Abstract
Aim: Optimal use of scarce resources is a focus in the healthcare sector, as resources devoted to health care are limited. Costs and health economic analyses can help guide decision-making concerning treatments. One important factor is the choice of cost perspective that can range from a focus on narrow drug budget costs to broader economic perspectives. In the case of treatment with oral anticoagulants in patients with venous thromboembolism (VTE), encompassing deep vein thrombosis and pulmonary embolism, the aim of this cost analysis was to illustrate the differences in costs when applying different cost perspectives.
Methods: In a cost analysis, pairwise comparisons of average costs of 6 months standard treatment with either a low molecular weight heparin parenteral anticoagulant (LMWH) and a Vitamin K Antagonist (VKA) versus one of the non-vitamin K oral anticoagulants [NOACs; dabigatran etexilate, rivaroxaban, apixaban, and edoxaban) used in daily clinical practice in Denmark for VTE patients were carried out. Each analysis included the results from five different cost analyses with increasingly broader cost perspectives going from the narrowest “drug cost only” perspective to the broadest “societal” perspective.
Results: Focusing on “drug costs only”, LMWH/VKA was associated with the lowest costs compared to all NOACs. However, including the economic impact of preventing recurrent VTE and limit bleedings, apixaban and rivaroxaban resulted in slightly lower health care costs than LMWH/VKA. When applying the “societal perspective”, the total costs saved with apixaban and rivaroxaban compared to LMWH/VKA further increased, with apixaban having the lowest total costs.
Conclusions: The present study’s case of oral anticoagulants in VTE treatment illustrated the importance of the cost perspective in the choice of therapy. If decision-making were based on drug costs only, instead of applying a health care sector or societal cost perspective, suboptimal decisions may be likely.
Introduction
Venous thromboembolism (VTE) is a common cardiovascular disorder that includes deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE is the third most common cause of vascular death after acute coronary syndrome and strokeCitation1. The incidence is estimated at a 1∼2 per 1,000 person-years – a number that is increasingCitation2–4. In addition, the risk of recurrence of VTE is highCitation5,Citation6. The healthcare and societal costs of VTE and recurrent VTE are substantialCitation7–9. Due to this focus is on optimal use of scarce resources.
Originally, the treatment of VTE consisted of a low molecular weight heparin parenteral anticoagulant (LMWH) (e.g. enoxaparin) as daily subcutaneous injections for at least 5 days, combined with a Vitamin K Antagonist, VKA (e.g. warfarin) initiated during this time and continued for at least 3 months. VKA therapy requires frequent monitoring of the international normalized ratio (INR). Several studies have established that non-vitamin K oral anticoagulants (NOACs) such as apixaban, dabigatran etexilate, edoxaban and rivaroxaban are efficacious alternatives in VTE treatment with or without initial LMWHCitation10–13. They do not require monitoring of INR levels like VKA. All treatments with anticoagulants (both LMWH/VKA or NOACs) are associated with a risk of bleedingCitation10–13.
Health economic analyses provide information to decision-makers on the costs, and in case of a cost-effectiveness analysis outcome of different treatment alternatives, in order to optimize the best use of scarce resources. Health economic analyses, including cost analyses, can apply different cost perspectives. Considering drug treatment, the perspective can range from a focus on the drug costs only, making it wider with the inclusion of all health care costs, home and social care costs at the local level, and further into the societal level, e.g. inclusion of production lost. To avoid suboptimal decisions, literature on health economic evaluation recommend application of the societal perspective (i.e. the broadest possible perspective)Citation14,Citation15. Specific budget owners and decision-makers in the healthcare sector though tend to increasingly require more narrow cost perspectives as seen today at the national levels in Sweden, United Kingdom and Denmark, where indirect costs are excluded from the calculation of costsCitation16–19. At regional and hospital budget levels focus maybe even more narrow to only consider the consequences for a single budget, e.g. the drug budget, being referred to as a drug silo mentalityCitation14,Citation20.
