Abstract
Aim: To evaluate the relative cost-effectiveness of using rivaroxaban vs apixaban for the initial treatment plus extended prevention of venous thromboembolism (VTE) in the UK. Extended prevention was assessed using a 10-mg rivaroxaban dose, as the 20-mg dose has already been evaluated.
Methods: A Markov model compared the health outcomes and costs of treating VTE patient cohorts with either rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily for 6 months, then extended prevention with 10 mg once daily) or apixaban (10 mg twice daily for 1 week, followed by 5 mg twice daily for 6 months, then extended prevention with 2.5 mg twice daily) over a lifetime horizon. The model included an initial acute treatment and prevention phase (0–6 months) and an extended prevention phase (6–18 months). Efficacy and safety data were derived from two network meta-analyses. Reference treatment comparators were derived from the EINSTEIN-Pooled study and EINSTEIN-CHOICE trial. Healthcare costs and utility data were derived from published literature.
Results: The rivaroxaban regimen was associated with increased quality-adjusted life years (QALYs) and slightly lower total costs compared with apixaban over a lifetime horizon. Deterministic and probabilistic sensitivity analyses demonstrated that rivaroxaban remained a cost-effective alternative to apixaban over a wide range of parameters. Incremental cost-effectiveness ratio estimates were below the £20,000 per QALY threshold in 74.1% of 2,000 model simulations. Scenario analyses further supported that rivaroxaban is a cost-effective alternative to apixaban.
Limitations: Clinical and safety inputs were derived from network meta-analysis, which are subject to inherent limitations whereby small differences between study designs may severely impact efficacy and safety outcomes. Furthermore, these inputs were based on data from clinical trials, which may not reflect real-world data.
Conclusions: Rivaroxaban was associated with a slightly lower total cost and increased QALYs compared with apixaban for VTE management in the UK over a lifetime horizon.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE) is a common disorder, affecting ∼1–3 people per 1,000 per year in Western EuropeCitation1. VTE has a substantial burden on patients and healthcare systems due to its high mortality, especially among hospital patients, and considerable morbidity in terms of recurrent VTE (rVTE) and long-term complications such as post-thrombotic syndrome (PTS) and chronic thromboembolic pulmonary hypertension (CTEPH)Citation2,Citation3.
Patients who experience a symptomatic VTE event remain at high risk of recurrence despite initial anticoagulation therapyCitation4,Citation5. The risk of recurrence varies with time from the initial event, but is highest in the first 6–12 months, independent of initial treatment durationCitation4–7. The cumulative incidence of rVTE is reported to be as high as 17.5% after 2 years and 30.3% after 8 years from the initial event with the risk higher among patients with unprovoked VTE compared with provoked VTE (28.4% vs 20.5% when assessed over a 10-year period)Citation7,Citation8. PTS is an important potential complication following DVT. Patients who experience ipsilateral recurrent DVT (rDVT) have an ∼6-fold increase in their risk of developing PTS when compared to patients without recurrenceCitation8,Citation9. Patients who develop PTS have an ∼3-fold higher risk of rVTE than patients without PTSCitation9.
The economic impact of treating VTE and its associated complications is considerableCitation10. In the UK, the total direct and indirect costs of managing VTE was estimated to be ∼£640 million in 2005Citation11. Reducing the risk of rVTE and its complications could help alleviate the substantial health burden to patients with VTE and consequential resource burden on the UK healthcare system.
Treatment of VTE generally consists of three phases: the initial acute treatment phase to stop the thrombotic process, an intermediate prevention phase to reduce the risk of rVTE, and an extended prevention phase for the secondary prevention of rVTECitation12. An option for initial treatment of VTE and extended prevention of recurrences, as recommended by the National Institute for Health and Care Excellence (NICE), is initial treatment with heparins or fondaparinux, overlapping with vitamin K antagonists (VKAs) followed by continued treatment with VKAs for a duration determined by assessment of risks and benefitsCitation13,Citation14. However, treatment with VKAs is challenging, with its slow onset of action and multiple drug and food interactions necessitating the use of routine coagulation monitoring and frequent dose adjustmentCitation15. Furthermore, prolonged VKA treatment is associated with a relatively high risk of major bleedingCitation16,Citation17.
More recently, direct oral anticoagulants (DOACs) have become available for the management of thromboembolic disorders including the initial treatment of VTE and extended prevention of recurrences. DOACs including rivaroxaban, apixaban, edoxaban, and dabigatran are currently recommended by NICE for this indicationCitation13. DOACs offer a simple practical alternative to VKAs, achieving a faster onset of action in adults using single fixed-dosing regimens without the need for continual monitoring. Consequently, these DOACs allow VTE to be increasingly managed in an outpatient settingCitation18. Of all anticoagulants prescribed in the UK in 2015 for VTE (based on an analyses of GP practices using The Health Improvement Network [THIN] database), DOACs accounted for 47% of those prescribed for initial VTE treatment (within 30 days of a VTE event) and 45% of those prescribed for extended prevention of rVTE (after 1 year following a VTE event)Citation19. Of these DOAC prescriptions, rivaroxaban was the most commonly prescribed (92.5% of initial treatments and 76.8% of extended preventions) followed by apixaban (6.6% of initial treatments and 21.6% of extended preventions) and dabigatran (0.8% of initial treatments and 1.6% of extended preventions)Citation19.
Rivaroxaban is an oral direct factor Xa inhibitor, proven to be clinically-effective in the initial treatment of VTE and extended prevention of recurrences through a series of phase III randomized controlled trials (RCTs): EINSTEIN-DVTCitation20, EINSTEIN-PECitation21, EINSTEIN-EXTCitation20, and EINSTEIN-CHOICECitation22.
