Abstract
Aims: To assess healthcare resource utilization (HCRU) and costs in patients with non-small cell lung cancer treated with the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors afatinib or erlotinib as first-line treatment.
Materials and methods: This retrospective analysis used data from three large administrative claims databases in the US: Truven MarketScan, IMS PharMetrics Plus, and Optum Clinformatics Data Mart. Patients with diagnosis codes of lung cancer treated with afatinib or erlotinib were included in the sample. Treatment cohorts were matched on baseline characteristics using propensity scores to account for potential selection bias. HCRU and healthcare costs were compared between the matched afatinib and erlotinib cohorts.
Results: In total, 3,152 patients met the study inclusion criteria; propensity score matching of the afatinib and erlotinib patients yielded 525 matched pairs with well-balanced baseline characteristics. The afatinib cohort had significantly fewer patients with ≥1 inpatient visits (40.4% vs 52.2%, p = 0.0001) and outpatient emergency room (ER) visits (45.7% vs 54.1%, p = 0.0066). Per patient per month (PPPM) visits were significantly different between afatinib compared to erlotinib for inpatient visits (0.1 vs 0.2, p = 0.0152), other outpatient visits PPPM (2.6 vs 3.0, p = 0.022) and outpatient office visits (2.0 vs 1.7, p = 0.0059). Although costs of outpatient office ($1,624 vs $1,070; p = 0.0086) and pharmacy ($6,709 vs $5,932; p < 0.0001) visits were higher for afatinib vs erlotinib, total costs did not differ significantly between cohorts ($14,972 vs $14,412; p = 0.4415).
Limitations: Retrospective claims data can be subject to coding errors or data omissions; patients were required to have continuous health plan enrolment; EGFR mutation status was not confirmed.
Conclusions: Patients treated with afatinib as first-line monotherapy experienced fewer inpatient stays and ER visits compared with erlotinib. Total costs were not significantly different between the two treatment cohorts.
Introduction
Lung cancer is the leading cause of cancer deaths in the US. In 2019, it is estimated that nearly 230,000 new cases of lung cancer will be diagnosed, and ∼140,000 people will die from the diseaseCitation1. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for ∼80–85% of cases; in these patients, epidermal growth factor receptor (EGFR) mutations are found in ∼10% of Caucasian patients and up to 50% of Asian patients. Healthcare resource utilization (HCRU) in patients with NSCLC is high and includes diagnostic testing, treatments, frequent office visits, and hospitalization, as well as supportive, and potentially hospice, careCitation2. The associated cost burden of care is an increasingly important component of healthcare decision-making for both patients and payers. Lung cancer is one of the costliest cancers to treatCitation3.
EGFR tyrosine kinase inhibitors (TKIs) are standard first-line treatment for patients with EGFR mutation-positive NSCLCCitation4,Citation5. Three generations of EGFR TKIs are approved for the first-line treatment of EGFR mutation-positive NSCLC in the US: the first-generation reversible TKIs, erlotinib and gefitinib; the second-generation irreversible TKIs: afatinib (ErbB family blocker) and dacomitinib (an EGFR, HER2 and ErbB4 inhibitor); and the third-generation irreversible EGFR/T790M inhibitor osimertinib. These agents have different pharmacological characteristics and mechanisms of action. The National Comprehensive Cancer Network in the US recommends any of the EGFR TKIs as first-line therapy for metastatic NSCLC with EGFR del19 and L858R mutations, based on high-level evidence from randomized controlled trialsCitation5.
Evidence suggests that second-generation EGFR TKIs may be more effective than first-generation TKIs as first-line treatment in EGFR mutation-positive NSCLCCitation6–8, although no randomized, controlled clinical trials have been conducted that have directly compared afatinib with erlotinib in this setting. To address this gap, and to provide insights into the comparative effectiveness of afatinib and erlotinib, a real-world study was undertaken using data from three administrative claims databases in the USCitation9. Results showed that patients prescribed afatinib had a significantly longer median duration of treatment compared to those prescribed erlotinib (12.1 vs 9.9 months, p = 0.035) and experienced a 14% reduction in risk of discontinuing therapy (adjusted hazard ratio = 0.86, confidence interval = 0.75–0.99).
