA, Wu EQ, et al. The costs of Crohn’s disease in the United States and other Western countries: a systematic review. Curr Med Res Opin. 2008;24:319–328.
- Cohen RD, Larson LR, Roth JM, et al. The cost of hospitalization in Crohn’s disease. Am J Gastroenterol. 2000;95:524–530.
- Lichtenstein GR, Yan S, Bala M, et al. Remission in patients with Crohn’s disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. Am J Gastroenterol. 2004;99:91–96.
- Armstrong AW, Betts KA, Signorovitch JE, et al. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018;34:1325–1333.
- Betts KA, Griffith J, Song Y, et al. Network meta-analysis and cost per responder of tumor necrosis factor-α and interleukin inhibitors in the treatment of active ankylosing spondylitis. Rheumatol Ther. 2016;3:323–336.
- Gissel C, Repp H. Cost per responder of TNF-α therapies in Germany. Clin Rheumatol. 2013;32:1805–1809.
- Olivieri I, Fanizza C, Gilio M, et al. Efficacy, safety and cost per responder of biologics in the treatment of non-radiographic axial spondyloarthritis. Clin Exp Rheumatol. 2016;34:935–940.
- Liu Y, Wu EQ, Bensimon AG, et al. Cost per responder associated with biologic therapies for Crohn’s disease, psoriasis, and rheumatoid arthritis. Adv Ther. 2012;29:620–634.
- NIH U.S. National Library of Medicine. ClinicalTrials.gov.
- University hospital Medical Information Network. UMIN Clinical Trials Registry (UMIN-CTR). [cited Jan 29]. Available from: https://www.umin.ac.jp/ctr/
- Japan Pharmaceutical Information Center. Clinical trial information (Japic Clinical Trials Information). [cited 2019 Jan 29]. Available from: https://www.clinicaltrials.jp/cti-user/common/Top.jsp.
- The Japan Medical Association Center for Clinical Trials. JMACCT Clinical Trials Registry. [cited 2019 Jan 29]. Available from: https://dbcentre3.jmacct.med.or.jp/jmactr/Default_Eng.aspx.
- NPO Japan Medical Abstracts Society. ICHUSHI. [cited 2019 Jan 29]. Available from: https://www.jamas.or.jp/english/.
- Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Ther. 2014;39:1349–1362.
- Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology. 2015;148:344–354 e345. quiz e314-345.
- Cholapranee A, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45:1291–1302.
- Singh S, Fumery M, Sandborn WJ, et al. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther. 2018;48:394–409.
- Vermeire S, van Assche G, Rutgeerts P. Review. article: Altering the natural history of Crohn’s disease–evidence for and against current therapies. Aliment Pharmacol Ther. 2007;25:3–12.
- Japan Ministry of Health Labour and Welfare. The Medical Fee Schedule 2018. https://www.mhlw.go.jp/index.html
- Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
- Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis. 2012;6:160–173.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960.
- Imafuku S, Nakano A, Dakeshita H, et al. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe plaque psoriasis in Japan. J Dermatolog Treat. 2018;29:24–31.
- IBM Watson Health. Micromedex RED BOOK – a comprehensive, consistent drug pricing resource. [2018 Sep]. Available from: http://truvenhealth.com/Products/Micromedex/Product-Suites/Clinical-Knowledge/RED-BOOK.
- MIMS. The monthly index of medical specialities. [2018 Sep]. Available from: https://www.mims.co.uk/.
- Rote Liste. Rote Liste Service GmbH. [2018 Sep]. Available from: https://www.rote-liste.de/.
- Kanatani Y, Tomita N, Sato Y, et al. National registry of designated intractable diseases in Japan: present status and future prospects. Neurol Med Chir (Tokyo). 2017;57:1–7.
- The Central Social Insurance Medical Council. Draft outline of drug pricing system reform. [2018 May]. Available from: http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000188706.pdf.
- Saito S, Nakazawa K, Suzuki K, et al. Paradigm shift of healthcare cost for patients with inflammatory bowel diseases: a claims data-based analysis in Japan. Gastrointestin Disord. 2019;1:120–128.
- Kasper LH, Everitt D, Leist TP, et al. A phase I trial of an interleukin-12/23 monoclonal antibody in relapsing multiple sclerosis. Curr Med Res Opin. 2006;22:1671–1678.
- Cornillie F, Shealy D, D’Haens G, et al. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn’s disease. Aliment Pharmacol Ther. 2001;15:463–473.
- den Broeder A, van de Putte L, Rau R, et al. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol. 2002;29:2288–2298.
- Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141.
- Organization for Economic Co-operation and Development (OECD). Purchasing Power Parities. [2018 May]. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm.