?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims: The objective of the study is to evaluate the cost-effectiveness of percutaneous mitral valve repair (TMVr) with the MitraClip NT system (MitraClip procedure) for patients with symptomatic severe mitral regurgitation (MR) at high surgical risk in line with the methodological guideline for cost-effectiveness evaluation by the Ministry of Health, Labour and Welfare.
Material and Methods: The cost-effectiveness of MitraClip procedure was evaluated using a Markov model. Patients are classified into four New York Heart Association classes in each cycle. The model considered MitraClip complication (“major vascular complication”, “major bleeding complication”, “non-cerebral thromboembolism”), adverse events, re-implantation with MitraClip device, mitral valve surgery, and congestive heart failure hospitalization. For the evidence on additional benefits, a study compared with propensity score-matched medical therapy group was used in the analysis. The analysis was conducted from the perspective of a public healthcare payer with a discount rate of 2% for both cost and effectiveness.
Results: In the base-case analysis, total cost and quality-adjusted life year (QALY) gained (Life year (LY) gained) were 7,541,151 JPY and 3.23 QALYs (3.85 LYs) for MitraClip group, and 4,699,692 JPY and 1.79 QALYs (2.43 LYs) for medical therapy group, respectively. The incremental cost-effectiveness ratio (ICER) of MitraClip procedure versus medical therapy was 1.97 million JPY/QALY (US$18,570/QALY, US$1 = 106 JPY), which was evaluated to be cost-effective. The probability of ICER of MitraClip procedure versus medical therapy being 5 million JPY/QALY was 96.7%.
Limitations: There are two limitations. Firstly, the parameters for the comparators were based on some assumptions. However, it was a conservative setting against MitraClip group. Secondary, the mortality rate and adverse events of MitraClip group in a lifetime were estimated from data during a year after the procedure.
Conclusions: MitraClip procedure improved life-years and quality of life in patients at high surgical risk and it was also a cost-effective treatment option.
Introduction
Mitral regurgitation (MR) occurs when the heart’s mitral valve leaflets do not coapt or close properly, allowing the backward flow of blood from the left ventricle (LV) into the left atrium (LA) during systole, decreasing forward flow into the aorta and the systemic circulation. There is inadequate coaptation of the mitral valve leaflets, resulting in regurgitation of blood from the LV into the LA during systole that leads to LV volume overload, reduction in LV afterload, and increase in LV pressure. Severe acute MR presents with cardiogenic shock secondary to pulmonary congestion and lower cardiac output associated with rapid increase in LV volume load. Severe chronic MR is asymptomatic for a while but, over time, LV dilatation occurs followed by remarkable pulmonary congestion and worsened LV ejection fraction (EF). The majority of patients present with chronic MR, and their quality of life (QOL) is worsened as they experience heart failure (HF) symptoms. If worsened, the condition can lead to chronic HF and ultimately, stroke, atrial fibrillation or sudden death.
The management of MR will vary based on the onset and severity of symptoms, the severity of MR, and the degree of LV dysfunction. For most patients with mild to moderate MR, medical management is instituted. Oral medications include angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blocker (ARB), or beta-blockers to inhibit the neuroendocrine system in order to slow progression of LV dilatation and worsening of contraction (LV remodeling), and diuretics or anti-aldosterone to improve symptoms associated with congestion. For an acute-on-chronic state, intravenous injection of vasodilators, diuretics or cardiotonic agents are used. Assisted circulation may be required when the pumping function of the heart is significantly worsened and the patient is poorly responsive to medications. However, medical management is not a radical treatment, and MR progresses over time. Patients with symptomatic severe MR or asymptomatic severe MR with evidence of LV dysfunction or dilation are considered candidates for surgery (valve repair or replacement). Despite its effectiveness in many patients, surgery involves significant trauma and risks, including operational complications, physical impairment, and death, some of which are related to the use of cardiopulmonary bypass. Surgical risks increase with specific co-morbidities such as prior cardiothoracic surgery and advanced age. Additionally, many patients experience an extended post-operative recovery period, which can translate into significant post-discharge healthcare costs, and in some cases, re-hospitalizations. According to Flameng et al., the reoccurrence rate of severe MR (3+/4+) is 3.7%Citation1. The complications, including both morbidity and mortality associated with re-operation, are substantially greater than for the initial surgical repair or replacement procedure. Surgery is delayed or not an option (i.e. only medically managed) due to its inherent factors explained above, and severe MR in many patients are considered not effectively treated. The number of patients with valvular disease including MR is increasingCitation2. In addition, the number of patients at increased risk for surgery is increasing as the overall population agesCitation3. Surgery is the only radical treatment option for MR. As explained above, however, there has been no effective treatment option for patients with contraindication for surgery due to advanced age, comorbid condition, or severe HF.
