Abstract
Aims: The costs associated with insulin therapy and diabetes-related complications represent a significant and growing economic burden for healthcare systems. The aim of this study was to evaluate the cost-effectiveness of switching to insulin degludec (degludec) vs continuing previous basal insulin, in Italian patients with type 1 (T1D) or type 2 (T2D) diabetes, using a long-term economic model.
Materials and methods: Data were retrieved from a real-world population of patients from clinical practice in Italy. Clinical parameters included in the base-case model were change from baseline in HbA1c, rates of hypoglycemia, and basal and bolus insulin dose, at 6 months following switch to degludec. Costs of treatments were taken from official Italian pharmaceutical list prices and costs of hypoglycemia were based on the literature. The data were used to populate a long-term (lifetime) IQVIA CORE Diabetes Model to evaluate the incremental cost-effectiveness ratio (ICER) – cost per quality-adjusted life-year (QALY). The robustness of these results was tested with extensive sensitivity analyses by varying the time horizons and abolishing each of the treatment differences and previous basal insulins.
Results: The total incremental cost for degludec vs previous basal insulin was €–6,310 and €–2,682 for patients with T1D and T2D, respectively; the switch to degludec resulted in a QALY gain of 0.781 and 0.628. The long-term ICER for degludec vs continuing the previous basal insulin regimen showed that degludec was dominant for both T1D and T2D, meaning that patient health was improved in terms of QALYs with lower healthcare costs. Sensitivity analyses showed that degludec remained dominant in most scenarios including after elimination of any benefit in non-severe hypoglycemia and insulin dose, in both T1D and T2D.
Conclusions: Under routine care, switching to degludec is dominant, compared with continuing previous basal insulin, in Italian patients with T1D or T2D.
Introduction
Insulin is required in the treatment of patients with type 1 diabetes (T1D) and is commonly used in patients with type 2 diabetes (T2D) as the disease progresses. Hypoglycemia is a frequent side-effect of insulin treatmentCitation1 and is associated with avoidable healthcare costs and decreased quality-of-life for patientsCitation2.
Various basal insulin treatments are available, including insulin degludec (degludec), insulin glargine 100 units/mL (glargine U100), insulin glargine 300 units/mL (glargine U300), and insulin detemir (IDet). Degludec is a long-acting basal insulin with a flat pharmacokinetic profile and a unique mode of protraction, which provides a stable, long-lasting blood glucose-lowering effectCitation3. Degludec was approved in Europe in 2013 for use as a basal insulin therapy in patients with T1D or T2D. In numerous randomized controlled trials (RCTs), degludec has demonstrated appreciable reductions in the risk of hypoglycemia compared with glargine U100 and nocturnal confirmed hypoglycemia compared with detemirCitation4–7. This has financial implications, as hypoglycemia is associated with avoidable healthcare costs and decreased quality-of-life for patientsCitation2.
The prevalence of diabetes is increasing and, as a result, the direct and indirect costs of treatment (for example, the cost of insulin therapy, treatment for severe and non-severe hypoglycemia and other diabetes-related complications) represent a significant and growing economic burden for healthcare systemsCitation8–10. A cost-effectiveness analysis (CEA) can be used as a tool for economic decision-makers, payers, and clinicians to assess the cost of treating diabetes with newer therapies compared with existing treatments. CEAs are requested for Health Technology Assessments and by reimbursement agencies as part of price and reimbursement decisionsCitation11. These results are often expressed as an incremental cost-effectiveness ratio (ICER), which is derived from dividing the cost difference between the two treatments by the difference in effectivenessCitation9.
Although RCTs are normally used for CEAs, real-world evidence (RWE) related to drug effectiveness and healthcare utilization is increasingly important to assess the effectiveness of new interventions in clinical practice. RCTs often use restrictive inclusion and exclusion criteria, resulting in high internal validity, but lower generalizability to clinical practiceCitation12. RWE studies have higher external validity and reflect how treatments are administered in everyday clinical practice. Therefore, the CEA using data from RWE may more closely reflect the cost of treating diabetes than those using data from RCTs. It is important, however, to ensure that, when interpreting data from RWE, the limitations of these studies should be considered. These limitations include sources of bias, the quality of data collection, and the need for analytic approaches adjusting for confoundersCitation13.