The EINSTEIN-Pooled study used data from the EINSTEIN-DVT and EINSTEIN-PE trials to compare rivaroxaban (15 mg twice-daily [bd] for 3 weeks followed by 20 mg once-daily [od]) with standard therapy (enoxaparin 1.0 mg/kg bd and VKA) for up to 12 months of treatment in patients with acute symptomatic VTECitation23. Compared to standard therapy, rivaroxaban was associated with a significantly lower rate of major bleeding and a non-inferior reduction in symptomatic rVTE. Both the EINSTEIN-EXT and the EINSTEIN-CHOICE trials evaluated treatment for the extended prevention of rVTE in patients with symptomatic VTE who had previously completed 6–12 months of anticoagulation therapy and were in equipoise regarding the need for continued anticoagulation. The EINSTEIN-EXT trial compared rivaroxaban 20 mg od with placebo and the EINSTEIN-CHOICE trial compared rivaroxaban (20 mg od or 10 mg od) with aspirin 100 mg od. Rivaroxaban 20 mg od significantly reduced the risk of rVTE compared to placebo with an acceptable risk-to-benefit profile. Compared to aspirin, both the 20 mg od and 10 mg od doses of rivaroxaban significantly reduced the risk of rVTE, and the rates of major bleeding were low and similarCitation20,Citation22.
Rivaroxaban 15 mg bd for 3 weeks followed by 20 mg od (dosing regimen hereon referred to as 15 mg bd/20 mg od) has been recommended as a cost-effective option for the initial treatment of VTE and prevention of rVTE by NICECitation24,Citation25 and the Scottish Medicines Consortium (SMC)Citation26,Citation27. Since the EINSTEIN-CHOICE trial found that a 10 mg od rivaroxaban dose had similar clinical benefits to the 20 mg od dose when used for extended preventionCitation22, inclusion of this dose in the license provides clinicians with more choice when planning individualized patient treatments regimens. The license recommends the lower dose of 10 mg od for the extended prevention of recurrences, but a 20 mg od dose should be considered for those patients judged to have a high risk of recurrences, such as those with complicated comorbidities, or who have developed rDVT or rPE on extended prevention with rivaroxaban 10 mg odCitation28. Although the clinical benefits of the 10 mg od dose have been established, the cost-effectiveness of this option has yet to be evaluated.
Since apixaban is the second most prescribed DOAC in the UK for this indicationCitation19, it was considered the most appropriate comparator treatment to rivaroxaban for a cost-effectiveness analysis. Like rivaroxaban, apixaban is an oral direct factor Xa inhibitor, which was proven to be clinically-effective in the initial treatment of VTE and extended prevention of recurrences through two phase III RCTs: AMPLIFYCitation29 and AMPLIFY-EXTCitation30.
The AMPLIFY trial compared apixaban (10 mg bd for 1 week followed by 5 mg bd) with standard therapy (enoxaparin 1 mg/kg bd and warfarin) over 6 months of treatment in patients with acute symptomatic VTECitation29. Compared to standard therapy, apixaban was associated with a significantly lower rate of major bleeding and a non-inferior risk of symptomatic rVTE or death related to VTE. The AMPLIFY-EXT trial compared two different doses of apixaban (2.5 mg bd and 5 mg bd) with placebo over 12 months of treatment to evaluate the extended prevention of rVTE in patients with symptomatic VTE who had previously completed 6–12 months of anticoagulation therapy and were in equipoise regarding the need for continued anticoagulationCitation30. Compared to placebo, the risk of rVTE or death related to VTE was significantly lower in patients treated with apixaban (2.5 mg bd or 5 mg bd) and the rates of major bleeding were low and similar.
Apixaban 10 mg bd for 1 week, then 5 mg bd for 6 months, followed by extended prevention with 2.5 mg bd (dosing hereon referred to as 10 mg bd/5 mg bd/2.5 mg bd) has been recommended for the initial treatment of VTE and prevention of rVTE by NICECitation31 and the SMCCitation32.
The objective of this study was to evaluate the relative cost-effectiveness of rivaroxaban (15 mg bd for 3 weeks, then 20 mg od for 6 months, followed by extended prevention with 10 mg od [hereon referred to as 15 mg bd/20 mg od/10 mg od]) vs apixaban (10 mg bd/5 mg bd/2.5 mg bd) for the initial treatment of VTE and extended prevention of rVTE from the UK National Health Service (NHS) and Personal Social Services (PSS) perspective.
Methods
Model overview
Rivaroxaban (15 mg bd/20 mg od) has been recommended as a cost-effective option for the initial treatment of VTE and prevention of rVTE by NICECitation24,Citation25 and the SMCCitation26,Citation27. In this study, the initial acute treatment and prevention regimen remained the same as those used in previous evaluations. However, in order to represent the overall management of the disease, the model included both the initial acute treatment and prevention regimen and the extended prevention regimen to reflect the full treatment pathway. This approach was in-line with the SMC and NICE evaluations for apixabanCitation31,Citation32.
The cost-effectiveness analysis was conducted assuming a UK NHS and PSS perspective over a lifetime horizon (40 years). In the absence of head-to-head comparative trials of rivaroxaban vs apixaban, clinical efficacy and safety data used to inform the cost-effectiveness analysis was derived from two network meta-analyses (NMAs). NMA 1 provided data on the initial acute treatment of VTE and prevention of rVTE, and NMA 2 provided data on the extended prevention of rVTE.
The primary clinical outcome of the analysis was quality-adjusted life years (QALYs). The cost-effectiveness of rivaroxaban relative to apixaban was assessed using an incremental cost-effectiveness ratio (ICER), expressed as the incremental cost per QALY. A willingness-to-pay threshold was set at £20,000 per QALY based on the cost-effectiveness threshold set by NICECitation33.
Population
The model cohort was based on the study populations of patients with symptomatic DVT and/or PE from the EINSTEIN-Pooled study and EINSTEIN-CHOICE clinical trialCitation22,Citation23. The mean patient age at model entry was set to 57. Patient age was tracked throughout the model, which allowed the use of age-dependent mortality inputs and, thus, all-cause mortality was accurately accounted for.
Model structure
The cost-effectiveness analysis was conducted using a de novo Markov model built using Microsoft Excel 2016. The model structure included two phases, the initial acute treatment and prevention phase (0–6 months) and the extended prevention phase (6–18 months). The initial acute treatment and prevention phase was fixed to a duration of 6 months to reflect the usual duration of treatment in UK practiceCitation24,Citation25 and the majority of trial endpoints included in NMA 1. In the base-case, the extended prevention phase was modeled for a period of 12 months, again to reflect the trial endpoints included in NMA 2. Once the full 18-month initial acute treatment and prevention and extended prevention period ended, patients remained off-treatment for their lifetime unless they experience a rVTE. The model used a cycle length of 3 months.