MitraClip NT system (Abbott Vascular Co., Ltd., Tokyo, Japan) (MitraClip procedure) is a system providing a less invasive treatment to mitigate MR with long-term double orifices (two valve orifices) formed by applying the surgically established suture of double-orifice formation (edge-to-edge repair) and joining the anterior leaflet and posterior leaflet of the mitral valve with percutaneously inserted clipCitation4. In Japan, the MitraClip system were reviewed for marketing approval based on the clinical evaluation of the data from the following clinical studies: the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) II randomized controlled trial (RCT), an overseas study which compared MitraClip and surgery in surgical candidates; the EVEREST II High Risk Registry (HRS) and the EVEREST II Real World Expanded Multicenter Study of the MitraClip System (REALISM) Continued Access Study, overseas studies in patients with severe MR (3+/4+) at high surgical risk; the Integrated High Risk Cohort, the pooled analysis of these two studies; the AVJ-514 Japan clinical trial. As result, the review concluded that the percutaneous treatment with the MitraClip system is lower invasive than surgery and beneficial as a novel treatment option for patient with severe symptomatic MR at high surgical risk. Overseas, the MitraClip system has been approved in over 90 countries including the European Union (March 2008) and the United States (October 2013).
As medical technologies advance, the attention to cost-effectiveness of medical technologies has now increased. Actually, the pilot introduction of economic evaluation targeted for new/existing technologies (medical drugs, medical devices) was started in April 2016Citation5. Active discussions on price adjustment using the results of the cost-effectiveness analysis is being conducted in the Ministry of Health, Labour and Welfare (MHLW)Citation6.
The study is a cost-effectiveness analysis of percutaneous mitral valve repair (TMVr) with MitraClip procedure for patients with symptomatic severe MR at high surgical risk in line with the methodological guideline for cost-effectiveness evaluation by the MHLW.
Methods
Overview and model structure
The target population for this analysis was patients with symptomatic severe MR at high surgical risk. The comparator was patients with medical therapy. The analysis was conducted from the perspective of public healthcare payers, and quality-adjusted life year (QALY) was used as an economic outcome. According to the guideline for cost-effectiveness evaluation in Japan (second edition)Citation7, a discount rate of 2% was set in both cost and effectiveness, and lifetime simulation was conducted.
The cost-effectiveness of TMVr with MitraClip procedure was evaluated using a Markov model constructed based on a previous study by Cameron et al.Citation8 The analytical model consists of two states: “alive” and “death”. The analysis starts with patients in the “alive” state. They conduct a TMVr with MitraClip procedure (MitraClip group) or continue medical therapy without any additional intervention (medical therapy group). After the start of analysis, patients in the “alive” state are classified into four New York Heart Association (NYHA) classes and gain a utility value by NYHA class in each cycle. The proportions of the patients with each NYHA class were assumed not to change over times and remain to be constant during lifetime. The cycle length of 1 month was used for the Markov model.
The model considered MitraClip complications (“major vascular complication”, “major bleeding complication”, and “non-cerebral thromboembolism”), adverse events, re-implantation with MitraClip device for failed MitraClip procedure, mitral valve (MV) surgery, and, CHF hospitalization. Costs for those events were calculated. Utility decrement for each event was also considered for re-implantation with MitraClip device, MV surgery, and congestive heart failure (CHF) hospitalization.