The EUropean TREsiba AudiT (EU-TREAT) study evaluated the clinical effectiveness of switching to degludec in a real-world population of insulin-treated patients with either T1D or T2D in conditions that reflected routine clinical careCitation14. Based on the data from the Italian cohort of the EU-TREAT study, the current analysis aimed to evaluate the cost-effectiveness of switching to degludec vs continuing the previous basal insulin regimen, in patients with T1D or T2D in everyday clinical practice using a long-term economic model and various sensitivity analyses.
Materials and methods
EU-TREAT study overview
This secondary analysis used data from an Italian sub-population (n = 550, 21.6%) of EU-TREAT (n = 2550; ClinicalTrials.gov NCT02662114) – a European, multi-center, retrospective, non-interventional chart review study, which investigated the clinical impact of switching from any basal insulin to degludec (100 units/mL and 200 units/mL, Novo Nordisk, Denmark) ± bolus insulin in patients with T1D or T2D (± oral antidiabetic drugs [OADs] in patients with T2D)Citation14. Data reported in medical records were collected for patients who had switched from any basal insulin to degludec with a minimum of 6 months of follow-up, across six European countries (Austria, Denmark, Germany, Greece, Italy, and Switzerland). Previous basal insulin regimens included glargine U100, IDet, and other insulins. Baseline was defined as the most recent recording during the 3-month period prior to degludec initiation. At baseline, 49.9% of patients with T1D and 62.4% of patients with T2D were on a once-daily basal insulin regimen, and 45.8% of patients with T1D and 28.1% of patients with T2D were on a twice-daily basal insulin regimen, with the remainder unknown or missingCitation14. Outcome data, including HbA1c, daily basal and bolus insulin doses, and number of hypoglycemic episodes, were recorded at 6 ± 3 and 12 ± 3 months in the time periods before and after the switch, respectively. Data for the pre- and post-switch time periods were collected simultaneously. The detailed study design and methodology for EU-TREAT have been described previouslyCitation14 (Supplementary Figure S1). Results from the EU-TREAT study were robust and a similar trend in the clinical parameters was observed at 6 months. Therefore, only data at baseline and 6 months post-switch were used in this secondary analysis.
The inclusion criteria specified patients (aged ≥18 years at the time of starting degludec) with T1D or T2D, treated with insulin for >12 months, who were switched to degludec (± bolus insulin [± OADs in T2D]) from any other basal insulin (± bolus insulin [± OADs in T2D]) at least 6 months prior to data collection, and were treated with basal insulin for at least 6 months before switching. The following exclusion criteria were applied: previous participation in this study or a diabetes clinical trial or receipt of any investigational medicinal product up to 12 months before or any time after the initiation of degludec; current participation in another non-interventional study on degludec; patients treated by continuous subcutaneous insulin infusion or premix insulin in the 6 months prior to receiving degludec.
The study protocols were approved according to local regulations by appropriate health authorities and by institutional review boards at all participating institutions, and conducted in accordance with the Declaration of HelsinkiCitation15. A list of independent ethics committees for participating institutions was provided in Supplementary Table S1 of the EU-TREAT study manuscriptCitation14. Written informed consent from all patients was obtained before enrollment.
Base case: long-term cost-effectiveness modeling
Model overview
The IQVIA CORE Diabetes Model (CDM) version 9.0 (Supplementary Figure S2) was used to simulate the long-term (patient lifetime) impact of switching to degludecCitation16,Citation17. Briefly, CDM is a lifetime Markov simulation model that predicts diabetes complications over time, and the resulting impact on cost and effects. The CDM takes into account cost and quality-adjusted life-year (QALY) parameters, treatment effects, and baseline patient characteristics to project outcomes for a given population. QALYs were calculated by applying the relevant disutility values. Values were assigned to typical diabetes complications and hypoglycemic events, and totals obtained by multiplying these values by the number of complications or hypoglycemic events observed in each treatment group. Outcomes included direct healthcare costs, life expectancy, QALYs, and ICERs. Future costs and clinical benefits were discounted with a discount rate of 3%. Net monetary benefit (NMB) was calculated as a willingness-to-pay threshold multiplied by the difference in QALYs, minus the difference in costsCitation18. The commonly used willingness-to-pay threshold of €30,000 was implemented in these calculationsCitation19–21.
