?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims: Allergic rhinitis is caused by sensitivity to environmental allergens that can significantly impact quality-of-life. The objective of this analysis was to estimate health state utilities and quality-adjusted life days (QALDs) for a tree allergy immunotherapy trial, TT-04 (EudraCT No.2015-004821-15). Health-state utilities are a measure of patient preference for health states and are necessary to derive QALDs for cost-utility analysis. Preference-based utilities were not collected in the TT-04 trial, so a mapping algorithm was developed based on a similar grass allergy immunotherapy trial, GT-08 (EudraCT No. 2004-000083-27), to estimate utilities.
Methods: A two-part model was developed to predict utilities for the GT-08 trial and applied to the TT-04 trial to estimate the difference in mean utility and QALDs between SQ tree sublingual immunotherapy (SLIT)-tablet and placebo.
Results: Mean utility difference between SQ tree SLIT-tablet and placebo was 0.030 [95% CI = 0.015–0.046] during the birch pollen season (BPS), 0.019 [95% CI = 0.007–0.030] during the tree pollen season (TPS) and 0.018 [95% CI = 0.007–0.030] during the full trial. The treatment showed a QALD benefit of 1.26 [95% CI = 0.619–1.917] during the BPS, 1.90 [95% CI = 0.692–3.047] during the TPS, and 2.47 [95% CI = 0.930–4.101] during the full trial.
Limitations: The generalizability of this algorithm is limited to allergy trials containing the same covariates as those present in the model. The analysis also assumes that grass and tree pollen allergy have the same relationship with EQ5D utilities, which is supported by the fact that both grass and tree pollen induce similar symptoms.
Conclusions: Application of the mapping function enabled the calculation of QALDs associated with the treatment, with the caveat that data were extrapolated from grass seasonal allergy to tree seasonal allergy. The results showed a significant QALD benefit of the treatment over placebo in treatment of tree pollen-induced rhinoconjunctivitis.
Introduction
Allergic rhinitis is a common inflammatory disorder of the nasal mucosa affecting more than 20% of the European population. Allergic rhinitis is caused by sensitivity to environmental allergens such as tree or grass pollenCitation1–5. Symptoms of allergic rhinitis include a runny, itchy, or blocked nose and itchy, gritty, or watery eyes. An estimated 10–40% of patients also suffer from concomitant allergic asthmaCitation2,Citation3,Citation5,Citation6. In addition to physical symptoms, patients often experience reduced sleep quality, emotional problems such as depression, and social difficultiesCitation3,Citation5,Citation6. Patients often require additional general practice services and medication, which can be a financial burden to patients, healthcare providers, and societyCitation3,Citation6–8.
Treatments for allergic rhinitis include the use of symptom-relieving medications such as oral antihistamines, intranasal corticosteroids, and allergen avoidance, all of which may provide temporary relief from symptomsCitation1,Citation5,Citation9. Allergy immunotherapy (AIT) is a treatment for patients with evidence of Immunoglobulin E (IgE) sensitization whose moderate-to-severe allergic rhinitis is uncontrolled by symptom-relieving medicationsCitation10,Citation11. AIT may be administered subcutaneously or sublingually, and contains allergen extracts from the target allergenCitation5,Citation9,Citation12. In addition to providing relief from daily symptoms, AIT induces a long-term immune tolerance that improves quality-of-life for years past treatment discontinuationCitation10,Citation13–15.
The effect of allergic rhinitis on quality-of-life may be assessed by preference-based measures (PBMs) or disease-specific measures. Generic PBMs assess general quality-of-life with standardized dimensions broad enough to capture quality-of-life differences in most disease areas. PBM responses may be used to generate health-state utilities, which are relative preference weights for different health states measured on a cardinal scale and may be used to calculate quality-adjusted life years (QALYs), a common outcome in cost-effectiveness studies and a requirement for cost-utility analysis. However, the domains of generic questionnaires may be too broad to be sensitive to condition-specific symptoms such as ocular or nasal symptomsCitation16–18. Disease-specific measures of quality-of-life such as the Rhinoconjunctivitis Quality-of-Life Questionnaire (RQLQ) have the advantage of greater sensitivity to condition-specific symptoms, but may not be used to calculate QALYsCitation16–20.