Adverse events and CHF hospitalization were considered over a lifetime. MitraClip complications and re-implantation with MitraClip device were considered for 1-month post-procedure. Adverse events included the following 8 events; myocardial infarction (MI), stroke, renal failure, non-elective cardiovascular surgery, mechanical ventilation (≥48 h), gastrointestinal (GI) complication requiring surgery, septicemia, and blood transfusion (≥2 units). The adverse events were considered only in MitraClip group as there was no report on those events for medical therapy group. CHF hospitalization was considered in both groups. MV surgery for 1 year from the start of analysis was considered in both groups. All the incidences of MV surgery occurring in the year were calculated in the first cycle of the analytical model. The analytical model is shown in .
Data source
Base-case analysis
Evidence for additional benefits of MitraClip procedure compared with medical therapy was collected by a systematic literature review (SLR). Among the collected evidence, direct comparison studies or propensity score-matched comparison studies were selected. As a result of the SLR, two studies were selected as candidateCitation9,Citation10. Both of them were propensity score-matched study, the study by Velazquez et al.Citation10 was selected as evidence for base-case analysis because its larger sample size and it included both functional and degenerative MR.
Velazquez et al. pooled the data of two studies on clinical efficacy of MitraClip procedure for patients with symptomatic severe MR (MR severity 3 or 4+) at high surgical risk; EVEREST II HRSCitation11 and REALISMCitation12. With respect to the comparator, patients without procedure were extracted from two databases; Duke Echocardiography Laboratory Database (DELD) and Duke Databank for Cardiovascular Disease (DDCD). Among those, patients with matched background characteristics were selected for the comparator by propensity score-matched using age, sex, a history of MI, stroke, chronic obstructive pulmonary disease, renal failure and diabetes, previous cardiac surgery, NYHA class III/IV and LVEF of both groupsCitation10. There was no report on outcomes other than 30-day and 1-year mortality. Therefore, although the report by Velazquez et al. was used for mortality, other clinical outcomes of MitraClip group were obtained from re-analysis of the same group by Abbott Vascular Japan Co., Ltd.
Although the length of stay (LOS) was 14.4 days in AVJ-514 trialCitation13, as this is the data in clinical trial, it is expected to be shorter in actual clinical practice. By reference to a medical expert’s experience that a clinical-path of hospitalization for MitraClip procedure was the same as for percutaneous coronary angioplasty (PCI) surgery in the United States, 5.8 days, which was the average LOS of Diagnosis Procedure Combination (DPC) hospitalization for “Percutaneous coronary angioplasty” (K 546) in Japan, was used for the LOS of hospitalization for MitraClip procedureCitation14,Citation15. The average age of target population in AVJ-514 trial (80 years old) tended to be older because one of the eligibility criteria for the trial was the Society of Thoracic Surgeons (STS) score ≥8%.Citation13 In the actual indication, there is no criterion for STS score ≥8% and the average age is considered to be lower. Therefore, in the base-case analysis, the initial age was set to 74 years old as the average age reported by Velazquez et al.Citation10
Scenario analysis
An analysis using clinical evidence used in a paper of cost-effectiveness analysis of MitraClip procedure published by Cameron et al.Citation8 was conducted as scenario analysis.
Cameron et al. used the results of EVERST II HRS, the study on the clinical efficacy of MitraClip procedure for patients with symptomatic severe MR (MR severity 3 or 4+) at high surgical risk. Regarding the comparator, patients without procedure were retrospectively reviewed after recruiting the patients with severe MR at high surgical risk for the trial described above. Mortality was estimated by applying Weibull distribution to each survival curve. The initial age was 77 years old, which is the average age in EVEREST II HRS used in the analysis by Cameron et al.
Model inputs
Transition probabilities
Transition probabilities used for the analyses are summarized in .
Table 1. Transition probability.