Clinical input and parameters
A total of 550 patients from the Italian cohort of EU-TREAT were included in this analysis (T1D = 397, T2D = 153; ).
Table 1. Baseline demographics of patients with T1D and T2D included in the Italian cohort of the study.
We assumed scenarios where patients either continued their baseline insulin regimen (and baseline data) or switched to degludec, to simulate the long-term impact on HbA1c, rates of hypoglycemia, and basal and bolus insulin doses. Only parameters that were significantly different pre- and post-switch to degludec were used for the modeling of the degludec scenario. Otherwise, a ratio (dose ratio or rate ratio [RR]) of 1, i.e. the baseline value, was used. The HbA1c value at baseline was assumed to be the same as that for the hypothetical cohort who continued their basal insulin, and the HbA1c value at 6 months of follow-up was used to represent the cohort who switched to degludec. Therefore, the relative effectiveness in terms of HbA1c, of degludec vs previous basal insulin, was evaluated as the difference between these two values. In patients with T1D, a significant decrease in HbA1c was observed at 6 months compared with baseline (estimated treatment difference [ETD] = −0.35 [95% CI = −0.44; −0.27], p < 0.0001; and Supplementary Table S1). A similar result was observed for patients with T2D (ETD = −0.50 [95% CI = −0.65; −0.35], p < 0.0001; and Supplementary Table S1).
Figure 1. HbA1c, insulin dose, and number of hypoglycemic events in patients with T1D at baseline and 6-months of follow-up. *p < 0.0001; **p = 0.011. Numbers of hypoglycemic events were based on data from 6 months pre-switch to switch vs switch to 6 months post-switch. Abbreviations. ETD, estimated treatment difference; n, number of patients; NS, not significant; RR, rate ratio; T1D, type 1 diabetes.
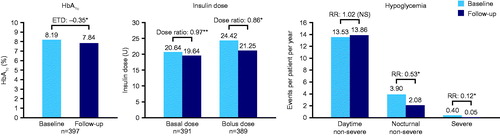
Figure 2. HbA1c, insulin dose, and number of hypoglycemic events in patients with T2D at baseline and 6 months of follow-up. *p < 0.0001; **p = 0.049; ***p < 0.05. Numbers of hypoglycemic events were based on data from 6 months pre-switch to switch vs switch to 6 months post-switch. Abbreviations. ETD, estimated treatment difference; n, number of patients; RR, rate ratio; T2D, type 2 diabetes.
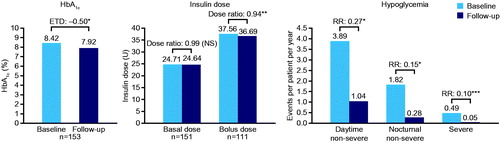
Dose ratios for degludec vs previous basal insulin were estimated using analysis of covariance adjusted for the following covariates: type of diabetes, age, body mass index (BMI), gender, diabetes duration, duration of insulin therapy, and number of daily injections. Basal and bolus insulin doses were reduced at 6 months compared with baseline in patients with T1D (basal dose ratio = 0.97 [95% CI = 0.95; 0.99], p = 0.011; bolus dose ratio = 0.86 [95% CI = 0.84; 0.89], p < 0.0001; and Supplementary Table S1). In patients with T2D, there was no significant change in basal insulin dose between baseline and 6 months of follow-up (ETD = 0.99 [NS]; and Supplementary Table S1), but the change was significant for bolus insulin dose (ETD = 0.94 [95% CI = 0.89; 0.99], p = 0.049; and Supplementary Table S1).