Real-world studies can complement data from randomized, controlled trials by providing additional information on treatment use in everyday practice in a population of patients that might otherwise have been excluded from participation in controlled trials. Analysis of data from electronic medical records can help to provide information on healthcare resource use, and data from claims databases can inform practitioners and researchers regarding the cost of care for individuals with lung cancer. There is currently limited information on the HCRU and costs associated with treating patients with EGFR mutation-positive NSCLC; this information would help payers to make more informed decisions for managing EGFR mutation-positive NSCLC in the US. As such, this analysis used data collected from three administrative claims databases in the US to evaluate the all-cause HCRU and costs among patients with NSCLC who initiated first-line treatment with afatinib or erlotinib.
Methods
Data sources and study design
This analysis combined data from three large, administrative claims databases in the US that include patient demographics, enrolment history, and medical and pharmacy claims for more than 110 million members of commercial and Medicare Advantage/supplemental plans. Databases used for this study included Truven MarketScan (spanning January 1, 2013‒March 31, 2017)Citation10, IMS PharMetrics Plus (spanning January 1, 2013‒September 30, 2017)Citation11 and Optum Clinformatics Data Mart (spanning January 1, 2013‒September 30, 2017)Citation12. Full details of databases included in this analysis have been reported in full previously; algorithms used information on age, sex, US region, hospital admission date, discharge date, and primary discharge diagnosis to remove duplicate information, and only the most complete records were retained for analysisCitation9. Ethics committee approval was not required for this study.
For inclusion in analysis, patients were required to have at least one prescription claim for afatinib or erlotinib during the identification period defined for each database (July 1, 2013 to February 28, 2017 [Truven MarketScan] or August 31, 2017 [IMS PharMetrics Plus and Optum Clinformatics Data Mart]). The index date was defined as the initiation date of EGFR TKI therapy. Patients were also required to have continuous health plan enrolment with medical and pharmacy benefits for ≥6 months pre-index date (baseline) and ≥30 days post-index date. Patients needed to have at least one inpatient or outpatient claim with a diagnosis of lung cancer (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 162.x or ICD-10-CM: C34.xx) within 30 days before or after the index date. Patients were excluded from the study if they were under 18 years old or had received other systemic NSCLC treatment prescription fills during the 6-month baseline period; prescriptions for chemotherapy that were accompanied with a code for lung surgery were permitted and were considered as (neo)adjuvant therapy and not as a line of treatment. Details of codes used in the inclusion/exclusion criteria are provided in the Supplementary Methods.
Patients were followed for at least 30 days and then until health plan disenrollment or end of the study period, whichever occurred first (follow-up period). Patients whose treatments were used until disenrollment from the healthcare plan or end of the study period were censored at the end of follow-up.
Patients were stratified into two cohorts based on the index drug: the afatinib cohort or the erlotinib cohort. Patients identified in 2013 (from July to December) were excluded to reduce channeling bias arising from selecting patients in the same year that afatinib was approved (July 2013) by the US Food and Drug Administration, as used previouslyCitation13–15.
Propensity score matching
Patients in the afatinib and erlotinib cohorts were matched 1:1 using propensity scores, calculated using a logistic regression with age, sex, geographic region, Deyo-modified Charlson Comorbidity Index (CCI) scoreCitation16,Citation17, and individual comorbidities included as covariates. The matching algorithm selected the closest matches between cohorts, where propensity scores were within ±0.001 units of each other. Characteristics with standardized differences <10% were considered to be balanced.
Outcomes
HCRU was assessed over the follow-up period and reported on a per patient per month (PPPM) basis for all patients in each treatment cohort, including those without any HCRU visits. HCRU outcomes included number of inpatient admissions and length of stay, as well as emergency room (ER) visits, outpatient visits (both office and other, such as urgent care, laboratory, and radiology services), and pharmacy services. ER visits that resulted in hospitalization were counted as inpatient visits to avoid double counting of HCRU events. The proportion of patients in each cohort with at least one HCRU encounter in the follow-up period was also evaluated.