The proportions of patients with NYHA class from the clinical trial used in each analysis were applied. For MitraClip group, the proportions were at 1 year post-operative. As there was no report for the medical therapy group, the proportions of patients before MitraClip procedure (baseline) were used. The mortality rates (/month) for the setting assuming a hazard ratio (HR) to be constant (the base-case analysis, Scenario 1, 3) were estimated by the following formula:
(1)
(1)
(Cumulative mortality rate at T months in each clinical study (x))
In the case that the mortality estimated by the above formula in the analytical model was below the average mortality in general population, the mortality in general population was selected and increase in mortality by age was considered.
As for MitraClip complications, the incidence rates at 30 days evaluated in the clinical study used for each analysis were set. The incidences of those complications were not considered for the medical therapy group as they were complications related to MitraClip procedure. The incidence rates at 30 days and 12 months evaluated in the clinical study used for each analysis were set for the adverse events rates. The 30-day results were used for at 1 month from the start of the analysis. From the second month onwards, the incidence rate per month of each event was estimated using the incidence rate through 2 to 12 months in the clinical trials. The incidence rates of the adverse events from the second month onwards were estimated by the following formula.
(2)
(2)
XT2: Incidence rate at T2 months in each clinical study.
XT1: Incidence rate at T1 months in each clinical study.
Although the adverse events are expected to occur in both of MitraClip group and medical therapy group, the incidence rate of the adverse events was assumed to be 0% as there was no report on the adverse events for a medical therapy group. The probability of MV surgery for MitraClip group was set using the rate of MV surgery until 12 months evaluated in the clinical study used for each analysis. The probability of MV surgery for medical therapy group was conservatively set to be the same as MitraClip group. The probability of CHF hospitalization for MitraClip group was set using the CHF hospitalization rate until 12 months evaluated in the clinical study used for each analysis. For medical therapy group, the rate before MitraClip procedure (baseline) in the same clinical study was used.
Costs
Cost parameters used in the analysis are summarized in .
Table 2. Cost.
In order to set costs related to MitraClip procedure for the analysis, a standard treatment flow was discussed with medical expert and costs were estimated for hospitalization, MitraClip procedure, drug, and outpatient follow-up. Costs for MitraClip procedure include material cost (3,057,535JPY)Citation16, (average 1.77 clips used per case), technical fee (K559-3) (349,300JPY)Citation17 and other cost for procedure. Other cost for procedure was assumed to be a cost of interatrial septum paracentesis. Drug cost (concomitant cardiac drugs administering during hospitalization) was set based on prescription data of concomitant drugs in AVJ-514 trial. Outpatient follow-up cost was calculated based on the expert’s opinion. Patients were assumed to make follow-up visits monthly and take a blood test, electrocardiogram, transthoracic echocardiography, chest X-ray examination at 1-, 6-, and 12-month visit, and then once a year. Medical fee points in April 2017 were used as unit costs for each resource usage.
Hospitalization cost for MV surgery, cost for CHF hospitalization, hospitalization cost for major adverse event (MI, stroke, renal failure) treatment and treatment cost for adverse events (mechanical ventilation (≥48 h), blood transfusion (≥2 units)) were calculated using claims database provided by Medical Data Vision (MDV) (April 2008 through December 2016).
With reference to the expert’s opinion, medical treatment for adverse events (non-elective cardiovascular surgery due to adverse events related to a device or procedure) was assumed to be “percutaneous pericardial drainage”, and medical fee for the corresponding procedure was calculated. With reference to the expert’s opinion, medical treatment for adverse events (GI complication requiring surgery) was assumed to be “endoscopic gastrointestinal hemostasis (K654)”. As for hospitalization cost for septicemia, points for corresponding DPC (A419) was usedCitation14. The details of the type of MitraClip complications were cited from the report of Velazquez et al.Citation10. Costs associated to those complications were set to be costs of representative treatments based on the expert’s opinion.
Health utility
Health Utility in the analysis was set as the same as the study evaluating the cost-effectiveness of MitraClip procedure by Cameron et al. In the study by Cameron et al., utility by NYHA class in an “alive” state, utility decrement for CHF hospitalization, MitraClip procedure and MV surgery were considered and cited from other literatureCitation8. The utility parameters used in the analysis are summarized in .