When PBMs are not used in a clinical trial it is possible to “map” disease-specific scores to preference-based utilities that may be used to calculate QALYs in cost-effectiveness studies. A common mapping strategy takes advantage of an “estimation” dataset, which is a dataset with recorded PBM data that is similar to the dataset of interest that does not contain PBM data. Regression modeling is applied to the estimation dataset to develop a mapping function that quantifies the statistical relationship between the PBM and the other outcomes measured in the study. The two datasets must be similar because the mapping function assumes that the statistical relationship between the estimated utilities and the covariates are the same in both the estimation dataset and the study dataset. The regression developed from the estimation dataset is applied to the study dataset in order to estimate preference-based health-state utilitiesCitation19–22.
This analysis developed an algorithm to map from the disease-specific measure RQLQ to the generic PBM European Quality-of-Life in 5-Dimensions (EQ5D). The algorithm was developed from grass allergy immunotherapy trial data (GT-08), in which both RQLQ and EQ5D data were collected. The algorithm was applied to a similar tree allergy immunotherapy trial (TT-04) that did not collect EQ5D data to estimate health-state utilities and QALYs.
Methods
Analysis set
The GT-08 trial (EudraCT No. 2004-000083-27) was a randomized, double-blind, placebo-controlled, 5-year Phase III trial designed to assess the efficacy and safety of the SQ grass sublingual immunotherapy (SLIT)-tablet to treat rhinoconjunctivitis in subjects with seasonal grass pollen allergyCitation14,Citation23. The grass pollen season was defined between the first and last day of 3 consecutive days with pollen count larger than or equal to 10 grains/m3. At the start of the trial, 634 subjects were enrolled and randomized to active treatment or placebo groups. Eligible subjects included healthy adults with a clinical history of grass pollen-induced allergic rhinoconjunctivitis suffering from moderate-to-severe symptoms despite pharmacotherapy use. Throughout the trial, all subjects had access to symptom-relieving medications including desloratadine, olopatadine, budesonide, prednisone, and asthma inhalers to control residual symptoms.
Subjects were asked to complete a daily record of rhinoconjunctivitis symptoms and medication use in an electronic diary. The six recorded symptoms included runny nose, blocked nose, sneezing, itchy nose, itchy eyes, and watery eyes. Subjects scored their rhinoconjunctivitis and asthma symptoms on a scale from 0–3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). Daily medication use was measured on a scale from 0–36 according to the type and dosage of medication used. Subjects were also asked to complete two quality-of-life instruments every week: the EQ5D and the RQLQCitation24. EQ5D is a common PBM recommended by the National Institute for Health and Care Excellence (NICE) for estimation of health-state utilities. EQ5D measures quality-of-life in five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depressionCitation25. EQ5D scores are only accurate for the day on which they are completed, so only daily scores recorded on the same day as the EQ5D scores were included in the analysis. The RQLQ is a disease-specific questionnaire consisting of 28 questions in seven domains, including activity limitation, sleep problems, nose symptoms, eye symptoms, non-nose/eye symptoms, practical problems, and emotional function. Subjects rate their health in these dimensions on seven levels from no impairment to severe impairmentCitation23.
The TT-04 trial (EudraCT No. 2004-000083-27) assessed the efficacy and safety of the SQ tree SLIT-tablet in subjects with allergic rhinitis and/or conjunctivitis caused by pollen from the birch homologous groupCitation9,Citation26. The birch homologous group includes birch, hazel, alder, hornbeam, beech, and oak pollen, which are classified based on similarities in chemical structure and cross-reactivityCitation9,Citation26. Accordingly, patients with a sensitivity to birch pollen are likely to react to other allergens within the homologous groupCitation27,Citation28. The birch pollen season (BPS) is the primary season in the study, and the birch, hazel, and alder seasons are considered the tree pollen season (TPS). The TPS was defined between the first and last day of 3 consecutive days with tree pollen count larger than or equal to 10 grains/m3, excluding the days between the pollen seasons where pollen counts fall below 10 grains/m3. The BPS was defined by the same criteria, though the pollen threshold was 30 grains/m3.
At the start of the trial, 634 subjects were randomized and treated with the SQ tree SLIT-tablet or placebo for 1 year. Similar to the GT-08 trial, subjects provided a daily diary of symptoms and medication use and RQLQ was registered weekly. The same symptoms and medications were recorded in both the GT-08 and TT-04 trials, with the exception of prednisone, which was not one of the symptom-relieving medications provided to patients in the TT-04 trial. Prednisone made up only 1% of the overall medication scores in the GT-08 trial and was assumed to have little effect on the daily medication scores. Baseline characteristics for both trials are presented in .