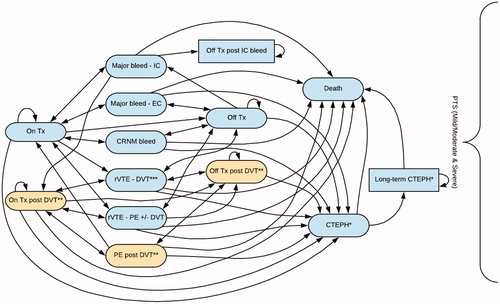
For the initial acute treatment and prevention phase of the model, a total of 16 health states describing the management and complications of all therapy and rVTE events 0–6 months after the index event were used (). Patients entered the model after their index VTE event to an “on-treatment” state in which they received treatment with either rivaroxaban (15 mg bd/20 mg od) or apixaban (10 mg bd/5 mg bd).
Figure 1. Model structure of the initial acute treatment and prevention phase. *Patients could only transition to this health state if their index event was a PE or if they had experienced a rPE. **Patients could only transition to this health state if they had an index PE (i.e. PE only heath state). ***This health state included both ipsilateral and contralateral DVT. Note: The model separated the two types of DVT into separate health states. Abbreviations. CTEPH, chronic thromboembolic pulmonary hypertension; DVT, deep vein thrombosis; EC, extracranial; IC, intracranial; PE, pulmonary embolism; PTS, post-thrombotic syndrome; rVTE, recurrent venous thromboembolism; Tx, treatment.
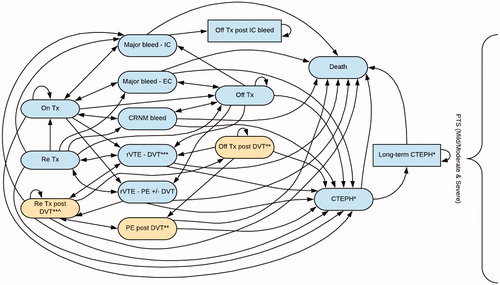
For the extended prevention phase of the model, a total of 17 health states describing the management and complications of all therapy and rVTE events once extended prevention commenced were used ().
Figure 2. Model structure of the extended prevention phase. *Patients could only transition to this health state if their index event was a PE or if they had experienced a rPE. **Patients could only transition to this health state if they had an index PE (i.e. PE only heath state). ***This health state included both ipsilateral and contralateral DVT. ^This health state included retreatment from the 1st DVT and subsequent DVT. Note: The model separated re-treatment post-DVT into two separate health states based on the two types of DVT. Abbreviations. CTEPH, chronic thromboembolic pulmonary hypertension; DVT, deep vein thrombosis; EC, extracranial; IC, intracranial; PE, pulmonary embolism; PTS, post-thrombotic syndrome; rVTE, recurrent venous thromboembolism; Tx, treatment.
After having received anticoagulation treatment for at least 6 months following an index VTE event, patients entered the extended prevention phase of the model to an “on-treatment” state where they received treatment with either 10 mg od rivaroxaban or 2.5 mg bd apixaban.
During each model cycle, patients either remained in their current on-treatment state, moved to an event state (major intracranial [IC] bleed, major extracranial [EC] bleed, clinically relevant non-major [CRNM] bleed, rVTE-DVT, rVTE-PE with or without DVT, or PE post-DVT), transitioned to an off-treatment state, entered a CTEPH state, or died. Each health state was associated with particular resource use and utility weighting expressed as a QALY.
Transition probabilities governing a patient’s progression between health states were derived from the NMAs. Patients who transitioned to the off-treatment state remained there until they experienced another event. Patients in a VTE-related event state remained there for one cycle before transitioning to the acute re-treatment health state to restart the treatment process. Patients could only transition to the CTEPH state if they had experienced a PE event.
The impact of PTS in patients who had an index DVT event was modeled by applying relevant costs and consequences to all states within the model. However, since patients with an index DVT had a higher risk of PTS than patients who had an index PECitation34, health states exclusively for index PE patients were included (on-treatment post DVT, off-treatment post DVT, re-treatment post-DVT, and PE post-DVT). The model assumes the same impact for rDVT events.
Although the extended prevention phase was modeled for a period of 12 months in the base-case, different treatment durations ranging from 3–6 months could be assessed. Estimates of costs, outcomes beyond the 12-month extended prevention period, was extrapolated from the published literature. Following treatment discontinuation, patients were transferred to an “off-treatment” health state in the model at which point they were exposed to a risk of rVTE.
Model inputs
Clinical efficacy and safety data
Clinical efficacy and safety data used in the model was derived from two NMAs (network diagrams can be found in the Supplementary Materials). The NMAs examined the efficacy and safety of a number of anticoagulants used for the initial acute treatment of VTE and prevention of rVTE (NMA 1) and the extended prevention of rVTE (NMA 2) and were informed from a systematic literature review (SLR) of RCTsCitation35. NMA 1 consisted of 5 studies: EINSTEIN-PooledCitation23, AMPLIFYCitation29, RE-COVERCitation36, RE-COVER IICitation37, and HOKUSAI-VTECitation38. NMA 2 consisted of 13 studies: EINSTEIN-EXTCitation39, EINSTEIN-CHOICECitation22, RE-MEDYCitation40, RE-SONATECitation40, AMPLIFY-EXTCitation30, ASPIRECitation41, ELATECitation42, LAFITCitation43, PREVENTCitation44, WARFASACitation45, WODIT-DVTCitation46, WODIT-PECitation47, and PADIS-PECitation48. The NMAs were conducted with Bayesian analysis software using WinBUGS version 1.4.3. The extent to which the included studies were subject to effect modification was assessed to help inform both the modeling approach and interpretation of results post-analysis. Study level effect modifiers included differences in patient population, differences in outcome definition, and differences in study design. For both NMAs, the network did not contain enough evidence to accurately estimate a random-effects model and, therefore, a fixed-effects model was used. Efficacy and safety outcomes derived from the NMAs and used in the model included DVT, PE, rVTE, IC bleed, EC bleed, CRNM bleed, VTE-related death, and treatment discontinuation. In the extended prevention phase, limited available evidence on IC or EC bleeds meant these outcomes could not be accurately estimated. Consequently, these bleeds were classified together as a major bleed and its nature (whether IC or EC) was assumed to be the same, regardless of treatment. The probability of IC bleeds was assumed to be 21.4%, and the remainder assumed to be EC bleeds based on a pooled analysis of randomized trialsCitation49. This assumption was consistent with the NICE apixaban submissionCitation31.