Table 3. QOL value.
Analysis
The incremental cost-effectiveness ratio (ICER) was calculated by dividing incremental costs by incremental QALYs between MitraClip group and the medical therapy group. The threshold for ICER, i.e. the threshold to be judged as cost-effective, was set as 5 million JPY/QALY in the pilot cost-effectiveness evaluation in JapanCitation23.
Both one-way sensitivity analysis (1-way SA) and probabilistic sensitivity analyses (PSA) were conducted to explore the uncertainty around model input parameters. A range in 1-way SA was set as the 95% confidence interval (CI) of each parameter for the incidence rates, costs, and utility values. The range was set as 20% for variables without 95% CI reported or not be able to estimate it. In the PSA, beta distribution was applied to transition probabilities and utilities, and gamma distribution was applied to cost parameters. Log normal distribution was applied to a HR.
Results
In the base-case analysis, total cost and QALY gained were 7,541,151 JPY and 3.23 QALYs for MitraClip group, and 4,699,692 JPY and 1.79 QALYs for medical therapy group, respectively. The ICER of MitraClip procedure versus medical therapy was 1.97 million JPY/QALY (US$18,570/QALY, US$1 = 106 JPY), which is within the range that MitraClip procedure was evaluated to be cost-effective compared with medical therapy.
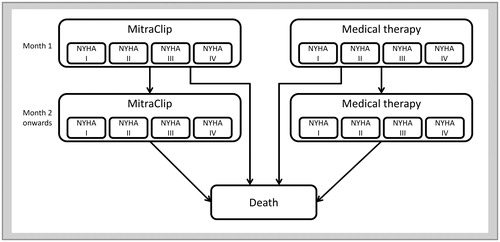
The results of the 1-way SA are shown in . As a result of 1-way SA, the ICER was above 5 million JPY/QALY if the HR of Overall Survival (OS) for MitraClip procedure against medical therapy exceeds 0.97. There was no other variable exceeding 5 million JPY/QALY within the setting range of the analysis. The results of PSA clarified that the probability of ICER of MitraClip procedure versus medical therapy being 5 million JPY/QALY was 96.7%. The cost-effectiveness plane and cost-effectiveness acceptability curve are shown in .
Figure 2. Tornado diagram. For parameters with asterisk (*), 95% CI is used as a range of sensitively analysis. The range of ±20% of baseline value is chosen for the rest of parameters. Abbreviations. ICER, incremental cost-effectiveness ratio; QALY, quality adjusted life year; HR, hazard ratio; OS, overall survival; CHF, congestive heart failure; NYHA, New York Heart Association; MI, myocardial infarction; MV, mitral valve; CI, confidence interval.
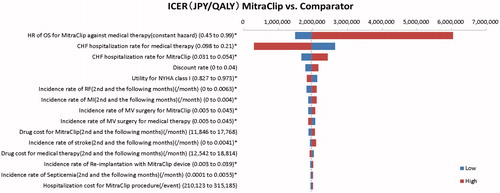
Figure 3. Probabilistic sensitivity analysis. (A) Cost-effectiveness plane, and (B) cost-effectiveness acceptability curve. Abbreviations. QALY, quality adjusted life year; WTP, willingness to pay.
In the scenario analyses, ICERs of MitraClip procedure versus medical therapy were also below 5 million JPY/QALY. The results of base-case and scenario analysis are reported in .
Table 4. Results of base-case and scenario analysis.
Discussions
In Japan, as the national medical expenditure continues to expand due to the aging population and the soaring prices by the increase of development expenses with the advancement of medical technology, concerns about the continuation of universal health insurance are increased year by year. The total population of Japan as of 2016 is 126.93 million, continuously decreasing since 2011, while the percentage of the population aged 65 or over in the total population is a record high of 27.2%. Japan has the highest percentage of the population aged 65 or over among the major countries (as of 2015). In terms of the national medical expenditure, it reached 42 trillion and 364.4 billion yen in 2015, and it is steadily increasingCitation24. Japan has an urgent issue in improvement in the tightening of social security expenses due to the imbalance between the income decrease and the expense increase, caused by the decrease in the labor force population.