Table 1. Baseline characteristicsa of GT-08 and TT-04 trials.
Model development
The GT-08 model was developed using a two-part modeling approach because the EQ5D scores were strongly left skewed, and 83% of EQ5D responses were clustered at 1, indicating perfect health. This skewness is common with EQ5D data, and a high percentage of subjects in perfect health were expected in this case because subjects were required to be healthy aside from rhinoconjunctivitis symptomsCitation19. Most standard statistical methods (e.g. Gamma and Poisson distributions) are only appropriate for right-skewed data. However, by transforming our data to a disutility scale, we converted our data from left-skewed to right-skewedCitation16. We developed the model in terms of disutility (d), then transformed the data back to the original utility scale (u) using the following equation:
In the first stage of the GT-08 model, EQ5D utilities were modeled as a binary variable (0 = imperfect health, 1 = perfect health). In the second stage, EQ5D utilities were modeled as a continuous variable conditional upon having imperfect health. Both generalized estimating equations (GEE) models and mixed effects models are appropriate to analyze repeated measures in longitudinal data. We tested several types of GEE models for the second stage of the model, including identity and log link functions with Gaussian, Poisson, and Gamma distributions. The variables subject and year were tested as random effects.
Covariates were chosen for inclusion in the model based on their availability in both the GT-08 and TT-04 trials and their expected clinical relevance. The candidate covariates included daily symptom score (DSS), daily medication score (DMS), total combined symptom and medication score (TCS), RQLQ index score, age, gender, asthma symptom score, history of asthma, and whether the measure was taken during or outside of a pollen season. An interaction term between DSS and DMS was also tested because subjects taking higher doses of symptom-relieving medication are likely to experience reduced symptoms and vice versa. All candidate covariates were included in a preliminary model. A more parsimonious model was developed using backward selection and only the statistically significant variables (p < 0.05) were retained in the final model.
Calculation of quality-adjusted life days (QALDs)
In this analysis, QALDs and utilities were analyzed over the range of relative days with a least one EQ5D response in each treatment arm within the pollen season in order to accurately capture the utility difference. The range of dates included in our analysis is longer than the duration of the seasons recorded in TT-04 because it includes data from the gaps between pollen seasons where pollen counts fall below the defined thresholds, and because pollen seasons from different regions overlap. The BPS and TPS are defined in order to capture all EQ5D responses, which is necessary in order to capture the full difference in quality-of-life.
Results
The first stage of the two-part model predicted the probability of imperfect health, and the second stage of the two-part model predicted disutility conditional on imperfect health. The best fit first stage model was a mixed effects logistic model. A mixed effects model provided a better fit for the second stage model as compared to the GEE model. The subject was included as a random effect in both stages of the model. Both parts of the model are summarized in .
Table 2. Summary of GT-08 model.
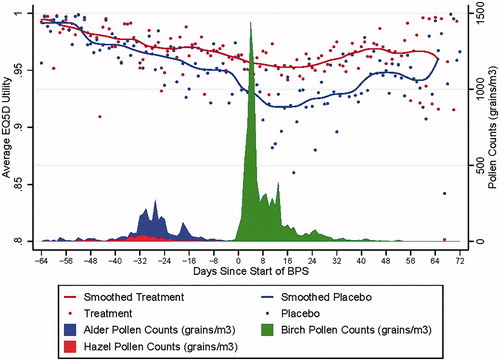
The model developed from the GT-08 trial data was then applied to the TT-04 trial data to predict EQ5D utilities. shows the predicted pooled mean utilities separated by treatment arm for the TT-04 trial. As in the clinical trial, the tree pollen season was defined between the first and last of 3 consecutive days with a pollen count above 10 grains/m3.
In the TT-04 trial, the duration of the BPS ranged from 10 days in length to 42, and the duration of the TPS ranged from 14 days in length to 50, depending on the region of the pollen exposure.
Dates were defined relative to the first day of the BPS. The full dataset analysis included all days over which there was at least one EQ5D response in each treatment arm, regardless of whether the pollen count of the respondent’s region was above the threshold. Using this definition, the length of the BPS season spanned 42 days, TPS spanned 100 days, and the full data set spanned 137 days. Mean utility difference between SQ tree SLIT-tablet and placebo was 0.030 [95% CI = 0.015–0.046] during the BPS, 0.019 [95% CI = 0.007–0.030] during the TPS, and 0.018 [95% CI = 0.007–0.030] over the duration of the trial.