VKA data from the EINSTEIN-Pooled study served as the reference treatment comparator for the initial acute treatment and prevention phase of the modelCitation23 and acetylsalicylic acid (ASA) data from the EINSTEIN-CHOICE trial was the reference treatment comparator in the extended prevention phase of the modelCitation22. To estimate the treatment effects for rivaroxaban and apixaban, the relative risks of each efficacy and safety outcome derived from the NMAs were applied to the baseline probabilities for VKA for the initial acute treatment and prevention phase, and for ASA for the extended prevention phase. The resultant relative risks for each event during both phases are presented in . In the base-case analysis, the distribution of bleed events was dependent on the treatment arm.
Table 1. Relative risks of clinical events in the initial acute treatment and prevention phase and extended prevention phase used in the base-case analysis.Table Footnotea
Long-term complications
An SLR was conducted to identify trial-based observational studies that provided evidence on rates of long-term complicationsCitation35. Risks of long-term complications such as PTS, rVTE, and CTEPH were derived from the published literature identified by the SLR and incorporated into the cost-effectiveness model. The values obtained from the literature used to calculate the risks applied to the model are shown in .
Table 2. Long-term complication values used to estimate risk.
Although PTS is an important potential complication following DVT, there is no evidence to indicate that the risk of PTS changes with treatment. Therefore, it was not expected to be a key driver of the results, but was included in the model to ensure that the overall burden of disease was accurately represented. Thus, the impact of PTS on patients who had an index DVT event or a rDVT was modeled by applying the relevant costs and consequences to all states within the model. This approach ensured that all patients diagnosed with PTS were not precluded from the risk of alternate events. Furthermore, it was assumed that the presence of PTS did not impact upon treatment of rVTEs, i.e. patients remained on treatment if PTS was diagnosed during the rVTE treatment period. Data from a 1997 study by Prandoni et al.Citation50 was used to determine the probability of acquiring PTS (). The probability of a PTS diagnosis was differentiated for up to 1 year and beyond to account for the greater incidence of PTS in the first year following a VTE. This allowed for differentiation of costs between the first year and subsequent years. The risk of PTS between the treatment arms was considered equal. The impact of PTS associated with rVTE was modeled, which was achieved through the inclusion of a post-DVT state. PTS patients were considered to represent 29.6% of patients within this post-DVT state, which was differentiated into mild/moderate PTS and severe PTSCitation50. Furthermore, it was estimated that 58.8% of rDVT would be ipsilateral and that patients with an ipsilateral rDVT were at an increased risk of PTS compared to patients experiencing a contralateral eventCitation50. This increased risk was estimated and applied to the model ().
The risk of rVTE was determined using data from the 2007 study by Prandoni et al.Citation5 (). To account for the change in the risk of rVTE over time, the probability of a recurrent event over a 10-year follow-up (39.9%) was selected and used to calculate the 1.26% 3-month probability that was applied to the model. No limit was applied to the time over which patients were exposed to the risk of rVTE.
Patients who had an index PE event or a rPE were at risk of CTEPH. The probability of CTEPH was calculated using the 1.25% 2-year probability data from Miniati et al.Citation51 (). From this a 3-month probability of 0.16% was derived and applied to all states of the model for 2 years. Patients who experienced a rPE were exposed to a further 1.25% risk of developing CTEPH within a 3-month cycle periodCitation51. Since pulmonary thromboendarterectomy (PTE) is considered the standard treatment for CTEPH, the probability of having this treatment was estimated using data from Mayer et al.Citation52, and was included in the model to capture the additional cost associated with this treatment.
Mortality
Health state-related mortality was modeled for rPE events, major bleeds, and CTEPH. Minor bleeds, PTS, and rDVT events were assumed to not be associated with case fatalities. All event-related mortality rates are presented in .
Table 3. Event-related mortality estimates for model inputs.Table Footnotea
For rPE events, a distinction was made between on- and off-treatment PE related mortality. For the on-treatment population, overall trial mortalities were derived for each treatment arm from the EINSTEIN-Pooled study (for the initial acute treatment and prevention phase) and EINSTEIN-CHOICE trial (for the extended prevention phase). For the base-case analysis, the mortality rate was identically applied to each treatment. An off-treatment PE related mortality rate (when patients were off-treatment post event or after treatment discontinuation) of 33.1% was derived from Prandoni et al.Citation5.
The risk of mortality due to IC and EC bleeding events for VTE patients was derived from Linkins et al.Citation49.
The model did not distinguish survival of CTEPH patients by the treatment they received for CTEPH. All CTEPH patients were exposed to the long-term risk of mortalityCitation53. A 3-month mortality risk of 2.48% (95% confidence interval [CI] = 2.05–2.93%), was derived from Condliffe et al.Citation53.
Utilities
Each modeled health state was associated with a utility weight to estimate QALYs within the model. Patients with VTE entered the model with a baseline utility of 0.825, based on the UK population norm valueCitation54. Utility values associated with VTE events were derived from the published literature, identified by the SLR (). A treatment-specific disutility was not included for either rivaroxaban or apixaban.
Table 4. Utility values.Table Footnotea
Health benefits were discounted at a rate of 3.5% per annum in line with current Her Majesty’s (HM) Treasury guidanceCitation59.
Costs and resource use
Costs and resource use were broken down into those associated with the index event, the treatment period, treatment discontinuations, and with rVTE.
Costs associated with the index event were included as acute treatment during the initial acute treatment and prevention phase within the model. All costs associated with treatment and prevention were applied to the on-treatment health states for as long as treatment continued.
Patients who suffered an rVTE while on treatment were assumed to undergo an initial period of acute therapy similar to current American College of Chest Physicians (ACCP) guidelinesCitation60. Although the guidelines recommend acute therapy with low molecular weight heparin (LMWH), acute therapy with rivaroxaban or apixaban was used in this model. This meant that patients previously treated for extended prevention with the reduced dose (10 mg od rivaroxaban or 2.5 mg bd apixaban) received the acute dose (15 mg bd rivaroxaban for 3 weeks or 10 mg bd apixaban for 1 week) followed by the therapeutic dose (20 mg od rivaroxaban or 5 mg bd apixaban) until 6 months after the recurrent event, at which point they received reduced-dose preventative therapy again. For rVTEs, acute treatment was broken down into inpatient and outpatient treatment components. In the event of an rVTE, the cost of an inpatient or outpatient visit was applied in addition to the resource use associated with acute treatment and any other treatment costs within the cycle in which the rVTE occurs (e.g. cost of preventative therapy once it was re-initiated) (). For patients with a DVT it was assumed that 80% were treated as outpatients and 20% were treated as inpatients, whereas for patients with a PE, 10% were treated as outpatients and 90% were treated as inpatientsCitation66.