Under these circumstances, discussions towards the introduction of cost-effectiveness evaluation to the medical system have begun in Japan from May 2012, and verification towards full-scale introduction in the 2019 fiscal year is in progress. Therefore, as well as foreign countries, concern about the cost-effectiveness of medical drugs and medical devices is rising in Japan not only in government ministries but also at medical sites. Especially in innovative technologies, cost-effectiveness evaluation is important to evaluate quantitatively their values for money
Our base-case analysis showed that ICER of MitraClip procedure versus medical therapy was 1.97 million JPY/QALY (2.00 million/LY) and MitraClip procedure was evaluated as cost-effective. The results of the 1-way SA showed that the HR of OS for MitraClip procedure against medical therapy had a large influence on the analysis result. As the results of PSA clarified that the probability of ICER of MitraClip procedure versus medical therapy being 5 million JPY/QALY was 96.7%, the robustness of these results was confirmed. In the 1-way SA about the base-case analysis, it was shown that the long-term transition of OS has a large influence on the analysis result. Therefore, a scenario analysis quoting a transition probability using survival curves based on observation results in a longer-term by Cameron et al.Citation8 was conducted. The results of the analysis were similar to the results of the base-case analysis.
The cost-effectiveness of MitraClip procedure was evaluated by Haute Autorité de Santé (HAS)Citation25 in France, Health Quality Ontario (OHTAC)Citation26 in Canada, and Medical Services Advisory Committee (MSAC)Citation27 in Australia. Medical therapy was set as a comparator in the evaluations by all three organizations of health technology assessment (HTA).
This is the first analysis to evaluate the cost-effectiveness of MitraClip procedure in patients with symptomatic severe MR at high surgical risk in Japan. Newer data was used for the clinical evidence, and it was a comparison study with propensity score-matched with medical therapyCitation10. Since Velazquez et al. reported only 30-day and 1-year mortality as outcomes, other clinical outcomes for MitraClip group were newly obtained by re-analysis in the same population and used in the analysis.
The report of the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial, a multicenter, randomized, controlled, parallel-group, open-label trial, was recently publishedCitation28. In evaluation of long-term outcomes within 24 months of follow-up in the trial, it was shown that death from any cause occurred in 29.1% of the patient in the device group and 46.1% in the control group (HR, 0.62; 95% CI, 0.46 to 0.82; p < .001), and the annualized rate of all hospitalizations for HF in each group was 35.8% per patient-year and 67.9% per patient-year (HR, 0.53, 95% CI, 0.40 to 0.7, p < .001), respectively. Since the ICER value is affected by the HRs of both outcomes and the magnitudes of the event rates in the control group, the results cannot be judged unconditionally. Nevertheless, from the result of the 1-way SA shown that both outcomes have a large impact on the analysis results, it is expected that the cost-effectiveness of MitraClip procedure will be shown similarly to the result of this analysis. Cost-effectiveness analysis based on COAPT trial is expected in order to evaluate the robustness of the result of this analysis. Before the publication of the COAPT trial, Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA-FR trial), a multicenter, randomized, open-label, controlled phase 3 trialCitation29 was also reported. In the evaluation of the cost-effectiveness of MitraClip procedure, it might be needed to consider uncertainties of the analysis results caused by the learning curve effect on MitraClip procedureCitation30,Citation31, the follow-up period (12 months) of this trial and differences in the baseline disease stateCitation32.