Rates of hypoglycemia were inputted as three mutually exclusive groups: non-severe events occurring during the day (daytime non-severe), non-severe events occurring during the night (nocturnal non-severe), and severe events. In the EU-TREAT study, the American Diabetes Association definition of severe hypoglycemia was used, defined as events requiring the assistance of another person to actively administer carbohydrate, glucagon, or other corrective actionsCitation22. Non-severe hypoglycemia was defined as all other reported hypoglycemic events, and nocturnal non-severe hypoglycemia was defined as events captured by the words ‘nocturnal’, ‘night’, or their equivalent in the report. RRs of hypoglycemia with degludec vs previous basal insulin were analyzed using a negative binomial regression model adjusted for type of diabetes, age, BMI, gender, diabetes duration, and duration of insulin therapy. In patients with T1D, there was no significant change in the rate of daytime non-severe hypoglycemic events, but there was a significant reduction in the rate of nocturnal non-severe (RR = 0.53 [95% CI = 0.40; 0.72], p < 0.0001) and severe hypoglycemic events (RR = 0.12 [95% CI = 0.05; 0.28], p < 0.0001) from baseline to 6 months of follow-up ( and Supplementary Table S1). In patients with T2D, there was a significant reduction in the rate of all hypoglycemic events at 6 months of follow-up (daytime non-severe (RR = 0.27 [95% CI = 0.14; 0.50], p < 0.0001); nocturnal non-severe (RR = 0.15 [95% CI = 0.07; 0.33], p < 0.0001); and severe [analyzed based on the rule of threeCitation23; Supplementary Table S1] (RR = 0.10 [95% CI = 0.00; 0.10], p < 0.05); and Supplementary Table S1).
As a base case, the parameters remained constant for the duration of the two scenarios. A life-long perspective was used to capture all relevant costs and effects. Values used for the model parameters in the baseline state for age, gender, diabetes duration, and HbA1c were from the studied patient cohort. As it was not possible to obtain all parameters from the cohort, the rest were obtained from the literature, using Italian patients when available (Supplementary Tables S1 and S2).
Costs and utilities
Costs were based on a healthcare payer perspective in 2017 Euros (€). Costs of insulins were based on the official Italian list prices (Supplementary Table S3) in 2017Citation24. The price of “other insulins” was calculated as a weighted average according to the proportion of patients on each basal insulin. The unit costs were multiplied by the number of units per day. The costs of resource use, including needles and self-measured blood glucose (SMBG) tests, were taken from the literatureCitation25,Citation26 assuming one needle and one SMBG test per injection with degludec or previous basal insulin (Supplementary Table S4).
The costs associated with diabetes-related complications were identified through various sources and a literature review, and inflated to 2017 values (Supplementary Table S4). Sources comprised public tariffs, government databases, registries publications, physicians’ consortium publications, or health-economic technology appraisals. Additionally, literature searches were performed on the EMBASE and PUBMED databases in English and subsequently in the language of each country of interest. Complications associated with diabetes that were used in this model were: annual management costs and direct costs associated with cardiovascular disease (CVD) complications, renal complications, acute events (e.g. hypoglycemia), eye disease, neuropathy, foot ulcer, and amputation (Supplementary Table S4).
For international comparisons, costs were converted from € to purchasing power parity (PPP) dollars (PPP $) based on the conversion rate of 0.69628Citation27.
To evaluate the impact of diabetes-related complications on patients’ quality-of-life, event disutility values were applied in the year the event occurred, and health state utility values were applied thereafter (Supplementary Table S5).
Sensitivity analyses
A number of sensitivity analyses were conducted to evaluate the robustness of the outcomes in the base-case model. The influence of discount rates of 0% and 8% were investigated. Analyses were conducted using 30, 10, 8, 5, and 1 years to examine the impact of variations of time horizons on long-term outcomes. An additional sensitivity analysis taking hypoglycemia (daytime non-severe, nocturnal non-severe, and severe hypoglycemia) as the only complication was also conducted, i.e. no other diabetes-related complications or mortality were included in this analysis. The individual influences of the various treatment effects (changes in HbA1c, BMI, rates of non-severe and severe hypoglycemia and basal insulin dose) were analyzed by abolishing each of the differences between degludec and the previous basal insulin. Additionally, sensitivity analyses using the upper and lower 95% confidence interval limits of these treatment effects were conducted. Other sensitivity analyses included those with disutilities of ±10%, diminishing the hypoglycemia disutility, using the United Kingdom Prospective Diabetes Study (UKPDS) 82 HbA1c progression equationCitation28, and assuming a fresh needle and SMBG test for every injection. Analyses based on two pre-switch insulin sub-groups (glargine U100 and IDet at baseline) were also conducted.