QALDs were calculated by multiplying the difference in pooled mean utility by the length of the season of interest. The SQ tree SLIT-tablet showed an incremental QALD benefit of 1.26 (95% CI = 0.619–1.917) during the 42-day BPS, 1.90 (95% CI = 0.692–3.047) during the entire 100-day TPS, and 2.47 (95% CI = 0.930–4.101) over the full 137-day duration of the trial (). Confidence intervals were computed using bootstrap estimation, and all utility and QALD differences were statistically significant given the exclusion of zero in the 95% confidence intervals. All statistical analyses were performed using Stata version 14.
Table 3. Estimated utilities and QALDs for SQ-SLIT tablet.
Discussion
The pooled mean utilities presented in show a clear difference between the treatment arms, that is most pronounced during the BPS and appears to persist beyond the day when the birch pollen counts drop below the season threshold of 30 grains/m3. The difference in treatment arms beyond the season may be related to the priming effect. The alder and hazel seasons occur prior to the birch season, and early exposure to these cross-reactive pollens may prime patients to experience elevated symptoms, even after a reduction in pollen levelsCitation29. The data points on the outer edges of show a greater spread because fewer subjects provided data outside of the pollen season. The smoothed curves intersect when the pollen levels drop well below the threshold and the impact of pollen levels on EQ5D is negligible.
The SQ tree SLIT-tablet inhibits human IgE binding of birch, alder, hazel, and oak allergen extractsCitation9,Citation27,Citation28. Oak pollen counts were not included in the TPS because the peak of the oak pollen season occurs a month after the peak of the BPSCitation30. Although the pollen counts of the oak seasons were not reported in this trial, the extended treatment effect visible in may be caused by a treatment-related reduction in oak allergy symptoms or the priming effect.
The benefit of the algorithm is that it allows mapping of clinical results to the generic outcome of QALYs. However, the generalizability of this algorithm is limited to allergy trials containing the same covariates as those present in the model. The analysis also assumes that grass pollen allergy and tree pollen allergy have the same relationship with EQ5D utilities and other key covariates in the mapping algorithm. This assumption is supported by the fact that both grass and tree pollen induce similar allergic rhinitis symptomsCitation2,Citation29. The trials are also similar in terms of patient population, exclusion criteria, and baseline characteristics (). Both trials collected symptom, medication, and asthma scores daily, and quality-of-life data weekly, and symptoms were rated using the same scale. This algorithm may be applicable to other allergy trials of similar design, patient population, and symptomology.
A notable difference between the trials is the use of prednisone as a rescue medication. Prednisone was available to subjects in the GT-08 trial, but was not available to the subjects in TT-04. The impact of prednisone on symptoms and medication use is captured in the mapping function despite the fact that it was not used in the TT-04 trial. However, prednisone represents only 1% of the overall medication scores in the GT-08 trial and is unlikely to have significantly impacted medication scores. Another difference between the trials is the overall duration of treatment. Subjects in the GT-08 trial were treated for 3 years and followed for 2 years after treatment discontinuation, while subjects in the TT-04 trial were treated for an average of 32 weeks. The European Academy of Allergy and Clinical Immunology (EAACI) guidelines for allergy treatment recommend at least 3 years of AIT treatment to achieve a long-term treatment effect and improvement in quality-of-lifeCitation31. Additional treatment and follow-up is needed to assess sustained improvement in quality-of-life.
The algorithm developed in this analysis maps from the disease-specific measure RQLQ to generic EQ5D. RQLQ, which asks subjects to rate symptoms over the previous week, is used to predict EQ5D, which is a daily measure. The lack of concordance between the time frames of the two measures may dilute the association. Nevertheless, our results do show empirically that the measures are associated.
Conclusions
An effective mapping function was developed based on grass SLIT-tablet trial data for use in a tree pollen SLIT-tablet trial with the caveat that we are extrapolating data from grass seasonal allergy to tree seasonal allergy. The application of the mapping function allows the calculation of QALDs associated with the tree SLIT-tablet treatment, which avoids the cost of a new trial and can be used for future cost-utility analysis. The results show a significant QALD benefit of the SQ tree SLIT-tablet over placebo in treatment of tree pollen-induced rhinoconjunctivitis.
Transparency
Declaration of funding
The research was funded by ALK.