The total costs of VTE may differ depending on the choice of treatment and upon the cost, perspective applied in the analysis. Inspired by the methodology in a previous study focusing on atrial fibrillationCitation21 the aim of the present study was to estimate the average total costs of VTE over a 6 months period including all relevant treatment costs (e.g. costs of VTE prophylaxis including costs of recurrent VTE events and incurring bleeds), costs of home care and indirect costs to apply a societal perspective. The total cost result is presented for five different cost perspectives going from the narrow drug cost only to the broad societal perspective.
Methods
We performed an analysis of the average costs of VTE after the initial VTE incident. An Excel-based cost model was developed to calculate results for the different cost perspectives. The analyses were carried out as pairwise comparisons of 6 months standard treatment, with LMWH (enoxaparin) for the first 5 days along with 183 days of VKA (warfarin) compared with each of the four NOAC treatment regimens. In the case of dabigatran etexilate and edoxaban the 6 months NOAC treatment was initiated with 5 days of LMWH and followed with 178 days of either dabigatran etexilate or edoxaban. Specifically, the analyses were based on data on resource use, cost estimates, and phase III clinical trial data of the risk of recurrent VTE and major bleeding for each of the treatments, as described below.
Clinical input
In the 6 months period after the initial VTE incident, there is a risk of recurrence of VTE, why anticoagulant treatment is initiated. There is a risk of bleeding following anticoagulation treatment. The applied risk estimates are summarized in . The estimates were sourced by the phase III randomized controlled trials (RTC) in which each of the NOACs were compared with standard treatment regimens involving LMWH along with and followed by VKACitation10–13. shows that the relative risks in some of the pairwise comparisons are insignificant. In our base-case scenario, we did only include a cost difference if the relative risks are significant (i.e. differ significantly from 1.0). In addition to the base case, we also presented results where all relative risks were included, irrespective of significance.
Table 1. Six months risks and risk reductions in recurrent VTE and major bleedings, NOACs versus LMWH/VKA.
Time horizon
To ensure consistency, and because the applied risk data from the RCTs were all based on a 6 months period, the time horizon of the cost analysis is also set to 6 months.
Cost perspectives
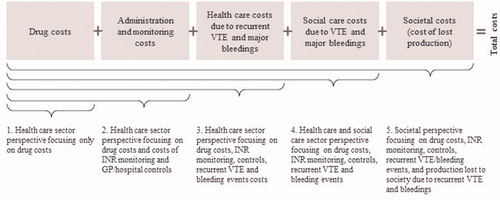
The different cost perspectives related to the analysis of VTE are illustrated in . The figure also provides an overview of the included costs. In the most limited cost perspective, only drug costs were included (i.e. the cost of LMWH/VKA and NOACs), whereas the societal perspective or the total costs perspective also includes home care costs and costs to society of lost production in addition to a broad spectrum of health care costs (drug costs, costs of INR monitoring and general practitioner (GP)/hospital visits, and costs of recurrent VTE and major bleeding events).
Applied cost estimates
The 6 months drug costs for the LMWH/VKA and NOAC regimens were based on the pharmacy selling prices (PSPs, source: www.medicinpriser.dk, accessed 14 May 2019) and dosing as in SPC (Summary of Product Characteristics)Citation22–27. The number of tablets and syringes (enoxaparin) were rounded up to whole packages. An average patient weight of 75 kg was assumed (relevant for enoxaparin). PSPs included 25% value added tax (VAT) and were converted from DKK to EUR (exchange rate: 1 EUR = 7.47 DKK, 15 May 2019).
Post-discharge, VKA (e.g. warfarin) and NOACs are prescription medicine purchased by the patient at a community pharmacy. Part of the costs for the patient is reimbursed (0–85%). In the analyses, we included the total drug costs implying that both the reimbursement cost and the patient’s co-payment were included.
The estimated costs related to anticoagulation therapy, drug administration and monitoring (e.g. INR) and the cost estimates for recurrent VTE and major bleedings are summarised in .