The cost-effectiveness of MitraClip procedure compared with medical therapy for patients with symptomatic severe MR at high surgical risk was evaluated in the present study. However, there are two limitations regarding result interpretation. Firstly, there was a limit to the setting of parameters for the comparator. In the base-case analysis, the result of medical therapy group with propensity score-matched to the result of the clinical trial of MitraClip procedure was used for the comparator. However, data of direct comparison for parameters other than mortality was not obtained because there was no report on other parameters. Therefore, the incidence rate of adverse events for medical therapy was set to be 0%, which was a conservative setting against MitraClip group. In addition, the proportions of patients with NYHA class, CHF hospitalization were assumed to be the same as before MitraClip procedure, and data of MitraClip group at baseline was used. Although the analysis using parameters of previous studyCitation8 was conducted in Scenario analysis, its comparability has limitation because EVEREST II HRS used by Cameron et al. was not an RCT nor propensity score-matched study. Secondly, the mortality rate and adverse events of MitraClip group in lifetime were estimated from data during a year after the procedure. As for the proportions of patients with NYHA class, the state at 1 year after procedure was assumed to continue over lifetime. Although the average of time horizon in lifetime simulation was short, which is 4.17 years for MitraClip group and 2.55 years for medical therapy, an analysis considering long-term prognosis after MitraClip procedure is required after accumulation of longer-term clinical data.
Conclusions
Using best available comparative studies of MitraClip procedure and medical therapy, the cost-effectiveness of MitraClip procedure compared with medical therapy for patients with symptomatic severe MR at high surgical risk was evaluated. The ICER in the base-case analysis was 1.97 million JPY/QALY, and the robustness of the result was confirmed in the sensitivity analysis and the scenario analysis. It was shown that MitraClip procedure improved life-years and QOL in patients at high surgical risk and it was also a cost-effective treatment option.
Transparency
Declaration of funding
This study was funded by Abbott Vascular Japan Co., Ltd.
Declaration of financial/other interest
The authors have no conflicts of interest. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design of this study. SI conducted the analyses. KN, TM, and HS were involved in the interpretation of data. All authors agree to be accountable for all aspects of the work.
Acknowledgements
None reported.
References
- Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation. 2003;107(12):1609–1613.
- Tanemoto K. Surgical indication and recent progress of mitral valve disease. Clinic All-Round. 2011;60:262–268. Japanese.
- Guidelines for Surgical and Interventional Treatment of Valvular Haert Disease (JCS 2012) [Internet]. Tokyo (Japan): The Japanese Association for Thoracic Surgery, the Japanese Society for Cardiovascular Surgery, Japanese College of Cardiology; 2012 [cited 2018 Aug 23]. Available from: http://www.j-circ.or.jp/guideline/pdf/JCS2012_ookita_h.pdf
- Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54(8):686–694.
- Shiroiwa T, Fukuda T, Ikeda S, et al. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value in Health: J Int Soc Pharmacoecon Outc Res. 2017;20(3):372–378.
- Central Social Insurance Medical Council [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2017 [cited 2017 Dec 18]. Available from: http://www.mhlw.go.jp/stf/shingi/shingi-chuo.html?tid=128157
- Research team (Team Leader: Takashi Fukuda) on cost-effectiveness evaluation supported by Health and Labour Science Research Grants (Strategic Integrated Scientific Research Project). Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council version 2.0. [Internet]. Tokyo (Japan): Center for Outcomes Research and Economic Evaluation for Health, National Institute of Public Health; 2019 [cited 2019 Aug 16]. Available from: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf
- Cameron HL, Bernard LM, Garmo VS, et al. A Canadian cost-effectiveness analysis of transcatheter mitral valve repair with the MitraClip system in high surgical risk patients with significant mitral regurgitation. J Med Econ. 2014;17(8):599–615.
- Giannini C, Fiorelli F, De Carlo M, et al. Comparison of percutaneous mitral valve repair versus conservative treatment in severe functional mitral regurgitation. Am J Cardiol. 2016;117(2):271–277.
- Velazquez EJ, Samad Z, Al-Khalidi HR, et al. The MitraClip and survival in patients with mitral regurgitation at high risk for surgery: a propensity-matched comparison. Am Heart J. 2015;170(5):1050.e3–1059.e3.
- Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol. 2012;59(2):130–139.
