Abstract
Aims: Bone complications (also known as skeletal-related events [SREs]) pose significant health and financial burdens on patients with bone metastases. Denosumab demonstrated superiority over zoledronic acid in delaying the time to first SRE. This study examined the lifetime cost-effectiveness of denosumab vs zoledronic acid from both US payer and societal perspectives.
Methods: This analysis used a lifetime Markov model and included patients with breast cancer, prostate cancer, and other solid tumors and bone metastases. The societal perspective included direct medical, direct non-medical, and indirect costs associated with denosumab and zoledronic acid; the payer perspective included direct medical costs only. Bone complication rates for each tumor type were estimated from three pivotal phase 3 studies and modified to reflect real-world incidence.
Results: From a societal perspective, compared with zoledronic acid, denosumab use resulted in an incremental cost of $9,043, an incremental benefit of 0.128 quality-adjusted life-years (QALYs), a lifetime cost per QALY of $70,730, and a net monetary benefit (NMB) of $10,135 in favor of denosumab. Direct drug costs for denosumab ($28,352) were higher than zoledronic acid/untreated ($578), but were offset by reduced costs associated with bone complications. From a payer perspective, denosumab use was associated with an incremental cost of $13,396, and an incremental benefit of 0.128 QALYs, for a cost of $104,778 per QALY and an NMB of $5,782 in favor of denosumab.
Limitations: Some model inputs had limited information and, given that the results may be sensitive to changes in these inputs, our findings should be interpreted within the context of the data inputs and modeling assumptions used in the analysis.
Conclusions: Denosumab is a cost-effective option to prevent bone complications in patients with solid tumors when considering both payer and broader societal perspectives.
Introduction
Bone is a common site of metastatic disease for a variety of solid tumors, with bone metastases occurring in up to 75% of patients with advanced metastatic breast and prostate cancersCitation1,Citation2. Patients with solid tumors and bone metastases are at an increased risk for bone complications (also known as skeletal-related events [SREs]): rates of bone complications are ∼40% (19 months) in patients with prostate cancer and 54% (24 months) in patients with breast cancerCitation3,Citation4. Bone complications are associated with decreased survival and considerable morbidity, pain, impaired mobility, and decreased quality-of-lifeCitation3,Citation5–10. Healthcare costs for patients with bone complications are substantialCitation3,Citation11–16.
Zoledronic acid is an intravenous bisphosphonate used to delay or prevent bone complications in patients with bone metastases; however, treatment can be associated with renal toxicity, requiring renal function monitoring, and is not recommended in patients with chronic renal impairmentCitation17. Denosumab (XGEVA®, Amgen Inc., Thousand Oaks, CA, USA) is a fully human monoclonal antibody against receptor activator of nuclear factor-kappa Β ligand (RANKL) that inhibits osteoclast-mediated bone destructionCitation18. An integrated analysis of data from three identically designed, randomized, double-blind, phase 3 trials of 5,723 patients with bone metastases and breast cancer, prostate cancer, or other solid tumors or multiple myeloma demonstrated that denosumab was superior to zoledronic acid in delaying the time to first SRE (hazard ratio = 0.83 [95% CI = 0.76–0.90])Citation19. The median (95% CI) time to first on-study SRE was 27.7 (24.2–not estimable) months for denosumab and 19.5 (18.5–21.4) months for zoledronic acid, a difference of 8.2 monthsCitation19. Both drugs are recommended in the current American Society of Clinical Oncology guidelines on the use of bone-modifying agents for patients with bone metastasesCitation20.
Studies comparing the cost-effectiveness of zoledronic acid and denosumab in patients with solid tumors have reported varying resultsCitation21–28. Cost-effectiveness analyses typically focus on direct medical costs and their short-term economic impactCitation29. Comprehensive clinical, humanistic, and downstream economic benefits are rarely considered and may provide a broader perspective on drug valueCitation29. Median overall survival of patients is increasing across many tumor types. For example, patients with metastatic human epidermal growth factor receptor 2-positive breast cancer are now living up to 4 or 5 yearsCitation30. Therefore, models should also extend far enough into the future to capture major health and economic benefits of the agents being compared.
The objective of this analysis was to estimate the incremental cost-effectiveness ratio (ICER) and net monetary benefit (NMB) of denosumab vs zoledronic acid in patients with bone metastases from breast, prostate cancer, and other solid tumors, but not multiple myeloma, using a broader societal perspective and direct-cost (payer) perspective in the US.