Healthcare costs from a payer’s perspective were computed based on the reimbursed claim amounts associated with each HCRU encounter during the follow-up period. All-cause medical (i.e. costs associated with inpatient and outpatient care) and pharmacy costs (all pharmacy costs, including costs of other drugs that may be prescribed for adverse events or other reasons) were assessed on a PPPM basis and inflated to 2017 US dollars using the annual medical care component of the Consumer Price IndexCitation18.
Statistical analyses
Due to sufficient sample sizes in both cohorts post-PPPM, normality was assumed, due to the central limit theorem, and parametric tests for significance were employed, as has been done in previous researchCitation19. Baseline characteristics, HCRU, and healthcare costs in the afatinib and erlotinib cohorts were summarized descriptively using mean values, and confidence intervals for continuous variables and frequency distributions for categorical variables. HCRU and healthcare costs were compared between the matched afatinib and erlotinib cohorts using differences in mean number of visits, length of stay, and costs. Student t-tests and Chi-square tests were used to assess the significance of differences observed between cohorts for continuous and categorical variables, respectively.
This was a retrospective study with no a priori hypothesis testing; therefore, we did not undertake a formal calculation of sample size and statistical power. All analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, NC).
Results
Overall, 3,152 patients with NSCLC met the study selection criteria: 550 patients who initiated treatment with afatinib and 2,602 patients who initiated treatment with erlotinib (). There were differences in several baseline characteristics between the two treatment cohorts; patients prescribed afatinib were younger and had greater mean Deyo-modified CCI scores than those prescribed erlotinib (). Patients prescribed afatinib were also less likely to have comorbid cerebrovascular disease, peripheral arterial disease, chronic kidney disease, or chronic obstructive pulmonary disease, than those prescribed erlotinib (). Propensity score matching yielded 525 matched pairs with well-balanced baseline characteristics (). Mean (median) duration of follow-up was 351 (290) days in the afatinib cohort and 403 (319) days in the erlotinib cohort.
Figure 1. Sample selection. Abbreviation. NSCLC, non-small cell lung cancer. Reproduced with permission from Future Oncology.
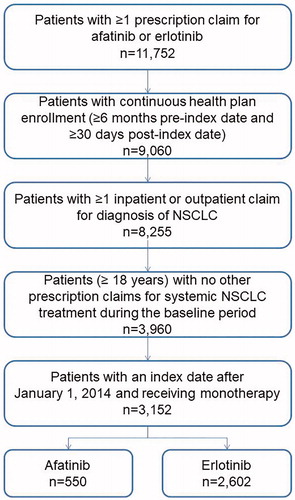
Table 1. Baseline characteristics among the cohort of patients treated with afatinib and erlotinib before and after propensity score matching.
The number of patients with ≥1 HCRU during first-line therapy is shown in . Among the matched cohorts, fewer patients in the afatinib cohort experienced at least one inpatient visit during the follow-up period (40.4%) compared to patients in the erlotinib cohort (52.2%; p = 0.0001). Similarly, the proportion of patients experiencing at least one outpatient ER visit was lower in the afatinib cohort compared to the erlotinib cohort (45.7% vs 54.1%; p = 0.0066). There was no difference between cohorts in the proportion of patients with at least one outpatient office visit (afatinib 92.0% vs erlotinib 91.6%; p = 0.8219) or other outpatient visit (95.8% vs 97.0%; p = 0.3215).
Table 2. Adjusted healthcare resource utilization and number of visits per patient per month among the cohort of patients treated with afatinib and erlotinib.
Patients treated with afatinib also had significantly fewer inpatient visits PPPM (0.1 vs 0.2, p = 0.0152) and other outpatient visits PPPM (2.6 vs 3.0, p = 0.022). Nevertheless, a significantly higher number of outpatient office visits PPPM was observed for afatinib patients, compared with erlotinib patients (2.0 vs 1.7, p = 0.0059) ().
Healthcare costs are also shown in . Although costs of outpatient office ($1,624 vs $1,070; p = 0.0086) and pharmacy ($6,709 vs $5,932; p < 0.0001) visits were higher for afatinib vs erlotinib, total costs did not differ significantly between cohorts ($14,972 vs $14,412; p = 0.4415).
Table 3. Adjusted healthcare costs among the cohort of patients treated with afatinib and erlotinib.