Table 2. Applied cost estimates, EUR.
In the LMWH/VKA regimen, it was assumed that the average patient over a period of 6 months had one GP contact every month (six contacts in total) for INR monitoring as well as 3 outpatient controls in a hospital settingCitation28. For the NOAC regimens it was assumed that over the 6 months period the average patient will have two GP controls as well as two controls in an outpatient clinicCitation28. National fees for GPs and Diagnosed-Related Group (DRG) tariffs were used as unit cost estimates for these contactsCitation29,Citation30.
The average costs per event of recurrent VTE and major bleedings (3-year total attributable societal costs of a first event) were sourced from a Danish population-based retrospective register study applying nationwide Danish registriesCitation31. We did use the present value of the 3-year average extra cost of these incident events as found in this study. These average event costs were combined with the absolute and relative risks of events for the average patient coming from the clinical studies (cf. ). This means that if an event occurred within the 6 months period of the economic analysis then the present value of the 3-year average cost of this event was added to the total costs.
Results
present the 6 months average total costs per VTE-patient for the five different cost perspectives – drug costs only, drug costs and administration/monitoring visit costs, healthcare sector perspective, healthcare and social care perspective and finally societal perspective. The results are presented as pair-wise comparisons of LMWH/VKA compared with each of the NOACs: apixaban, rivaroxaban, dabigatran etexilate and edoxaban.
Figure 2. Six months costs per VTE patient receiving LMWH/VKA versus apixaban (EUR). *Including the costs of recurrent VTE and major bleedings.
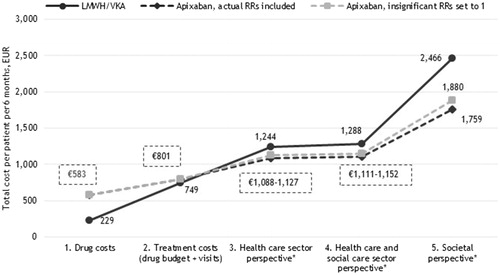
Figure 3. Six months costs per VTE patient receiving LMWH/VKA versus rivaroxaban (EUR). *Including the costs of recurrent VTE and major bleedings.
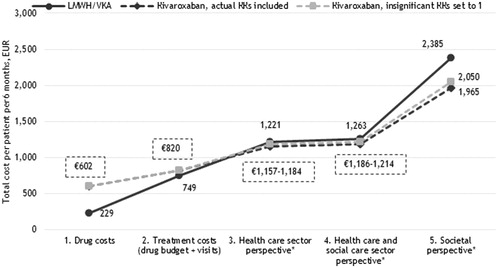
Figure 4. Six months costs per VTE patient receiving LMWH/VKA versus dabigatran etexilate (EUR). *Including the costs of recurrent VTE and major bleedings.
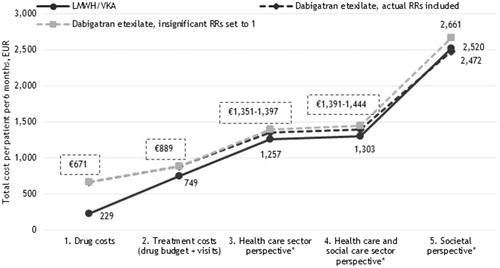
Figure 5. Six months costs per VTE patient receiving LMWH/VKA versus edoxaban (EUR). *Including the costs of recurrent VTE and major bleedings.
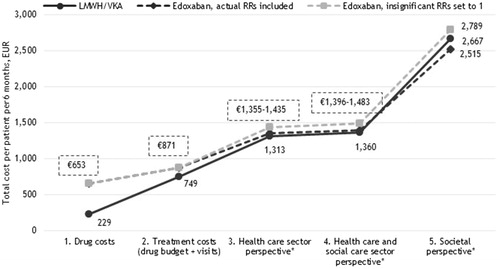
When only drug costs were considered in the analysis, LMWH/VKA was associated with lower costs than all NOAC treatment regimens (€229 versus €583–€671). However, when adding the costs of INR monitoring and GP/hospital control visits to the analyses (second cost perspective in ), the costs of LMWH/VKA did nearly reach the same level as the costs of NOACs in most cases (€801–€889 with apixaban having the lowest costs (€801)).