Table 5. Cost inputs.Table Footnotea
Unit and daily costs were taken from relevant published sources (). Drug costs and doses were based on UK 2017 list prices obtained from the British National Formulary (BNF)Citation61. Inpatient and outpatient treatments were taken from NHS Reference Costs 2015/16Citation62. Neither treatment option incurred monitoring costs. Outpatient treatment costs were applied as either a composite cost or as the sum of the costs associated with multiple outpatient treatment components. In the base-case analyses, outpatient treatment costs were estimated based on the cost of a combination of the treatment items listed in .
Future costs and health benefits were discounted at a rate of 3.5% per annum in line with current HM Treasury guidanceCitation59.
Treatment discontinuation
The relative risks of treatment discontinuation were derived from the NMAs and were relative to VKA for the initial acute treatment and prevention phase, and ASA for the extended prevention phase. Index event bleed-specific discontinuation rates were taken from the EINSTEIN-Pooled study and EINSTEIN-CHOICE trialCitation22,Citation23. Patients with an EC or CRNM bleed were assumed to discontinue treatment for 1 month only, after which they incurred treatment costs for the remainder of the cycle. All patients discontinued treatment permanently following an IC bleed, regardless of index event type.
Sensitivity and scenario analyses
A series of deterministic one-way sensitivity analyses were undertaken to assess the impact of uncertainty around key model inputs. The one-way analyses were conducted over a lifetime horizon and used ICER (QALY base) as the outcome of interest. All resource, risk, and cost input parameter values were varied one at a time from the lower and upper bounds of their 95% CIs to show the impact of each variable on the model results. A variation of 30% was applied to parameters where the range was not available from a relevant source. The impact of the top ten drivers on the model results (ICERs) is presented in the form of a tornado diagram.
Probabilistic sensitivity analysis, conducted to assess overall uncertainty in the economic model, was implemented by assigning a probability distribution to key input parameters to represent the uncertainty around its mean base-case value. Probability of events, mortality rates, treatment discontinuation, utilities, and discounting rates were varied according to beta distributions; rates of events and costs and resource use were varied according to gamma distributions; and relative risks of events were varied according to a log-normal distribution. One value was randomly sampled from each parameter distribution and the process repeated 2,000 times. The resulting outputs were presented graphically on a cost-effectiveness plane and transformed into a cost-effectiveness acceptability curve. From this, we estimated the likelihood of rivaroxaban being cost-effective compared with apixaban at a willingness-to-pay threshold of £20,000 per QALY.
Scenario analyses were performed to test the effect of changing the duration of the extended prevention period (3 months and 6 months), setting the probability of bleeds to a combined figure for both treatment arms (based on the overall probability of bleeds in the trial; in other words, assuming that the probability of bleeding events are independent of treatment), setting the probability of bleeds and VTE events to be treatment-specific (assuming that the probability of both bleeding and VTE events are treatment dependent), setting the on-treatment mortality to be treatment specific, and applying a 5-year time horizon.
Results
Base-case analysis
Base-case results predicted that initial acute treatment and prevention plus extended prevention with rivaroxaban (15 mg bd/20 mg od/10 mg od) was associated with a slightly lower total cost and increased QALYs compared with apixaban (10 mg bd/5 mg bd/2.5 mg bd) over a lifetime horizon ().
Table 6. Base-case results of the cost-effectiveness of rivaroxaban (15 mg bd/20 mg od/10 mg od) vs apixaban (10 mg bd/5 mg bd/2.5 mg bd), per patient over a lifetime horizon.
The slightly higher QALYs observed in the rivaroxaban arm were driven by the lower rates of mortality, rVTEs, and PTS/CTEPH compared with the apixaban arm.
Sensitivity analyses
The one-way sensitivity analysis results showed that initial acute treatment and prevention plus extended prevention with rivaroxaban (15 mg bd/20 mg od/10 mg od) remained a cost-effective option across a wide range of parameters, with either dominance over apixaban (10 mg bd/5 mg bd/2.5 mg bd) or an ICER below the willingness-to-pay threshold of £20,000 per QALY shown for most parameters. The only exception to this was the probability of a major bleed in the first 3 months of the extended prevention phase, whereby the ICER was close to £35,000 when the upper 95% CI was applied ().
Figure 3. One-way sensitivity analysis tornado diagram for rivaroxaban (15 mg bd/20 mg od/10 mg od) vs apixaban (10 mg bd/5 mg bd/2.5 mg bd), (ICER, QALY based). Abbreviations. Apix, apixaban; bd, twice-daily; CIs, confidence intervals; DVT, deep-vein thrombosis; HR, hazard ratio; IC, intracranial; ICER, incremental cost-effectiveness ratio; od, once-daily; OWSA, one-way sensitivity analysis; PE, pulmonary embolism; QALY, quality-adjusted life year; Riva, rivaroxaban; RR, relative risk; rVTE, recurrent venous thromboembolism; VKA, vitamin K antagonist.

The ICER was particularly sensitive to the relative risk of an IC bleed when treated with rivaroxaban during the extended prevention phase, the probability of a major bleed within the first 3 months of the extended prevention phase, and the relative risk of a PE event when treated with either apixaban in the extended prevention phase or rivaroxaban in the initial acute treatment and prevention phase.