- Glower DD, Kar S, Trento A, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol. 2014;64(2):172–181.
- Abbott Vascular Co., Ltd. MitraClip STED 8.1.7 Clinical Results of AVJ-514 trial. Tokyo: Abbott Vascular Co., Ltd. (Japan); 2016.
- DPC tensuu hayami hyou 2017-nen 4-gatsu ban [DPC point table (April 2017 edition)]. Tokyo: Igakutsushinsha Co.; 2017. Japanese.
- The 4th medical fee survey specialized organization DPC evaluation subcommittee in FY2008 [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2017 [cited 2017 Jun 28]. Available from: http://www.mhlw.go.jp/stf/shingi2/0000150723.html
- Insurance Reimbursement for Medical Devices (to be listed in April 2018) [Internet]. Tokyo (Japan): Central Social Insurance Medical Council; 2018 [cited 2019 Aug 16]. Available from: https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000192797.pdf
- Shinryou tensuu hayami hyou 2017-nen 4-gatsu ban [Medical treatment fee point April 2017]. Tokyo: Igakutsushinsha Co,; 2017. Japanese.
- Nihon iyakuhin shuu DB 2017-nen 4-gatsu ban [Japanese Drugs DB April 2017]. Tokyo: Jiho, Inc.; 2017. Japanese.
- Gohler A, Geisler BP, Manne JM, et al. Utility estimates for decision-analytic modeling in chronic heart failure–health states based on New York Heart Association classes and number of rehospitalizations. Value in Health: J Int Soc Pharmacoecon Outc Res. 2009;12:185–187.
- Chaplin S, Scuffham PA, Alon M, et al. Secondary prevention after PCI: the cost-effectiveness of statin therapy in the Netherlands. Netherlands Heart J. 2004;12:331–336.
- Marwick TH, Scuffham PA, Hunink MG. Selection for early surgery in asymptomatic mitral regurgitation: a Markov model. Int J Cardiol. 2013;165(2):266–272.
- Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–420.
- Price Adjustment in the Pilot Introduction [Internet]. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2017 [cited 2017 Dec 18]. Available from: http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000182066.pdf
- Demographics [Internet]. Tokyo (Japan): National Institute of Population and Social Security Research; 2018 [cited 2018 Sep 25]. Available from: http://www.ipss.go.jp/syoushika/tohkei/Popular/Popular2016.asp?chap=2&title1=%87U%81D%94N%97%EE%95%CA%90l%8C%FB
- MITRACLIP [Internet]. Saint-Denis (France): Haute Autorité de Santé; 2017 [cited Dec 2017 18]. Available from: https://www.has-sante.fr/portail/jcms/c_2028916/fr/mitraclip
- Mitral Valve Clip for Treatment of Mitral Regurgitation [Internet]. Ontario (Canada): Health Quality Ontario; 2017 [cited 2017 Dec 18]. Available from: http://www.hqontario.ca/Evidence-to-Improve-Care/Health-Technology-Assessment/Reviews-And-Recommendations/Mitral-Valve-Clip-for-Treatment-of-Mitral-Regurgitation
- Medical Services Advisory Committee [Internet]. Canberra (Australia): MSAC. 1192.2 – The reduction of mitral regurgitation through tissue approximation using transvenous/transeptal techniques (resubmission); 2017 [cited 2017 Dec 18]. Available from: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1192.2-public
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379(24):2307–2318.
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379(24):2297–2306.
- Eleid MF, Reeder GS, Malouf JF, et al. The learning curve for transcatheter mitral valve repair with MitraClip. J Interv Cardiol. 2016;29(5):539–545.
- Schillinger W, Athanasiou T, Weicken N, et al. Impact of the learning curve on outcomes after percutaneous mitral valve repair with MitraClip and lessons learned after the first 75 consecutive patients. Eur J Heart Fail. 2011;13(12):1331–1339.
- Nishimura RA, Bonow RO. Percutaneous repair of secondary mitral regurgitation – a tale of two trials. N Engl J Med. 2018;379(24):2374–2376.