Results
Base-case analysis
In patients with T1D, treatment costs were higher for degludec, but were fully offset by the costs associated with diabetes-related complications, compared with previous basal insulin regimen (). QALYs were in favor of switching to degludec vs continuing previous basal insulin regimen with a QALY gain of 0.781 (). The long-term ICER for switching to degludec vs continuing previous basal insulin regimen in patients with T1D showed that switching to degludec was dominant compared with continuing the previous basal insulin regimen ().
Table 2. Base-case long-term cost-effectiveness analysis of degludec vs other basal insulins in T1D and T2D.
In patients with T2D, treatment costs were also higher for degludec, but were fully offset by the cost of severe hypoglycemia (). QALYs were also in favor of switching to degludec (QALY gain = 0.628; ). The long-term ICER for switching to degludec vs continuing previous basal insulin regimen also showed that degludec was dominant ().
Results from the base-case analysis are also presented in PPP $ (Supplementary Table S6).
In both T1D and T2D populations, switching to degludec was dominant compared with continuing the previous basal insulin regimen, meaning that patient health was improved in terms of QALYs for lower healthcare costs.
Sensitivity analyses
In patients with T1D, sensitivity analyses showed that shortening the time horizon to 5 years or 1 year and excluding the HbA1c difference between treatments had an impact on the cost-effectiveness outcomes showing that degludec is dominant compared with continuing the previous basal insulin regimen (). Using 5- and 1-year time horizons led to an increase in the ICER to €1,566 and €2,772 per QALY gained, respectively (). When only hypoglycemia was considered as the complication in the 1-year model, the QALY gain was estimated as €2,832 (). Abolishing the HbA1c difference between treatments led to the greatest impact, with €3,630 per QALY gained (). Outcomes for other sensitivity analyses remained dominant for switching to degludec compared with continuing the previous basal insulin regimen ().
Table 3. Sensitivity analysis results.
The NMB, based on a willingness-to-pay threshold of €30,000, of switching to degludec vs continuing previous basal insulin regimen was €29,710 for patients with T1D and €21,582 for patients with T2D (). The NMB results assuming a willingness-to-pay threshold of €0, €10,000, €20,000, €30,000, €40,000, and €50,000 are presented in Supplementary Table S7.
In patients with T2D, abolishing the difference in severe hypoglycemia led to the greatest impact, with an increase in the ICER to €4,606, and the sensitivity analysis based on the sub-group IDet at baseline resulted in an increase in the ICER to €2,237 ().
Discussion
The results from this long-term (lifetime) CEA showed that, in the Italian cohort of EU-TREAT patients with T1D or T2D, switching to degludec under conditions of routine clinical care was dominant compared with continuing previous basal insulins. Various sensitivity analyses demonstrated the robustness of the long-term modeling and identified that the HbA1c and severe hypoglycemia benefit gained by switching to degludec were the key drivers for improved outcomes in patients with T1D or T2D, respectively.
Results from the current study in an Italian setting are in line with those from previous CEAs that compared degludec with glargine U100 using data from phase 3 trials in a UK settingCitation2,Citation9. Using data from the BEGIN trials, degludec was shown to be dominant vs glargine U100 in patients with T1D and patients with T2D on a basal-only therapy (T2D-BOT) regimen, and cost-effective vs glargine U100 in patients with T2D on a basal−bolus regimen (T2D-BB)Citation9. In a CEA performed on data from the SWITCH trials, degludec was highly cost-effective compared with glargine U100 in participants with T1D using basal–bolus therapy and in T2D-BOTCitation2. Similarly, a CEA that used data taken from the patients in Sweden enrolled in a multinational study concluded that use of degludec was likely to be cost-effective compared with glargine U100 from a societal perspective in T1D, T2D-BOT, and T2D-BB over a 1-year time horizonCitation29.
The current findings emerge from real-world clinical practice, and are also consistent with the evidence from CEAs comparing degludec with other insulins using other real-world data. Degludec was shown to be highly cost-effective with an ICER of £10,754 vs glargine U100/IDet when including only the effects of hypoglycemia reduction in a UK settingCitation30. The short-term and long-term CEAs using data from a Swedish population of patients with T1D showed degludec was also dominant compared with other insulinsCitation31,Citation32.