Declaration of financial/other interests
KD, AB, and RO were paid as consultants for this project by ALK. TSG and SB are employees of ALK. The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript discloses their role as a scientific consultant for Stallergenes-Greer. The reviewers have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Data availability statement
The clinical trial data that support the findings of this study are proprietary to ALK and, therefore, cannot be made publicly available.
References
- Greiner AN, Hellings PW, Rotiroti G, et al. Allergic rhinitis. Lancet. 2011;378:2112–2122.
- Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764.
- Linneberg A, Dam Petersen K, Hahn-Pedersen J, et al. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016;14:12
- Lake IR, Jones NR, Agnew M, et al. Climate change and future pollen allergy in Europe. Env Health Persp. 2017;125:385–391.
- Larsen JN, Broge L, Jacobi H. Allergy immunotherapy: the future of allergy treatment. Drug Discov Today. 2016;21:26–37.
- Hoehle LP, Speth MM, Phillips KM, et al. Association between symptoms of allergic rhinitis with decreased general health-related quality of life. Am J Rhinol Allergy. 2017;31:235–239.
- Pitt AD, Smith AF, Lindsell L, et al. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol. 2004;11:17–33.
- Rønborg SM, Svendsen UG, Micheelsen JS, et al. Budget impact analysis of two immunotherapy products for treatment of grass pollen-induced allergic rhinoconjunctivitis. CEOR. 2012;4:253–260.
- Biedermann T, Kuna P, Panzner P, et al. The SQ tree SLIT-tablet is highly effective and well tolerated: Results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2019;143:1058–1066.e6.
- Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798.
- ALK-Abelló A/S. ALK obtains European approval for its tree SLIT-tablet against allergic rhinitis. 2019. GlobeNewswire, Inc. GlobeNewswire.com.
- Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72:1597–1631.
- Fiocchi A, Fox AT. Preventing progression of allergic rhinitis: the role of specific immunotherapy. Arch Dis Child Educ Pract Ed. 2011;96:91–100.
- Dahl R, Kapp A, Colombo G, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;118:434–440.
- Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948.
- Wolowacz SE, Briggs A, Belozeroff V, et al. Estimating health-state utility for economic models in clinical studies: an ISPOR good research practices task force report. Value Health. 2016;19:704–719.
- Kremer B. Quality of life scales in allergic rhinitis. Curr Opin Allergy Clin Immunol. 2004;4:171–176.
- Versteegh MM, Leunis A, Uyl-de Groot CA, et al. Condition-specific preference-based measures: benefit or burden? Value Health. 2012;15:504–513.
- Wailoo AJ, Hernandez-Alava M, Manca A, et al. Mapping to estimate health-state utility from non-preference-based outcome measures: an ISPOR good practices for outcomes research task force report. Value Health. 2017;20:18–27.
- Brazier JE, Yang Y, Tsuchiya A, et al. A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. Eur J Health Econ. 2010;11:215–225.
- Ara R, Rowen D, Mukuria C. The use of mapping to estimate health state utility values. Pharmacoeconomics. 2017;35:57–66.
- Longworth L, Rowen D. Mapping to obtain EQ-5D utility values for use in NICE health technology assessments. Value Health. 2013;16:202–210.
- Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21:77–83.
- Calderón M, Brandt T. Treatment of grass pollen allergy: focus on a standardized grass allergen extract – Grazax®. TCRM. 2008;4:1255–1260.
- Guide to the methods of technology appraisal 2013. 2013. Available from: https://www.nice.org.uk/process/pmg9/chapter/foreword.
- Makela MJ, et al. Immunotherapy with the SQ tree SLIT-tablet in adults and adolescents with allergic rhinoconjunctivitis. Clin Ther. 2018;40:574–586.e4.
- Lorenz AR, LÜTtkopf D, May S, et al. The principle of homologous groups in regulatory affairs of allergen products–a proposal. Int Arch Allergy Immunol. 2009;148:1–17.
- Heath MD, Collis J, Batten T, et al. Molecular, proteomic and immunological parameters of allergens provide inclusion criteria for new candidates within established grass and tree homologous groups. World Allergy Org J. 2015;8:21–21.
- D'Amato G, Cecchi L, Bonini S, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990.
- McInnes RN, Hemming D, Burgess P, et al. Mapping allergenic pollen vegetation in UK to study environmental exposure and human health. Sci Total Env. 2017;599–600:483–499.
- Muraro A, Roberts G, Halken S, et al. EAACI guidelines on allergen immunotherapy: executive statement. Allergy. 2018;73:739–743.