According to clinical trial data, apixaban and rivaroxaban are associated with less major bleeding events than LMWH/VKA (). Correspondingly, when major bleeding event cost was incorporated into the model, the costs associated with apixaban and rivaroxaban were estimated to be lower than that of LMWH/VKA. Adding the healthcare costs of recurrent VTE and major bleedings to the analysis (third cost perspective in ), apixaban and rivaroxaban both resulted in lower costs compared to LMWH/VKA. Adding the costs of home care from the municipalities (social care - fourth cost perspective in ) as well as the lost production due to disease (fifth cost perspective in ), the total costs saved with apixaban (€1,880 versus €2,466) and rivaroxaban (€2,050 versus €2,385) compared to LMWH/VKA, with apixaban having the lowest total costs.
Because treatment with dabigatran etexilate and edoxaban were not associated with a lower risk of major bleedings compared to LMWH/VKA (cf. ), treatment with these two NOACs in the base-case were not associated with lower costs irrespective of the choice of perspective.
However, in the alternative scenario, where also insignificant risk estimates from the clinical trials were applied, and with the inclusion of all relevant costs (i.e. the societal perspective), treatment with all four NOACs was associated with lower costs compared to LMWH/VKA opposed to the base-case analysis.
Discussion
In this analysis, we have estimated the 6 months total costs of VTE for five different cost perspectives. The results showed that the choice of cost perspective matters as different cost perspectives may lead to different conclusions regarding which drug to use based on economic arguments.
Health care authorities’ application of a health care sector perspective only or payers at the local hospital level with a focus on drug costs only are often seen, as payers may tend to focus only on the costs relevant for the health care sector or drug budgets only (silo thinking)Citation14,Citation20. However, ignoring the potential costs in the healthcare sector (e.g. costs of bleedings), other sectors as well as in society (productivity costs) may lead to suboptimal decisions.
Based on this economic analysis that when adding the administration/monitoring visits as well as other healthcare sector costs, treatment with apixaban and rivaroxaban are associated with lower costs than the LMWH/VKA alternative. This is similar to two cost-effectiveness analyses showing apixaban as well as rivaroxaban to be cost-saving in VTE compared to LMWH/VKACitation32,Citation33.
Limitations
The present analysis has several limitations. Not all relevant costs were included, e.g. the costs of short-term sick leave (less than 30 days), patients’ transportation time related to consultations, and non-prescription drugs, although the last two are expected to have a very limited impact on the total cost result. Moreover, possible costs of informal care provided by relatives or friends were not included in the estimation of the costs of recurrent VTE and major bleedings as these data are not available in the registers. Therefore, the presented total cost estimates remain conservative.
The analysis was based on risk estimates from four RCTs – one for each of the four NOACs. The limitation of these RCT study designs though is that the external validity may be lower than what is found in a real-world setting. To accomplish this evidence from real-world data studies could have been applied instead, although this would have been with the loss of some internal validity. Furthermore, the real-world evidence published varies to some degree both in terms of designs and results. Therefore, to secure that the evidence for the risk estimates originates from the same type of research studies, as well as it had high internal validity, we did choose the RCT studies for the present analysis.
Apart from the risk estimates from the RCTs and the scenario analysis, we only present single estimates (point estimates) for the drug costs, health care sector costs, social care costs and productivity costs and thus single estimates of the total costs of VTE (given different treatments). Some variation or uncertainty may be linked to these point estimates.
The total cost analyses were limited to the first 6 months covering the acute phase, which was related to the RCT evidence. The implication, however, is that a longer time horizon and associated costs are not covered.