RWE is increasingly being used to complement data from RCTs, and EU-TREAT demonstrated comparable outcomes in clinical practice to those of phase three trialsCitation5,Citation6,Citation33–35. A recent systematic review and network meta-analysis in adults with T1D based on 16 studies showed considerable uncertainty and significant network inconsistency with regard to severe hypoglycemiaCitation36. However, the majority of the studies demonstrated that, in patients with T1D and insulin-experienced patients with T2D, use of degludec significantly reduced the risk of overall and nocturnal symptomaticCitation5,Citation6, confirmed overall and nocturnalCitation33, and severe hypoglycemiaCitation5,Citation6,Citation35 compared with glargine U100. In insulin-naïve patients with T2D, the rates of nocturnal symptomatic and severe hypoglycemia were also significantly lower with degludec compared with glargine U100Citation34. The reproducibility of these results in terms of hypoglycemia demonstrates the credibility of the data from RWE and the current analyses based on data from EU-TREAT.
A recent RCT comparing degludec with glargine U300Citation37 contrasts with these multiple, consistent findings of the hypoglycemic benefit of degludec over glargine U100Citation4–6,Citation14,Citation35. While both insulins achieved similar glycemic control, the incidence and rates of hypoglycemia were comparable during the full study period, possibly due to the patient population selected in this trialCitation37. However, a recent study (CONFIRM) using a propensity-score matching method to compare the real-world effectiveness of basal insulins in insulin-naïve adults with T2D demonstrated significantly improved HbA1c and larger reductions in rates and likelihood of hypoglycemia with degludec vs glargine U300Citation38.
The relative weight of cost indicators in the current study was different for T1D compared with T2D. This is likely due to the different pathophysiology of the two diseases and the differing impact of glycemia on the development of complications. For example, while microangiopathy is a common feature of both types of diabetes, macroangiopathy is more frequent in T2D and is related to other factors, including lipid levels, hypertension, and obesityCitation39. Additionally, patients switch to degludec from other insulins at different ages for T1D and T2D. In this analysis, the greatest incremental cost savings for patients with T1D switching to degludec were CVD, renal complications, and eye disease. Whereas for patients with T2D, the greatest cost offsets to the treatment cost were due to differences associated with severe hypoglycemia and renal disease. Overall, the cost savings were greater for patients with T1D compared with patients with T2D, indicative of the lower mean age and resulting longer time for the benefits of the insulin to accumulate.
RWE is important to assess the effectiveness of new interventions in real-world clinical practice that could help inform the decision-making process for payers and clinicians. The real-world data presented in the current study, collected from Italian patients with T1D or T2D who switched to degludec from other basal insulins, represent a large population using degludec outside of a clinical trial setting. In terms of the benefits for healthcare systems, the evidence to date indicates that degludec provides greater effectiveness at lower costs compared with other insulins, for patients considered suitable for treatment with degludec. Cost savings are incurred with degludec due to a lower daily insulin dose and fewer hypoglycemic events compared with other insulins. These results are important for patients with T1D for whom fear of hypoglycemia is often a barrier to glycemic controlCitation40. For patients with T2D, the results of this cost-effectiveness analysis are relevant because direct medical costs related to hypoglycemia are generally greater than those for patients with T1D, due to more frequent hospitalizations and longer hospital staysCitation10.
This study has some limitations. First, the clinical data used to populate the economic model were retrieved from a retrospective observational study with no randomization or a comparator arm. Second, in terms of the long-term analysis using the CDM, it was assumed that there was a constant impact of the treatment throughout the lifetime of the patient, and the results were extrapolated over a longer period of time. Finally, the rates of non-severe hypoglycemia with degludec were lower than previously reported rates for both patients with T1D or T2DCitation5,Citation6,Citation33. However, as demonstrated by the sensitivity analysis, degludec was still dominant when non-severe hypoglycemic events were excluded from this analysis.
One of the strengths of this study is that the sensitivity analyses demonstrated the robustness of the results on various parameters, including hypoglycemia and insulin dose. Finally, the assumptions for the CEA were conservative, i.e. no additional resource utilization arising from twice-daily basal injections.