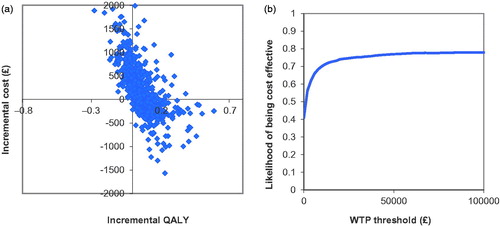
The probabilistic sensitivity analysis found that, for most of the 2,000 simulations, rivaroxaban (15 mg bd/20 mg od/10 mg od) showed either dominance compared with apixaban (10 mg bd/5 mg bd/2.5 mg bd), or an ICER below the £20,000 per QALY gained willingness-to-pay threshold (. The cost-effectiveness acceptability curve indicated that rivaroxaban (15 mg bd/20 mg od/10 mg od) is the cost-effective option compared with apixaban (10 mg bd/5 mg bd/2.5 mg bd), with a 74.1% likelihood of cost-effectiveness at a willingness-to-pay threshold of £20,000 per QALY (.
Figure 4. (a) Cost-effectiveness plane, rivaroxaban (15 mg bd/20 mg od/10 mg od) vs apixaban (10 mg bd/5 mg bd/2.5 mg bd), (lifetime horizon, QALY outcome). (b) Cost-effectiveness acceptability curve, rivaroxaban (15 mg bd/20 mg od/10 mg od) vs apixaban (10 mg bd/5 mg bd/2.5 mg bd), (lifetime horizon, QALY outcome).
Scenario analysis
Results of the scenario analysis presented in show that rivaroxaban (15 mg bd/20 mg od/10 mg od) was the dominant or cost-effective treatment option when the extended prevention phase was set to 3 months and 6 months. When the probability of bleeds was assumed independent of treatment the resulting ICER became £81, still significantly under the £20,000 willingness-to-pay threshold. Rivaroxaban (15 mg bd/20 mg od/10 mg od) remained dominant when the probability of bleeds and VTE events were treatment-specific, when the mortality rate whilst receiving treatment was treatment-specific, and when a 5-year time horizon was applied.
Table 7. Scenario analyses.
Discussion
To our knowledge, this is the first study to explore the cost-effectiveness of rivaroxaban compared with apixaban for both the initial acute treatment and prevention of VTE and the extended prevention of rVTE from a UK perspective. Our model found that rivaroxaban (15 mg bd/20 mg od/10 mg od) was associated with improved QALYs at a slightly lower total healthcare cost than apixaban (10 mg bd/5 mg bd/2.5 mg bd) over a lifetime. The NMAs showed that, compared to apixaban, treatment with rivaroxaban was associated with lower recurrent events and long-term complications. Rivaroxaban (15 mg bd/20 mg od/10 mg od) was either dominant or cost-effective over a wide range of parameters in the one-way sensitivity analyses. The cost-effectiveness of rivaroxaban (15 mg bd/20 mg od/10 mg od) relative to apixaban (10 mg bd/5 mg bd/2.5 mg bd) was further supported by a probabilistic sensitivity analysis where ICER estimates were below the £20,000 per QALY threshold in 74.1% of model simulations. The ICER was particularly sensitive to variations in IC bleeds, major bleeds, and the risk of PE, reflecting the considerable health impact and associated costs of these conditions. However, only when the upper 95% CI was applied to the risk of a major bleed in the first 3 months of the extended prevention phase did the ICER exceed the willingness-to-pay threshold.
Our scenario analysis further demonstrated the economic value of rivaroxaban (15 mg bd/20 mg od/10 mg od) whereby, even under varying treatment durations, rivaroxaban (15 mg bd/20 mg od/10 mg od) was dominant or cost-effective compared to apixaban (10 mg bd/5 mg bd/2.5 mg bd).
There are numerous published studies comparing the cost-effectiveness of rivaroxaban or apixaban with the standard VTE treatment of LMWH and VKA. These consistently show that rivaroxaban and apixaban are cost-effective treatments for VTE compared with standard LMWH/VKA therapyCitation67–73. However, only one study was found that compared the cost-effectiveness of different DOACsCitation74. The study by Lanitis et al.Citation74 evaluated the cost-effectiveness of apixaban vs rivaroxaban (and other anticoagulants) for treatment of VTE. Their UK study, exploring cost-effectiveness over the initial 6-month VTE treatment period, predicted that treatment with apixaban would result in fewer rVTE and bleeding events than treatment with rivaroxaban and, consequently, apixaban was found to be dominant vs rivaroxaban. Similarly, we found treatment with rivaroxaban would result in a greater risk of EC and CRNM bleeds compared with apixaban during the initial 6-month treatment phase, however, we also found that the risks of rPE events and IC bleeds would be lower for rivaroxaban vs apixaban during this period. Notably, Lanitis et al.Citation74 does not distinguish between recurrent PE or DVT events, nor between types of major bleeds (IC or EC).
Furthermore, in the absence of a head-to-head trial of rivaroxaban vs apixaban, clinical efficacy and safety inputs rely on conclusions drawn from indirect comparisons using NMAs. It is important when comparing anticoagulant trials to consider the effect of differences in trial design on outcomes. When Beyer-Westendorf et al.Citation75 compared the treatment duration and patient selection criteria of the AMPLIFY trial and EINSTEIN-Pooled study, which were used to compare reference treatments both in our study and that of Lanitis et al.Citation74, they found that modest differences in study design could substantially impact study outcomes. They suggest that had the treatment duration and exclusion criteria from the AMPLIFY trial been applied in the EINSTEIN-Pooled study, then rivaroxaban would have had a significantly reduced risk of rVTE and major bleeding compared with LMWH followed by VKA. Applying this adjustment may result in lower rivaroxaban risk ratios applied to the models, although the extent of this effect is not known.
The EINSTEIN-CHOICE trial shows that extended preventative treatment with either 20 mg od or 10 mg od rivaroxaban is effective at reducing the risk of recurrent VTE events when compared to ASACitation22. With previous economic evaluations already proving that the 20 mg od dose is a cost-effective treatment option for this indicationCitation24–27, this study provides validity that the rivaroxaban 10 mg od dose is also a cost-effective option. Furthermore, the addition of a 10 mg dose provides clinicians with more choice to individualize treatment regimens of patients with VTE.
As with any economic evaluation, there are several limitations to our study. First, since clinical and safety outcomes were derived from clinical trial settings, they may not reflect real-world observations.