Discussion
Research incorporating real-world evidence on HCRU and costs among patients initiating treatment with afatinib or erlotinib can provide insight into the comparative economic impact of these therapies. We had previously reported that patients prescribed afatinib had a longer median treatment duration and lower risk of discontinuing first-line treatment than patients prescribed erlotinib in a large cohort of patients with NSCLC treated in the US and that this may reflect improved benefit for patients treated with afatinib compared to erlotinib in clinical practice. Here, we show that patients treated with afatinib experienced fewer inpatient stays and ER visits, compared to erlotinib-treated patients.
Results of this study showed no significant difference in overall costs for both treatment cohorts. Afatinib patients incurred higher costs associated with outpatient office visits, in accordance with higher all-cause outpatient office visits PPPM compared to erlotinib patients, and higher pharmacy costs. However, these higher costs appear to be offset by lower inpatient and other outpatient costs among afatinib patients. A retrospective, real-world analysis of medical records from US community oncology practices had previously evaluated the costs during TKI treatment and following progression on TKI therapy, although costs were not further stratified by individual TKI treatmentCitation20. The total mean monthly cost in this study was $20,106, and healthcare costs during TKI treatment and following progression were similar and largely attributed to hospitalization and anti-cancer therapy. The study also showed that mean monthly cost was lower in patients who initiated TKI as first-line therapy than in those who initiated TKI as second-line therapy ($18,354 vs $23,221). Our study adds to these findings by providing insight into costs in a different population and with consideration of choice of treatment.
Although previous research has suggested a lower incidence of adverse events with erlotinib compared to afatinibCitation21, our analysis of real-world data showed no differences in HCRU that might have been expected given potential differences in tolerability profiles. Fewer afatinib patients experienced at least one inpatient or outpatient visit compared to erlotinib patients, whereas the proportion of patients requiring at least one outpatient office or other outpatient visit was similar between afatinib and erlotinib. Our study results suggest potential clinical benefits for afatinib and imply that, in the real-world treatment setting, patients treated with afatinib may have experienced a lower incidence of acute events requiring ER visits and hospitalization, compared with erlotinib patients.
Our analysis is one of the first real-world claims-based studies to evaluate HCRU and costs of the first-line use of afatinib or erlotinib in patients with NSCLC. As such, these data provide important insights into real-world resource use and costs of treatment in the US and useful information for cost-effectiveness modeling. Cost-effectiveness models frequently use cost estimates derived from controlled clinical trials or expert opinion, both of which are known to be associated with bias. Using data from real-world studies can help control for some of this bias.
A strength of this analysis is the use of data from three claims databases to overcome challenges related to data scarcity; this also enabled data to be derived from relatively large propensity score-matched cohorts of patients that were well balanced with regard to baseline characteristics. Combining data from different databases also helps to improve the generalizability of the results as the different databases cover data from employer-provided health insurance plans and Medicare/Medicaid. It should be noted that we only accounted for patients that remained in the healthcare system by following them until health plan disenrollment, or end of study period. As patients had variable follow-up periods, we presented data as PPPM to give an objective method of comparing utilization and costs.
Several limitations should also be considered when evaluating the findings reported here. Although the propensity scoring methodology helps to reduce confounding, this method does not balance unmeasured confounding variables as would be the case with a randomized controlled trialCitation22. As such, residual confounding from unknown variables, such as race (a clinical characteristic known to be associated with EGFR mutation-positive disease) and time from diagnosis to treatment initiation, may remain. It should also be considered that erlotinib was available prior to afatinib, resulting in differences in the time period of treatment between cohorts. As such, the impact of changing treatment practice over time on the reported findings is unknown, although we did limit the identification period to 2014–2017 to minimize the chances of channeling bias that might have occurred due to including afatinib patients with index dates close to the approval date for the drug in 2013. EGFR mutation status is not captured in administrative claims data and receipt of approved first-line treatments was used as a proxy for EGFR mutation-positive NSCLC. As with all retrospective database analysis, data can be subject to coding errors or data omissions and patients were required to have continuous health plan enrolment to be included in this study; this may have led to selection bias. Analyses also assumed that all pharmacy costs were billed and captured in the claims data; refills that may have occurred and that have not been directly billed to insurance (e.g. free samples, during an inpatient stay) would not have been accounted for in the cost calculations. Finally, our study focused on direct costs associated with care; the indirect costs associated with the burden of disease were not evaluated.