In conclusion, using data from 550 Italian patients with T1D or T2D switching to degludec from other basal insulins in a real-world Italian practice setting, it was demonstrated that degludec was a cost-effective option for patients with diabetes. In the case of T1D, the higher cost of degludec compared with other insulins was offset by the cost saving associated with improvements in CVD, renal complications, and eye disease. For patients with T2D, the greatest contributions to the dominance of degludec over other insulins were from lower rates of severe hypoglycemia and differences in renal complications.
Transparency
Declaration of funding
Sponsorship for the EU-TREAT study, this study, and article processing charges were funded by Novo Nordisk A/S.
Declaration of financial/other relationships
SH was an employee of Novo Nordisk at the time of the study; JG and MLW are employees of, and own stocks/shares in, Novo Nordisk A/S. AL declares no relevant conflict of interest. The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript discloses that they have consulted for Eli Lilly and Co. The reviewers have no other relevant financial relationships or otherwise to disclose.
Previous presentations
The data in this manuscript have not been previously published in this format. Some of the data in this manuscript have been presented in a poster at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 20th Annual European Congress 2017, 4–8 November 2017, Glasgow, UK (https://www.ispor.org/ScientificPresentationsDatabase/Presentation/76435?pdfid=52786).
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Material
Download MS Word (1.2 MB)Acknowledgements
The authors thank Deniz Tutkunkardas, Novo Nordisk A/S, for his review of, and input to, the manuscript, and also thank Barnaby Hunt, Ossian Health Economics and Communications GmbH, for the CORE Diabetes Model simulations. Medical writing and editorial support, under the guidance of the authors, was provided by Jin Heppell and Richard McDonald, of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk.
References
- Barnett AH, Cradock S, Fisher M, et al. Key considerations around the risks and consequences of hypoglycaemia in people with type 2 diabetes. Int J Clin Pract. 2010;64(8):1121–1129.
- Evans M, Mehta R, Gundgaard J, et al. Cost-Effectiveness of insulin degludec vs. insulin glargine U100 in Type 1 and Type 2 diabetes mellitus in a UK setting. Diabetes Ther. 2018;9(5):1919–1930.
- Heise T, Hermanski L, Nosek L, et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14(9):859–864.
- Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15(2):175–184.
- Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with Type 1 diabetes: the SWITCH 1 randomized clinical trial. JAMA. 2017;318(1):33–44.
- Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA. 2017;318(1):45–56.
- Davies M, Sasaki T, Gross JL, et al. Comparison of insulin degludec with insulin detemir in type 1 diabetes: a 1-year treat-to-target trial. Diabetes Obes Metab. 2016;18(1):96–99.
- Levin P. The cost-effectiveness of insulin glargine vs. neutral protamine Hagedorn insulin in type 2 diabetes: a focus on health economics. Diabetes Obes Metab. 2008;10(s2):66–75.
- Evans M, Chubb B, Gundgaard J. Cost-effectiveness of the long-acting basal insulin degludec (IDeg) compared with insulin glargine U100 (IGlar) in patients with type 2 diabetes: evidence from the SWITCH 2 trial. P396. Diabet Med. 2017;34(S1):152–153.
- Giorda CB, Rossi MC, Ozzello O, et al. Healthcare resource use, direct and indirect costs of hypoglycemia in type 1 and type 2 diabetes, and nationwide projections. Results of the HYPOS-1 study. Nutr Metab Cardiovasc Dis. 2017;27(3):209–216.
- Kristensen FB, Mäkelä M, Neikter SA, et al. European network for Health Technology Assessment, EUnetHTA: Planning, development, and implementation of a sustainable European network for Health Technology Assessment. Int J Technol Assess Health Care. 2009;25(S2):107–116.
- Rothwell PM. External validity of randomised controlled trials: ''To whom do the results of this trial apply?'' Lancet. 2005;365(9453):82–93.
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence – what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297.
- Siegmund T, Tentolouris N, Knudsen ST, et al. A European, multicentre, retrospective, non-interventional study (EU-TREAT) of the effectiveness of insulin degludec after switching basal insulin in a population with type 1 or type 2 diabetes. Diabetes Obes Metab. 2018;20(3):689–697.