Methods
The XGEVA® Global Economic Model (X-GEM) builds on the Markov model used previouslyCitation25, which evaluated the cost-effectiveness of denosumab vs zoledronic acid for prevention of bone complications in patients with solid tumors in the US. X-GEM considers direct medical, direct non-medical, and indirect costs; includes revised inputs to reflect the changing treatment landscape of patients with bone complications and for loss of patent or change in acquisition cost for zoledronic acid; and provides a broader perspective of value. Key terms and definitions used in the model are provided in Citation31–36. Approximately 8% of patients with bone metastases related to solid tumors have renal impairment (i.e. estimated glomerular filtration rate <30 mL/min per 1.73 m2)Citation37, precluding zoledronic acid useCitation17. Therefore, all analyses compared denosumab to a weighted average of zoledronic acid (92%) and no treatment (8%).
Table 1. Definitions of key terms used in the analysis.
Model design
A Markov model is a mathematical model containing a finite number of mutually exclusive and exhaustive health states of uniform time periods (cycles) in which the probability of movement from one state to another depends on the current state and remains constant over timeCitation25. The Markov model was used to assess the cost-effectiveness of denosumab vs zoledronic acid in patients with prostate, breast, and other solid tumors (including lung tumors) with bone metastases at risk of experiencing bone complications ()Citation25. For the “on treatment” Markov state, there was a risk for bone complications, bone-modifying agent treatment-related adverse events (AEs), and death. For the “off treatment” Markov state, there was a higher risk of bone complications, an unchanged risk of death, and no risk of bone-modifying agent treatment-related AEs.
Figure 1. Markov model used for the analysis. Abbreviations. AE, adverse event; SRE, skeletal-related event.
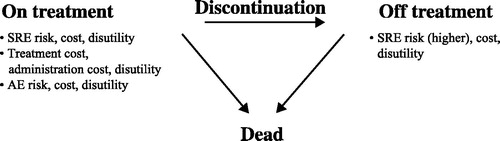
The model cycle length was 28 days (4 weeks), consistent with the labeled dose frequency of the two treatments. Because bone complications occur throughout the remaining lifespan of patients with bone metastases, the time horizon for this cost-effectiveness analysis was a lifetime to capture future major health and economic outcomes. Patient survival was modeled using parametric survival curves derived from the clinical trialsCitation38–40. Overall mean survival was 2.5 years (2.2, 3.7, and 1.7 years for prostate cancer, breast cancer, and other solid tumors, respectively) and median survival was 1.6 years (1.6, 2.8, and 0.9 years, respectively). The average duration of treatment was 1.3 years (1.2, 1.9, and 0.8 years, respectively). A statistically robust methodology was used to extrapolate mortality beyond clinical study durations, and the model was run for 200 cycles (15.33 years), after which more than 99.9% of patients had transitioned to the “dead” Markov health state. This timeline was chosen to model patients until the end of life for the entire cohort.
Model population
Patients with solid tumors and bone metastases at risk of a bone complication were included in the model. Data from the Oncology Services Comprehensive Electronic Records database of patients with solid tumors followed by a bone metastasis diagnosis were used for patient distribution by cancer type; for the model, patients were adults with breast (33%), prostate (26%), or other solid tumors (41% [26% lung; 15% other])Citation41.
Model event parameters
Clinical inputs
Bone complication rates were derived from three published Amgen-sponsored (Amgen Inc., Thousand Oaks, CA) phase 3, randomized controlled clinical trials that directly compared denosumab and zoledronic acid ()Citation38–40. These trials were utilized as similar study methodology was used across the trials simplifying data comparisons, and unpublished data related to the trials were available to inform this analysis. Rates were expressed using person-years at risk and total number of bone complications observed in patients with breast, prostate, and other solid tumors, excluding multiple myelomaCitation25,Citation38–40. The model calculated bone complication costs based on the proportion of each type of bone complication (radiation to bone, surgery to bone, spinal cord compression, and pathologic fracture) that occurred during the phase 3 studiesCitation38–40. The model considered the bone complication distribution from the trial data as a proxy of real-world distribution of bone complicationsCitation5,Citation6,Citation25. Rates of bone complications are higher in real-world studies than those reported in clinical trials because clinical trials typically recruit healthier patients owing to patient eligibility criteria and have higher levels of treatment complianceCitation42,Citation43. Therefore, a real-world adjustment relative-rate ratio of 2.84 was applied to the clinical trial results for both drugs to account for this difference (real-world bone complication rate [0.3167] divided by the clinical trials rate [0.1117])Citation11,Citation44. The bone complication rate for patients with no treatment (1.75) was calculated by dividing the bone complication rate in the zoledronic acid arm by the rate ratio of bone complications in treated and untreated patientsCitation45,Citation46.