Second, in the absence of head-to-head data, clinical efficacy and safety inputs were derived from NMAs, which are subject to inherent limitations, as indicated by Beyer-Westendorf et al.Citation75. Our analysis did not adjust for the key differences in duration of therapy and patient selection between trials that Beyer-Westendorf et al.Citation75 proved would have a significant impact on efficacy and safety outcomes. Had we done so, the cost-effectiveness benefits associated with rivaroxaban may have been higher. Furthermore, we did not control for differences in patient baseline characteristics. For example, 89.8% of patients treated with apixaban in the AMPLIFY trial were reported as having an unprovoked index VTE event compared to 63.1% of patients treated with rivaroxaban from the EINSTEIN-Pooled studyCitation23,Citation29. Since unprovoked VTEs are associated with a higher recurrence rate,Citation12 this difference suggests that, compared to rivaroxaban, a higher proportion of patients treated with apixaban were at increased risk of a recurrence. Our study found that patients treated with rivaroxaban experienced a lower risk of recurrence, so it is unclear if this difference had an impact on outcomes.
Since treatment effects were not adjusted in our model it may be considered more robust with less risk of bias had adjustments been made. Furthermore, this uncertainty and variation surrounding the model inputs from the NMAs and other sources were evaluated in our sensitivity analysis and demonstrated that, despite these uncertainties, most of the conclusions remained the same.
Finally, a simplified approach was used to calculate the risk of PTS, which did not take account of the changing risk of PTS over time or exclusion of PTS costs when patients exited the post-DVT state. However, the additional computational burden and model complexity required to reflect these factors was considered unwarranted due to the minimal difference in DVT events, and hence PTS risk, expected between arms. Furthermore, although most PTS occurs within 1 year of an event, due to the 3-month cycle length the model assumed that PTS occurred 3 months following the event. This assumption led to a small over-estimation of the risk of PTS, which did not meaningfully impact the results of the cost-effectiveness analyses.
Conclusions
Rivaroxaban 10 mg provides a simple once-daily treatment option for the extended prevention of rVTE. This economic evaluation shows that, for the management of VTE, rivaroxaban (15 mg bd/20 mg od/10 mg od) was associated with a slightly lower total cost and increased QALYs compared to apixaban (10 mg bd/5 mg bd/2.5 mg bd) in the base-case and was dominant or cost-effective in the sensitivity analyses. Rivaroxaban (15 mg bd/20 mg od/10 mg od) therefore represents value for money when used to treat VTE and prevent long-term recurrences from the UK NHS and PSS perspective. Further observational studies on real-world data is required to confirm these outcomes.
Transparency
Declaration of funding
This study was sponsored by Bayer, the manufacturer of rivaroxaban.
Declaration of financial/other relationships
JB and US are employees of Bayer Plc. KF is an employee of Bayer AG. SM is an employee of IQVIA, which received funding from Bayer for conducting this study and for the development of this manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data sharing statement
Unpublished data from this study can be made available to editors and reviewers upon request to the corresponding author.
Supplemental Materials
Download MS Excel (68.1 KB)Acknowledgements
Writing assistance in the preparation of this manuscript was provided by Dr Heidi Eriksen of Aandoo Ltd. Support for this assistance was funded by IQVIA.
References
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134:931–938.
- Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764.
- Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin. 2010;26:2465–2473.
- van Dongen CJ, Vink R, Hutten BA, et al. The incidence of recurrent venous thromboembolism after treatment with vitamin K antagonists in relation to time since first event: a meta-analysis. Arch Intern Med. 2003;163:1285–1293.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199–205.
- Heit JA. Predicting the risk of venous thromboembolism recurrence. Am J Hematol. 2012;87(Suppl 1):S63–S67.
- Martinez C, Cohen AT, Bamber L, et al. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost. 2014;112:255–263.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7.
- Stain M, Schonauer V, Minar E, et al. The post-thrombotic syndrome: risk factors and impact on the course of thrombotic disease. J Thromb Haemost. 2005;3:2671–2676.
- Ruppert A, Steinle T, Lees M. Economic burden of venous thromboembolism: a systematic review. J Med Econ. 2011;14:65–74.
- House of Commons Health Committee. The Prevention of Venous Thromboembolism in Hospitalised Patients. Second Report of Session 2004-05. 2005 [cited 2018 13 June]. Available from: https://publications.parliament.uk/pa/cm200405/cmselect/cmhealth/99/99.pdf.
- Barnes GD, Kanthi Y, Froehlich JB. Venous thromboembolism: Predicting recurrence and the need for extended anticoagulation. Vasc Med. 2015;20:143–152.
- National Institute for Health and Care Excellence. Nice Pathways: Treating Venous thromboembolism[cited 2018 30 May]. Available from: https://pathways.nice.org.uk/pathways/venous-thromboembolism.
- National Institute for Health and Care Excellence. Clinical guideline: Venous thromboembolic diseases: diagnosis, management and thrombophilia testing thrombophilia testing. 2012. [cited 2018 13 June]. Available from: https://www.nice.org.uk/guidance/cg144.
- Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl):e44S–e88S.
- Hutten BA, Prins MH. Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst Rev. 2000;3:CD001367.
- Klok FA, Kooiman J, Huisman MV, et al. Predicting anticoagulant-related bleeding in patients with venous thromboembolism: a clinically oriented review. Eur Respir J. 2015;45:201–210.
- Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206–232.
- IQVIA. Audit of anticoagulantion usage in the UK in 2015. Data on File 2018.
- Einstein Investigators, Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510.
- Einstein-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297.
- Weitz JI, Lensing AW, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–1222.
- Prins MH, Lensing AW, Bauersachs R, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thrombosis J. 2013;11:21.
- National Institute for Health and Care Excellence. Final appraisal determintion - Rivaroxaban for treating pulmonary embolism and preventing recurrent venous thromboembolism. 2013. [cited 2018 June 13]. Available from: https://www.nice.org.uk/guidance/ta287/documents/pulmonary-embolism-acute-treatment-vte-prevention-rivaroxaban-final-appraisal-determination-document2.
- National Institute for Health and Care Excellence. Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism, Technology appraisal guidance [TA261]. 2012. [cited 2018 June 13]. Available from: https://www.nice.org.uk/guidance/ta261/chapter/2-The-technology.
- Scottish Medicines Consortium. Rivaroxaban 15 and 20mg film-coated tablets (Xarelto®) SMC No. (755/12) 2012 [cited 2018 June13]. Available from: https://www.scottishmedicines.org.uk/files/advice/rivaroxaban_Xarelto_for_DVT_FINAL_Jan_2012_for_website.pdf.