VTE is associated with excess mortalityCitation1 However, given the relatively short time horizon (6 months), we have not included mortality in the analysis. However, due to the risk reduction of recurrent VTE events using a NOAC is higher as compared to LMWH/VKA this is conservative for the analyses showing that NOACs like apixaban and rivaroxaban are cost-saving.
Finally, Danish VAT (25%) was included in the costs of drugs in the analyses due to the focus on budgets. However, VAT is not a cost or use of resources but reflects a transfer payment, and it could thus have been omitted. Inclusion of VAT, though, is a disadvantage for the NOAC treatment regimens due to the higher drug costs of NOACs. By excluding VAT from the analyses, the difference in costs between the LMWH/VKA and the NOAC alternatives would have been even larger, why it can be argued that the cost-saving results showed for NOACs like apixaban and rivaroxaban were conservative.
Perspectives
This economic analysis was carried out from a Danish setting using Danish cost estimates and practice. Although one should be careful about directly transferring such results to other countries, these may, however, be transferrable to other Nordic and Northern European countries with similar treatment patterns. This also due to the use of the robust analysis of the costs of eventsCitation31. More importantly though the case presented with the different cost perspectives and their implications for the overall cost result to be used for decision-making are useful illustrations for other countries even though the specific cost-results should not be transferable. It highlights the importance of the adaptations of a broad cost perspective to avoid potential decision errors.
Conclusions
This present analysis estimated the total costs of VTE via pairwise comparisons of treatment with LMWH/VKA versus each of the four available NOACs. The analysis demonstrated the importance of choice of cost perspective, with the narrow perspectives, e.g. the drug only cost perspective, underestimating the true total costs of VTE.
Given a drug costs perspective, treatment with LMWH/VKA is associated with the lowest costs compared with all NOACs. However, applying a broader cost perspective (e.g. a health care sector perspective) shows that apixaban and rivaroxaban have 10% and 3%, respectively, lower 6-month costs than LMWH/VKA comparing the alternatives with a healthcare sector perspective. This illustrates that too narrow a cost perspective may lead to suboptimal decision making.
Transparency
Declaration of funding
This study was financed by Pfizer, Denmark and Bristol-Myers Squibb, Denmark.
Declaration of financial/other relationships
Agnete Nielsen and Jens Olsen are employees of Incentive. The Incentive was a paid vendor to Pfizer Denmark and Bristol-Myers Squibb Denmark. Thomas Kümler was a paid clinical consultant of Pfizer Denmark and Bristol-Myers Squibb Denmark in the present study. Peter Bo Poulsen and Lars Dybro are employees of Pfizer Denmark and both own shares in Pfizer Inc. Bitten Kloster and Anette Lorentzen are employees of Bristol-Myers Squibb Denmark. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors were involved in the conception and design of the analysis. Agnete Nielsen and Jens Olsen performed the analyses and drafted the manuscript. All authors revised and commented the manuscript critically and approved the final version. All authors agree to be accountable for all aspects of the work.
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol. 1992;19(2):246–247.
- Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet.. 2012;379(9828):1835–1846.
- Arshad N, Isaksen T, Hansen J-B, et al. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur J Epidemiol. 2017;32(4):299–305.
- Lehnert P, Lange T, Møller CH, et al. Acute pulmonary embolism in a national Danish cohort: increasing incidence and decreasing mortality. Thromb Haemost. 2018;118(3):539–546.
- White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23):I4–I8.
- Albertsen IE, Nielsen PB, Søgaard M, et al. Risk of recurrent venous thromboembolism: a Danish nationwide cohort study. Am J Med. 2018;131(9):1067.e4–1074.e4.
- Grosse SD, Nelson RE, Nyarko KA, et al. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10.
- Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291–302.
- Willich SN, Chuang L-H, van Hout B, et al. Pulmonary embolism in Europe - burden of illness in relationship to healthcare resource utilization and return to work. Thromb Res. 2018;170:181–191.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
- Prins MH, Lensing AW, Bauersachs R, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thrombosis J. 2013;11(1):21.
- Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014; Feb 18 129(7):764–772.
- Hokusai V, Investigators Büller HR, Décousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013; Oct 10 369(15):1406–1415.
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. Oxford (UK):Oxford University Press; 2005.
- Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. Oxford (UK): Oxford University Press; 1996.
- Amgros. Guidelines for cost analyses of new medicines and indications in the hospital sector. Copenhagen: Amgros; 2018. (Report No.: 1.3).
- Hälsoekonomi – Tandvårds-Läkemedelförmånsverket [Internet]. Tandvårds-Läkemedelförmånsverket (TLV). 2019 [cited 2019 Jun 14]. Available from: https://www.tlv.se/lakemedel/halsoekonomi.html
- National Institute for Health and Care Excellence. Developing NICE guidelines: the manual. London (UK): NICE; 2014.
- Sundheds- og AEldreministeriet. Vejledning om udarbejdelse af sundhedsøkonomiske analyser af laegemidler. Copenhagen: Sundheds- og Ældreministeriet, Lægemiddelstyrelsen; 2018. (Report No.: VEJ nr 9153 af 09/03/2018).
- Garrison L, Towse AA. The drug budget silo mentality in Europe: an overview. Value Health. 2003;6(s1):S1.
- Poulsen PB, Johnsen SP, Hansen ML, et al. Setting priorities in the health care sector - the case of oral anticoagulants in nonvalvular atrial fibrillation in Denmark. CEOR. 2017;9:617–627.
- Danish Medicines Agency. PRODUKTRESUMÉ for Warfarin Orion [Internet]. Copenhagen (Denmark): DMA; 2019 [cited 2019 May 14]. Available from: www.produktresume.dk
- Danish Medicines Agency. PRODUKTRESUMÉ for Klexane [Internet]. Copenhagen (Denmark): DMA; 2009. [cited 2019 May 14]. Available from: www.produktresume.dk
- European Medicines Agency. Summary of Product Characteristics – Apixaban. Amsterdam: EMA; 2018 [cited 2019 May 14]. Available from https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_da.pdf.
- European Medicines Agency. Summary of Product Characteristics – Rivaroxaban. Amsterdam: EMA; 2018 [cited 2019 May 14]. Available from https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_da.pdf
- European Medicines Agency. Summary of Product Characteristics – Dabigatran. Amsterdam: EMA; 2018 [cited 2019 May 14]. https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_da.pdf
- European Medicines Agency. Summary of Product Characteristics – Edoxaban. Amsterdam: EMA; 2018 [cited 2019 May 14]. Available from https://www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_da.pdf
- Nielsen JD, Grove EL, Larsen TB, et al. Recidiv af venøs tromboemboli [Recurrence of venous thromboembolism]. Ugeskr Laeger 2018; 180(20A):V03180177.
- Organisation of General Practitioners in Denmark (PLO). Takstkatalog [Charge catalogue for the general practice in Denmark]. Copenhagen: PLO; 2019.
- Sundhedsdatastyrelsen. Takstsystem, vejledning 2017 [Tariff system, guideline]. DRG og Finansiering, Sundhedsdatastyrelsen. Version 26-1. Copenhagen, December 2016.
- Gustafsson N, Stallknecht SE, Skovdal M, et al. Societal costs due to meningococcal disease: a national registry-based study. Clinicoecon Outcomes Res. 2018;10:563–572.
- de Jong LA, Dvortsin E, Janssen KJ, et al. Cost-effectiveness analysis for apixaban in the acute treatment and prevention of venous thromboembolism in the Netherlands. Clin Ther. 2017;39(2):288.e4–302.e4.
- Bamber L, Muston D, McLeod E, et al. Cost-effectiveness analysis of treatment of venous thromboembolism with rivaroxaban compared with combined low molecular weight heparin/vitamin K antagonist. Thromb J. 2015; [cited 2019 Jun 2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4464718/