Table 2. Adjusted bone complication rates used as model inputs for the base case analysis.
Overall survival and treatment discontinuation rates
Overall survival was not significantly different between the two treatment groups in the denosumab solid tumor randomized controlled trials (ST-RCTs). Generalized gamma functions were fitted using mortality data pooled across both treatment arms for each tumor type to extrapolate mortality beyond the trial follow-up timeCitation25. Treatment discontinuation rates were calculated using rates from the three ST-RCTsCitation38–40.
Adverse event rates
Rates for clinically and economically important serious AEs (SAEs) were incorporated into the model and included osteonecrosis of the jaw (ONJ), acute phase reactions, hypocalcemia, and renal toxicity using rates from an integrated safety analysis for all three ST-RCTsCitation19.
Model utilities
Utility is the preference of a patient for a particular health outcome or health stateCitation33. Utility decrements assign a numeric value from 0–1 to a health state that allows quantification of that state or eventCitation33. In this analysis, utility decrements were due to bone complications, SAEs (as above), and mode of drug administration (subcutaneous injections vs intravenous infusions; )Citation25,Citation38–40,Citation47,Citation48. Bone complication and mode of administration utility values were derived from a general population sample participating in a utility study to establish the value that participants assigned to their quality-of-life by hypothetically varying life expectancies at different health statesCitation47. Our study used utility decrements from eight health states determined previously that were averaged from the four types of bone complications: spinal cord compression (two health states: with and without paralysis), pathologic fracture (three health states: rib [mild fracture], arm [moderate], and leg [severe]), radiation to bone (two health states: administered in two appointments and administered daily for 2 weeks), and surgery performed to stabilize bone (one health state)Citation47.
Table 3. Baseline utility and utility decrements.
Results from the utility study were also used for the assessment of the utility decrement associated with receiving a subcutaneous injection or intravenous infusion for bone complication prevention (e.g. denosumab or zoledronic acid)Citation47. The SAE utility decrements were based on analyses performed using a regression model by pooling data across solid tumor trials ()Citation25,Citation38–40,Citation47,Citation48.
Costs
The model takes a societal perspective focused on three main cost categories: direct medical, direct non-medical, and indirect costs. Direct medical costs included drug acquisition, drug administration, SAEs, and SRE management (hospital, outpatient, long-term care and hospice, strong opioid use, emergency department visits, physical therapy, and skilled nursing facility); direct non-medical costs included caregiver time and driving/parking time to attend medical appointments; and indirect costs included short-term disability and productivity loss (Citation8,Citation16,Citation25,Citation46–53). The cost year for the analysis was 2017.
Table 4. Summary of all cost inputs.
Drug acquisition costs were based on the average sale price (ASP) per dose (denosumab, $1,928; generic zoledronic acid, $45) from the Centers for Medicare & Medicaid Services (3rd quarter 2017)Citation53. Drug wholesale acquisition costs per dose (denosumab, $2,155; branded zoledronic acid, $922) in 2017 were used for an alternative scenario analysis to reflect the pricing of different clinics or pharmacies (). The administered doses were denosumab 120 mg every 4 weeks and zoledronic acid 4 mg every 4 weeks. The costs of drug administration for subcutaneous injection and intravenous infusion (including an additional renal monitoring fee for each zoledronic acid administration) were taken from the original modelCitation25.
The inpatient and outpatient costs associated with managing bone complications are summarized in . Costs were reported as incremental cost per patient per month, and adjustments were made for the number of bone complications per year. The costs of bone complication-related emergency department visits were calculated based on a study evaluating the costs of treating patients with vs without bone complications among patients with multiple myelomaCitation51. Adjustments were made to account for the number of bone complications during the follow-up period to estimate the cost per bone complication. The bone complication hospice cost inputs were calculated based on a study by Jayasekera et al.Citation49, and long-term care cost inputs were based on an internal analysis of the MarketScan® (Truven Health Analytics, Ann Arbor, MI, USA) claims databaseCitation48, as were bone complication, physical therapy, and device costs. Other bone complication-related costs, such as the use of skilled nursing facilities, strong opioid use, caregiver burden, and short-term disability and productivity loss, were calculated based on multiple sources, and are described in . All values were adjusted for inflation by multiplying the cost by the Consumer Price Index for Medical Care (March 2017).
The societal perspective analysis included all of the above costs, whereas the payer perspective included only direct costs. Because economic evaluations compare costs and outcomes over time, we discounted costs by 3% to take into account how the costs and outcomes are valued over timeCitation54.