- World Medical Association (WMA). Declaration of Helsinki – Ethical Principles fo rmedical Research Involving Human Subjects. 64th WMA General Assembly, Brazil. October. 2013.
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Sup1):S5–S26.
- McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–724.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford (UK): Oxford University Press; 2016.
- Capri S, Porta C, Delea TE. Cost-effectiveness of pazopanib versus sunitinib as First-line treatment for locally advanced or metastatic renal cell carcinoma from an italian national health service perspective. Clin Ther. 2017;39(3):567–580 e2.
- Kim K, Svedbom A, Luo X, et al. Comparative cost-effectiveness of bazedoxifene and raloxifene in the treatment of postmenopausal osteoporosis in Europe, using the FRAX algorithm. Osteoporos Int. 2014;25(1):325–337.
- Hunt B, Kragh N, McConnachie CC, et al. Long-term Cost-effectiveness of Two GLP-1 receptor agonists for the treatment of Type 2 diabetes mellitus in the italian setting: liraglutide versus lixisenatide. Clin Ther. 2017;39(7):1347–1359.
- American Diabetes Association. 6 Glycemic Targets: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61–S70.
- Eypasch E, Lefering R, Kum CK, et al. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ. 1995;311(7005):619.
- Bella Republiblica Italiana. Gazzetta Ufficiale 2017. [cited 2017]. Available from: http://www.gazzettaufficiale.it/. Last accessed in November 2017. [Italian]
- Tunis SL, Willis WD, Foos V. Self-monitoring of blood glucose (SMBG) in patients with type 2 diabetes on oral anti-diabetes drugs: cost-effectiveness in France, Germany, Italy, and Spain. Curr Med Res Opin. 2010;26(1):163–175.
- Afonso M, Ryan F, Pitcher A, et al. Evaluating drug cost per responder and number needed to treat associated with lixisenatide on top of glargine when compared to rapid-acting insulin intensification regimens on top of glargine, in patients with type 2 diabetes in the UK, Italy, and Spain. J Med Econ. 2017;20(6):633–639.
- Organisation for Economic Co-operation and Development (OECD). Purchasing power parities (PPP) [cited July 2019]. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm#indicator-chart
- Hayes AJ, Leal J, Gray AM, et al. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925–1933.
- Ericsson A, Pollock RF, Hunt B, et al. Evaluation of the cost-utility of insulin degludec vs insulin glargine in Sweden. J Med Econ. 2013;16(12):1442–1452.
- Vora J, Cohen N, Evans M, et al. Intensifying insulin regimen after basal insulin optimization in adults with type 2 diabetes: a 24-week, randomized, open-label trial comparing insulin glargine plus insulin glulisine with biphasic insulin aspart (LanScape). Diabetes Obes Metab. 2015;17(12):1133–1141.
- Gundgaard J, Landstedt-Hallin L, Ericsson Å, et al. Annual cost and effects of switching to insulin degludec from other basal insulins: Evidence from Swedish real-world data. Value Health. 2016;19(7):A673.
- Landstedt-Hallin L, Gundgaard J, Ericsson Å, et al. Cost-effectiveness of switching to insulin degludec from other basal insulins: evidence from Swedish real-world data. Curr Med Res Opin. 2017;33(4):647–655.
- Garber AJ, King AB, Prato SD, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498–1507.
- Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: A 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012;35(12):2464–2471.
- Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377(8):723–732.
- Dawoud D, O’Mahony R, Wonderling D, et al. Basal insulin regimens for adults with type 1 diabetes mellitus: a systematic review and network meta-analysis. Value Health. 2018;21(2):176–184.
- Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 Units/mL versus insulin degludec 100 Units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Dia Care. 2018;41(10):2147–2154.
- Tibaldi J, Hadley-Brown M, Liebl A, et al. A comparative effectiveness study of degludec and insulin glargine 300U/mL in insulin-naïve patients with type 2 diabetes. Diabetes Obes Metab. 2018;21(4):1001–1009.
- Sabatier F, Darmon P, Hugel B, et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51(9):2840–2845.
- Cryer PE. Hypoglycemia in type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2010;39(3):641–654.