- Scottish Medicines Consortium. Rivaroxaban 15mg and 20mg film-coated tablets (Xarelto®) SMC No. (852/13). 2013 [cited 2018 June13]. Available from: https://www.scottishmedicines.org.uk/files/advice/rivaroxaban_Xarelto_FINAL_FEBRUARY_2013_amended_04.03.13_for_website.pdf.
- Bayer X. 10 mg film-coated tablets Summary of Product Characteristics 2018. [cited 2018 September 03]. Available from: https://www.medicines.org.uk/emc/product/6402/smpc.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808.
- Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708.
- National Institute for Health and Care Excellence. Final appraisal determination -Apixaban for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism. 2015. [cited 2018 June 13]. Available from: https://www.nice.org.uk/guidance/ta341/documents/deep-vein-thrombosis-pulmonary-embolism-treatment-secondary-prevention-apixaban-id726-final-appraisal-determination-document2.
- Scottish Medicines Consortium. Apixaban, 2.5 mg & 5mg, film-coated tablets (Eliquis®) SMC No. (1029/15). 2015 [cited 2018 September 03]. Available from: https://www.scottishmedicines.org.uk/files/advice/apixaban_Eliquis_FINAL_February_2015_Revised_020315_for_website_Revised_120315.pdf.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26:733–744.
- Bova C, Rossi V, Ricchio R, et al. Incidence of post-thrombotic syndrome in patients with previous pulmonary embolism. A retrospective cohort study. Thromb Haemost. 2004;92:993–996.
- IQVIA. Systematic Literature Review of Randomized Control Trials. Data on File 2017.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352.
- Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–772.
- Hokusai V, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415.
- Romualdi E, Donadini MP, Ageno W. Oral rivaroxaban after symptomatic venous thromboembolism: the continued treatment study (EINSTEIN-extension study). Expert Rev Cardiovasc Ther. 2011;9:841–844.
- Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–718.
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–1987.
- Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631–639.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–907.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348:1425–1434.
- Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–1967.
- Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med. 2001;345:165–169.
- Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139:19–25.
- Couturaud F, Sanchez O, Pernod G, et al. Six months vs extended oral anticoagulation after a first episode of pulmonary embolism: the PADIS-PE randomized clinical trial. JAMA. 2015;314:31–40.
- Linkins L, O’Donnell M, Julian JA, et al. Intracranial and fatal bleeding according to indication for long-term oral anticoagulant therapy. J Thromb Haemost. 2010;8:2201–2207.
- Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica. 1997;82:423–428.
- Miniati M, Monti S, Bottai M, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine. 2006;85:253–262.
- Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710.
- Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1122–1127.
- Kind P, Dolan P, Gudex C, et al. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–741.
- Locadia M, Bossuyt PM, Stalmeier PF, et al. Treatment of venous thromboembolism with vitamin K antagonists: patients' health state valuations and treatment preferences. Thromb Haemost. 2004;92:1336–1341.
- Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc. 1997;4:49–56.
- Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making. 2010;30:341–354.
- Meads DM, McKenna SP, Doughty N, et al. The responsiveness and validity of the CAMPHOR Utility Index. Eur Respir J. 2008;32:1513–1519.
- HM Treasury. The Green Book: Central Government Guidance on Appraisal and Evaluation. 2018 [cited 2018 04 June]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/685903/The_Green_Book.pdf.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–352.
- National Institute for Health and Care Excellence. British National Formulary. 2017. [cited 2018 13 June]. Available from: https://bnf.nice.org.uk/.
- NHS Department of Health. Reference costs guidance 2015-16. 2016. [cited 2018 June 13]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/497127/Reference_costs_guidance_2015-16.pdf.
- NHS Department of Health. Reference Costs Publication 2009-2010. 2011. [cited 2018 13 June]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216305/dh_123501.pdf.
- Curtis L, Burns A. Unit Costs of Health and Social Care. 2015. [cited 13 June]. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2015/.
- National Clinical Guideline Centre. Venous Thromboembolism: Reducing the Risk of Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Hospital. London: National Institute for Health and Clinical Excellence: Guidance; 2010.
- Bayer. VTE-PE model report v4.0. Data on file. 2012.
- Bamber L, Muston D, McLeod E, et al. Cost-effectiveness analysis of treatment of venous thromboembolism with rivaroxaban compared with combined low molecular weight heparin/vitamin K antagonist. Thromb J. 2015;13:20.
- de Jong LA, Dvortsin E, Janssen KJ, et al. Cost-effectiveness analysis for apixaban in the acute treatment and prevention of venous thromboembolism in the Netherlands. Clin Ther. 2017;39:288–302 e4.
- Elias I, Oyaguez I, Alvarez-Sala LA, et al. Cost-effectiveness analysis of apixaban compared to low-molecularweight heparins and vitamin k antagonists for treatment and secondary prevention of venous thromboembolism. Farm Hosp. 2016;40:187–208.
- Gourzoulidis G, Kourlaba G, Kakisis J, et al. Cost-effectiveness analysis of rivaroxaban for treatment of deep vein thrombosis and pulmonary embolism in Greece. Clin Drug Investig. 2017;37:833–844.
- Heisen M, Treur MJ, Heemstra HE, et al. Cost-effectiveness analysis of rivaroxaban for treatment and secondary prevention of venous thromboembolism in the Netherlands. J Med Econ. 2017;20:813–824.
- Lanitis T, Leipold R, Hamilton M, et al. Cost-effectiveness of apixaban versus low molecular weight heparin/vitamin k antagonist for the treatment of venous thromboembolism and the prevention of recurrences. BMC Health Serv Res. 2017;17:74.
- Lefebvre P, Coleman CI, Bookhart BK, et al. Cost-effectiveness of rivaroxaban compared with enoxaparin plus a vitamin K antagonist for the treatment of venous thromboembolism. J Med Econ. 2014;17:52–64.
- Lanitis T, Leipold R, Hamilton M, et al. Cost-effectiveness of apixaban versus other oral anticoagulants for the initial treatment of venous thromboembolism and prevention of recurrence. Clin Ther. 2016;38:478–493 e1.16.
- Beyer-Westendorf J, Lensing AW, Arya R, et al. Choosing wisely: The impact of patient selection on efficacy and safety outcomes in the EINSTEIN-DVT/PE and AMPLIFY trials. Thromb Res. 2017;149:29–37.