Cost-effectiveness analysis
The cost-effectiveness of denosumab was calculated primarily in terms of the cost per QALY, dividing the difference in total cost (ΔC) between denosumab and zoledronic acid by the difference in health outcomes (ΔE). The difference in health outcomes was measured in QALYs between denosumab and zoledronic acid; therefore, cost per QALY = ΔC/ΔE.
The NMB for a drug is used to help understand the benefits of a drug when a newer therapy provides greater advantage over older ones at a greater costCitation55. To help define the NMB of denosumab, we applied a WTP threshold for health gains, reflecting the maximum amount that society is willing to pay for one additional QALY gained. The threshold used was $150,000, consistent with previous reportsCitation56. The NMB was calculated as follows: [(Outcomesdenosumab – Outcomeszoledronic acid) × WTP] − (Costdenosumab – Costzoledronic acid). Cost-effectiveness and NMB analyses were conducted from societal and direct-cost (payer) perspectives. For the societal analysis, 35% of patients were assumed to be eligible for short-term disability and productivity loss based on the proportion of patients assumed to be aged <65 years and, therefore, eligible to be employed full-time while being treatedCitation41.
Sensitivity and scenario analyses
Sensitivity analyses were conducted to evaluate potential uncertainty surrounding variability in the underlying modelCitation32. A number of one-way deterministic and probabilistic sensitivity analyses were conducted for the societal perspective analysis only. For all sensitivity analyses, the 95% CI relevant to the point estimate for the base case was used. If these data were unavailable, an assumption of 50% variation was considered. Parameters examined in sensitivity analyses included discount rates, time horizon, rates of renal impairment, compliance, bone complications, AEs, and mortality; treatment discontinuation; costs of administration, bone complications, and AEs; and utilities (baseline, administration, bone complication decrement, and AE decrement).
Scenario analyses are “what ifs” in which various situations are examined, providing cost information on best- and worst-case scenariosCitation34. Scenario analyses were conducted for the societal perspective analysis only and examined changes in assumptions to the annual bone complication rates, the prices of denosumab and zoledronic acid (based on wholesale acquisition cost), the disutility of ONJ, and the percentage of patients considered to be working while being treated (0% vs 35% in the base case).
Results
Base case: societal and payer perspectives
The base case results for the cost-utility analysis are presented in . From a societal perspective, based on ASP, use of denosumab instead of zoledronic acid resulted in an incremental cost of $9,043 and an incremental benefit of 0.128 QALYs, with a lifetime cost per QALY of $70,730 and an NMB of $10,135 in favor of denosumab (; . Incremental costs per QALY by tumor type were $18,480 for prostate, $101,228 for breast, and $94,635 for other solid tumors; corresponding NMB values were $22,716, $8,147, and $3,758. The incremental cost per bone complication avoided was $12,022 (weighted by prevalence of each tumor type). The incremental costs per bone complication avoided by tumor type were $17,720 for breast, $3,180 for prostate, and $14,933 for other solid tumors.
Figure 2. Net monetary benefit of denosumab vs ZA in patients with solid tumors from (A) societal and (B) payer perspectives. Cost differences were aggregated to provide a summary of the economic model results. Abbreviations. ASP, average sale price; IV, intravenous; SRE, skeletal-related event; ZA, zoledronic acid.
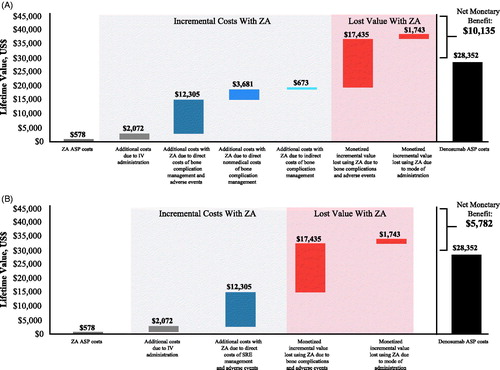
Table 5. Net monetary benefit and incremental cost-effectiveness results from societal and payer perspectives.
From the payer perspective, based on ASP, denosumab use was associated with an incremental cost of $13,396 and an incremental benefit of 0.128 QALYs for a cost of $104,778 per QALY and an NMB of $5,782 in favor of denosumab (; .
When only the combined total direct medical costs from the payer perspective were considered, the costs were $136,820 for denosumab and $123,423 for zoledronic acid, a cost difference of 10.9% in favor of zoledronic acid (). The combined total direct medical, direct non-medical, and indirect costs were $175,115 for denosumab and $166,072 for zoledronic acid, a cost difference of 5.4% in favor of zoledronic acid ().
Table 6. Total costs and cost differences between denosumab and zoledronic acid.