In conclusion, this retrospective claims-based study suggests that patients with NSCLC treated with afatinib as first-line monotherapy experienced fewer inpatient stays and ER visits, compared to erlotinib-treated patients. Costs of treatment were not significantly different. These findings may reflect improved clinical benefit for patients treated with afatinib, compared to erlotinib, resulting from the longer first-line treatment duration previously reported in this patient population.
Transparency
Declaration of funding
This study was supported by Boehringer Ingelheim.
Declaration of financial/other interests
JL, CS, AG, and IG are employees of Boehringer Ingelheim. SS and LW are employees of SIMR and received consultancy fees from Boehringer Ingelheim for this work. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental data for this article is available online at https://doi.org/10.1080/13696998.2019.1645681.
Download MS Word (37.4 KB)Acknowledgements
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Suzanne Patel during the preparation of this article. Statistical programming and analysis assistance were provided by Yigong Zhou and Christopher Dieyi of STATinMED Research.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
- Lee DH, Isobe H, Wirtz H, et al. Health care resource use among patients with advanced non-small cell lung cancer: the PIvOTAL retrospective observational study. BMC Health Serv Res. 2018;18:147.
- Institute. NC. Financial burden of cancer care; 2019. Available from: https://progressreport.cancer.gov/after/economic_burden.
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237.
- NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 1-2019; 2018 [cited 2019 Jul 18]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–589.
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466.
- Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36:2244–2250.
- Lim J, Samuelsen C, Golembesky A, et al. Duration of treatment among patients prescribed afatinib or erlotinib as first-line therapy for EGFR mutation-positive non-small-cell lung cancer in the USA. Future Oncol. 2019;15:1493–1504.
- Tung A, Hepp Z, Bansal A, et al. Characterizing health care utilization, direct costs, and comorbidities associated with interstitial cystitis: a retrospective claims analysis. J Manag Care Spec Pharm. 2017;23:474–482.
- Divino V, Karve S, Gaughan A, et al. Characteristics and treatment patterns among US patients with hairy cell leukemia: a retrospective claims analysis. J Comp Eff Res. 2017;6:497–508.
- Atzinger CB, Guo JJ. Biologic disease-modifying antirheumatic drugs in a national, privately insured population: utilization, expenditures, and price trends. Am Health Drug Benefits. 2017;10:27–36.
- Machado MA, Bernatsky S, Bessette L, et al. Hospitalization for musculoskeletal disorders in rheumatoid arthritis patients: a population-based study. BMC Musculoskelet Disord. 2016;17:298.
- Jalbert J, Gasse C, Bakshi S, et al. Channeling bias in comparative-effectiveness research of newly launched medications: a case study. Value in Health. 2016;19:A184.
- Ankarfeldt MZ, Thorsted BL, Groenwold RH, et al. Assessment of channeling bias among initiators of glucose-lowering drugs: a UK cohort study. Clin Epidemiol. 2017;9:19–30.
- de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56:221–229.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619.
- Bureau of Labor Statistics. Consumer price index-all urban consumers; medical care; 2018 [cited 2019 Jul 18]. Available from: https://data.bls.gov/cgi-bin/surveymost.
- Gehrman J, Bjorholt I, Angenete E, et al. Health economic analysis of costs of laparoscopic and open surgery for rectal cancer within a randomized trial (COLOR II). Surg Endosc. 2017;31:1225–1234.
- Skinner KE, Fernandes AW, Walker MS, et al. Healthcare costs in patients with advanced non-small cell lung cancer and disease progression during targeted therapy: a real-world observational study. J Med Econ. 2018;21:192–200.
- Isla D, De Castro J, Juan O, et al. Costs of adverse events associated with erlotinib or afatinib in first-line treatment of advanced EGFR-positive non-small cell lung cancer. Clinicoecon Outcomes Res. 2017;9:31–38.
- Streiner DL, Norman GR. The pros and cons of propensity scores. Chest. 2012;142:1380–1382.