Sensitivity analyses
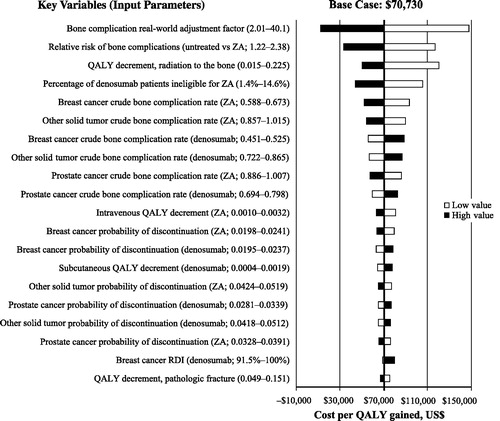
The univariate deterministic sensitivity analysis showed that the model results were relatively stable over a range of values for key variables, and the cost per QALY remained below the $150,000 threshold. The most influential parameters were the bone complication real-world adjustment factor, the rate ratio on bone complication rates for untreated patients, and the QALY decrement for radiation to the bone.
Probabilistic multivariate sensitivity analyses showed that, at a WTP threshold of $150,000, the probability of denosumab being cost-effective vs zoledronic acid was more than 74.2% from the societal perspective and 63.1% from the payer perspective. The top parameters modifying the cost per QALY are shown in .
Figure 3. Tornado diagram showing results of a one-way deterministic sensitivity analysis of key variables. The length of each bar represents the effects of individual parameter variation, with those parameters having the greatest effect at the top and those with the smallest effect at the bottom. Input parameters were varied according to the 95% CI boundaries or by 10% if 95% CIs were not available. The vertical line depicts the base case incremental cost-effectiveness ratio of the model. Black bars represent high value and white bars represent low value for cost per QALY gained. Abbreviations. QALY, quality-adjusted life-year; RDI, relative dose intensity; ZA, zoledronic acid.
Scenario analyses
Scenario analyses were performed for the societal perspective. Based on the wholesale acquisition cost, the same analysis resulted in an incremental cost of $8,740 per QALY gained. Adjustment of crude annual bone complication rates using the adjustment factor of 2.01, as described by Hatoum et al.Citation42, instead of the base case factor of 2.84Citation11, resulted in a cost per QALY of $148,223. For the scenario analysis of ONJ, in which the QALY decrement is greater compared with base case (0.024, 0.024, and 0.030 for breast, prostate, and other solid tumors, respectively, assuming renal toxicity is doubled), the ICER is $70,793 (vs base case $70,730) and the NMB is $10,118 (vs base case $10,135). The effect on base case results of considering greater disutility values for ONJ was <1% on both the ICER and the NMB.
In the scenario analysis in which 0% of patients are working, the ICER is $75,991 (vs base case $70,730) and NMB is $9,462 (vs base case $10,135; a 7% difference from base case on the ICER and the NMB).
Discussion
This analysis examined the economic value provided by denosumab vs zoledronic acid in patients with solid tumors using direct medical and non-medical as well as indirect costs accrued over a patient’s lifetime, and examined the effect on QALYs at a WTP threshold of $150,000/QALY. Although denosumab has a higher direct-acquisition cost, our results showed that its superior clinical profile prevents a greater number of SREs, which results in lower direct medical, non-medical, and indirect costs, thus providing additional benefits to the patient and society. The cost-effectiveness of denosumab compared with zoledronic acid is below the $150,000 WTP threshold used in this analysis, and less than half the $300,000 WTP threshold often used for cost-effectiveness analysis of oncology drugs and treatmentsCitation56–58. Scenario analyses supported the robustness of the model, sensitivity analysis results were also relatively stable over a range of values for key variables, and the cost per QALY for denosumab remained below accepted thresholds. Indeed, the key driver in the cost-effectiveness analysis was the number of bone complications prevented, for which denosumab is superior to zoledronic acid. The cost of zoledronic acid was not a key driver. Accordingly, when the cost of zoledronic acid was reduced to one-third its current cost in a sensitivity analysis, denosumab was still cost-effective ($104,400/QALY).
The present model extends our previously published analysis by adding the societal perspective and including the consideration for today’s costs and administration practices for zoledronic acidCitation25. Notably, although the rate of bone complications remained consistent, the cost per bone complication was higher than the previous model, reflecting the additional costs of bone complications from a societal perspective compared with the previous model. Previous studies assessing the cost-effectiveness of denosumab vs zoledronic acid for the prevention of bone complications in patients with solid tumors have provided mixed conclusions because of the differing methods and model inputs used for those analysesCitation21–28. Although the majority of those analyses reported that the use of denosumab vs zoledronic acid in patients with solid tumors resulted in fewer bone complications and increased QALYs, some indicated that ICERs were above the WTP thresholds of $150,000/QALY and $300,000/QALYCitation27,Citation28. Direct comparison among the analyses is difficult because of the different model designs and input data. However, an important difference between this analysis and previous analyses is the bone complication rate–adjustment factor used in our modelCitation59. The models used in previous analyses minimized the number of bone complications because they did not account for the difference between real-world vs clinical trial rates of bone complications (real-world rates are higher because clinical trials typically include healthier patients and have higher levels of treatment compliance), which would subsequently minimize the health benefits and cost offsets of denosumab vs zoledronic acid. The influence of this factor was tested in a sensitivity analysis (), with the value of 2.01 (from Stopeck et al.Citation25 and based on Hatoum et al.Citation42) used as the lower boundary, resulting in a WTP threshold below $150,000/QALY.
In the present analysis, we found a high level of variability in the cost per QALY for each tumor type. The number of bone complications avoided over a lifetime was higher for prostate and breast cancers compared with other solid tumors, leading to a higher difference in QALY and higher cost offsets due to the prevention of bone complications. Furthermore, the treatment duration was longer for breast cancer compared with prostate cancer or other solid tumors, leading to the higher difference in drug acquisition costs. Combining these two factors led to a significantly higher NMB for prostate cancer compared with breast cancer or other solid tumors. Furthermore, the reduction in long-term disability and increase in productivity in patients with metastatic prostate cancer and breast cancer are likely to be associated with clinically meaningful advantages for the patients who avoid bone-related complications. Although the utility decrements attempt to capture the quality-of-life impact associated with SAEs and bone complications, these are averages based on a survey of healthy patients and could under-estimate the utility decrement, particularly in more serious cases of ONJ or fracture. Given the currently evolving understanding of the risk factors associated with the development of ONJ, including the potential differences between the risks associated with denosumab and zoledronic acidCitation60,Citation61, data from clinical trials (rather than real-world data) were used to inform the risk of development of ONJ.
This analysis attempts to provide a holistic comparison of costs from both societal and direct-cost–only perspectives over a lifetime, an important distinction from previous analyses. Denosumab has greater efficacy compared with zoledronic acid, resulting in fewer bone complications and, therefore, lower overall bone complication management costs as well as reduced administration costs associated with easier administration. The value of denosumab is associated with greater QALYs, consistent with previous studiesCitation27,Citation28. However, denosumab has direct drug acquisition costs that are ∼40-fold greater than zoledronic acid. Nevertheless, given the efficacy and administration profile and its positive NMB despite higher direct acquisition costs, denosumab may be preferred over zoledronic acid, particularly by the individual patient.
This study has several strengths. First, input data were taken from three well-designed, large, double-blind, randomized controlled trials. Furthermore, the model incorporates direct non-medical and indirect costs that are typically not considered in other analyses, which provides a more practical view of the value of innovative drugs such as denosumab. This model also bridges the gap between the randomized controlled trials and the real world by accounting for important characteristics in the population, such as renal impairment, route of drug administration, and costs and burden for patients and caregivers. For this analysis, best modeling practices have been applied, and clinically and statistically motivated assumptions have been made as much as possibleCitation62. Finally, sensitivity analyses were used to test the robustness of the results.
This study also has several limitations. The results should be interpreted within the context of the data inputs and modeling assumptions used because the results may be sensitive to changes in these inputs. First, although the three trials used to inform input data were all well-designed published randomized controlled trials, all three were sponsored by Amgen Inc. Furthermore, several inputs, such as utility decrements, had limited information from either clinical or real-world studies and, therefore, may have been over-estimated or under-estimated (e.g. vertebral compression fractures are often asymptomatic and may not be fully reflected in the bone complication utility decrements). Although statistically robust methodology was used to extrapolate mortality rate beyond the clinical study durations to reflect a time horizon of 15 years, this is not guaranteed to accurately reflect actual outcomes. The bone complication costs in patients with other solid tumors were assumed to be the average of the costs for breast and prostate cancers. Furthermore, costs of health services were estimated from multiple sources that varied by tumor type, patient population, country, and other parameters, and some costs were assumed to be the same across tumor types. For example, the cost of emergency department visits for bone complications was determined from a study of patients with multiple myeloma, even though this patient group was excluded from the analysis. Furthermore, based on the source data, some costs (e.g. physical therapy and devices) were input as an incremental per patient per month value and some (e.g. parking and driving costs) were input as a single value per bone complication episode. Finally, this model did not include results from recent studies demonstrating the non-inferior efficacy of zoledronic acid treatment every 12 weeks compared with every 4 weeks in patients with breast, prostate, or other solid tumors and bone metastasesCitation63–65. However, decreasing the costs associated with zoledronic acid therapy by up to two-thirds did not diminish the cost-effectiveness of denosumab, which remained below the $150,000/QALY threshold.
Conclusions
In conclusion, this analysis demonstrates that, compared with zoledronic acid, denosumab is a cost-effective treatment option for the prevention of bone complications in patients with solid tumors. When taking all costs into account, the available evidence indicates that denosumab would remain cost-effective under a variety of scenarios and in different patient populations, providing value to patients, payers, and society.
Transparency
Declaration of funding
This study was funded by Amgen Inc.
Declaration of financial/other interests
AS has received grants and personal fees from Amgen Inc., consulting fees from Novartis, AstraZeneca, and Biothera, and speaking honoraria from Genomic Health; AB has received personal fees from Amgen Inc. and Novartis; LK has received personal fees from Amgen Inc.; SB, DB, JB, ND, and GH are employees and stockholders of Amgen Inc. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgements
The authors acknowledge Miranda Tradewell and Rick Davis (Complete Healthcare Communications, LLC, an ICON plc company, North Wales, PA), whose work was funded by Amgen Inc. (Thousand Oaks, CA), for medical writing assistance in the preparation of this manuscript, and Aurelien Jamotte (Amgen Inc., Zug, Switzerland) for modeling assistance in the preparation of this manuscript.
References
- Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9:14–27.
- Body JJ, Quinn G, Talbot S, et al. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit Rev Oncol Hematol. 2017;115:67–80.
- McDougall JA, Bansal A, Goulart BH, et al. The clinical and economic impacts of skeletal-related events among Medicare enrollees with prostate cancer metastatic to bone. Oncologist. 2016;21:320–326.
- Oster G, Lamerato L, Glass AG, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer. 2013;21:3279–3286.
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res Treat. 2012;131:231–238.
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis. 2011;14:177–183.
- Coleman R, Body JJ, Aapro M, et al. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25:iii124–iii37.
- von Moos R, Body JJ, Egerdie B, et al. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support Care Cancer. 2016;24:1327–1337.
- von Moos R, Body JJ, Egerdie B, et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer. 2013;21:3497–3507.
- Patrick DL, Cleeland CS, von Moos R, et al. Pain outcomes in patients with bone metastases from advanced cancer: assessment and management with bone-targeting agents. Support Care Cancer. 2015;23:1157–1168.
- Hechmati G, Cure S, Gouepo A, et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ. 2013;16:691–700.
- Zhong Y, Valderrama A, Yao J, et al. Economic evaluation of treating skeletal-related events among prostate cancer patients. Value Health. 2018;21:304–309.
- Body JJ, Chevalier P, Gunther O, et al. The economic burden associated with skeletal-related events in patients with bone metastases secondary to solid tumors in Belgium. J Med Econ. 2013;16:539–546.
- Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17:223–230.
- Hagiwara M, Delea TE, Saville MW, et al. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:23–27.
- Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. JMCP. 2010;16:693–702.
- Newland A, Godeau B, Priego V, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 2016;172:262–273.
- Body JJ, Facon T, Coleman RE, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228.
- Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–3092.
- Van Poznak C, Somerfield MR, Moy B. Role of bone-modifying agents in metastatic breast cancer: an American Society of Clinical Oncology-Cancer Care Ontario focused guideline update summary. JOP. 2017;13:822–824.
- Koo K, Lam K, Mittmann N, et al. Comparing cost-effectiveness analyses of denosumab versus zoledronic acid for the treatment of bone metastases. Support Care Cancer. 2013;21:1785–1791.
- Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17:1–386.
- Dellis A, Papatsoris A. Cost-effectiveness of denosumab as a bone protective agent for patients with castration resistant prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2016;16:5–10.
- Shapiro CL, Moriarty JP, Dusetzina S, et al. Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases: CALGB 70604 (Alliance). JCO. 2017;35:3949–3955.
- Stopeck A, Rader M, Henry D, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. 2012;15:712–723.
- Xie J, Diener M, Sorg R, et al. Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clin Breast Cancer. 2012;12:247–258.
- Snedecor SJ, Carter JA, Kaura S, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J Med Econ. 2013;16:19–29.
- Snedecor SJ, Carter JA, Kaura S, et al. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther. 2012;34:1334–1349.
- Garrison LP, Jr, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017;20:213–216.
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734.
- York Health Economics Consortium. A glossary of health economic terms. 2016 [cited 2018 Jul 19]. Available from: http://www.yhec.co.uk/tools-resources/glossary/.
- Adalsteinsson E, Toumi M. Benefits of probabilistic sensitivity analysis – a review of NICE decisions. J Mark Access Health Policy. 2013;1[doi: 10.3402/jmahp.v1i0.21240].
- Ara R, Wailoo A. Using health state utility values in models exploring the cost-effectiveness of health technologies. Value Health. 2012;15:971–974.
- Simoens S. Health economic assessment: a methodological primer. IJERPH. 2009;6:2950–2966.
- University of Maryland School of Pharmacy. Transferability of economic evaluation studies: is there a generally accepted alternative price benchmark to the WAC price? 2017 [cited 2018 May 23]. Available from: https://faculty.rx.umaryland.edu/jslejko/files/2016/05/ISPOR-2017-W14-Part-1.pdf.
- Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641.
- Arellano J, Hernandez RK, Wade SW, et al. Prevalence of renal impairment and use of nephrotoxic agents among patients with bone metastases from solid tumors in the United States. Cancer Med. 2015;4:713–720.
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822.
- Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132.
- Qian Y, Bhowmik D, Kachru N, et al. Longitudinal patterns of bone-targeted agent use among patients with solid tumors and bone metastases in the United States. Support Care Cancer. 2017;25:1845–1851.
- Hatoum HT, Lin SJ, Smith MR, et al. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer. 2008;113:1438–1445.
- Data on File. Study 20060392 (STARS). Thousand Oaks (CA): Amgen Inc; 2017.
- Cristino J, Finek J, Jandova P, et al. Cost-effectiveness of denosumab versus zoledronic acid for preventing skeletal-related events in the Czech Republic. J Med Econ. 2017;20:799–812.
- Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database of Systematic Reviews. 2005;(3):CD003474.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468.
- Matza LS, Chung K, Van Brunt K, et al. Health state utilities for skeletal-related events secondary to bone metastases. Eur J Health Econ. 2014;15:7–18.
- Data on File. Thousand Oaks (CA): Amgen Inc.; 2017.
- Jayasekera J, Onukwugha E, Bikov K, et al. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics. 2014;32:173–191.
- Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–396.
- Nash Smyth E, Conti I, Wooldridge JE, et al. Frequency of skeletal-related events and associated healthcare resource use and costs in US patients with multiple myeloma. J Med Econ. 2016;19:477–486.
- Qian DW, Bhowmik D, Kachru N. Utilization patterns of bone-targeting agents among patients with multiple myeloma: analysis of real-world data. Blood. 2015;126:4501.
- Centers for Medicare & Medicaid Services. Payment allowance limits for Medicare Part B drugs. Quarter 3. 2017 [cited 2017 Nov 9]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2017ASPFiles.html.
- Cancer Research Economics Support Team. Discounting in economic evaluations in health care: a brief review. 2015 [cited 2018 May 23]. Available from: http://www.crest.uts.edu.au/pdfs/FactSheet_Discounting.pdf.
- Tan MC, Regier DA, Esdaile JM, et al. Health economic evaluation: a primer for the practicing rheumatologist. Arthritis Rheum. 2006;55:648–656.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797.
- Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356.
- Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11:90–95.
- Kennedy L, Bhatta S, Hechmati G, et al. Response to cost-effectiveness analysis of zoledronic acid once per month, zoledronic acid once every 3 months, and denosumab once per month in women with breast cancer and skeletal metastases. JCO. 2018;36:1051.
- Nicolatou-Galitis O, Schiodt M, Mendes RA, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127:117–135.
- Otto S, Pautke C, Van den Wyngaert T, et al. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. 2018;69:177–187.
- Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II–an ISPOR Good Research Practices Task Force report. Value Health. 2015;18:161–172.
- Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14:663–670.
- Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317:48–58.
- Hortobagyi GN, Zheng M. Zoledronic acid dosing in patients with metastatic breast cancer–reply. JAMA Oncol. 2018;4:586.
- Xie J, Namjoshi M, Wu EQ, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm. 2011;17:621–643.
- Bell MJ, Miller JD, Namjoshi M. Comparative budget impact of formulary inclusion of zoledronic acid and denosumab for prevention of skeletal-related events in patients with bone metastases. Presented at: International Society For Pharmacoeconomics and Outcomes Research 16th Annual Meeting; 2011 May 21–25; Baltimore, MD.
- Gridelli C, Ferrara C, Guerriero C, et al. Informal caregiving burden in advanced non-small cell lung cancer: the HABIT study. J Thorac Oncol. 2007;2:475–480.
