Abstract
Aims: Cumulative exogenous factor VIII (FVIII) exposure is an important predictor of developing neutralizing antibodies (inhibitors) to FVIII in patients with persons with hemophilia A (PwHA). The aim of this study was to model the costs of emicizumab versus FVIII prophylaxis and total treatment costs for patients with severe HA.
Materials and Methods: An Excel-based decision model was developed to calculate cumulative costs in PwHA over a 20-year time horizon from the US payer perspective. The model considered persons with severe HA beginning at age 12 months with no prior FVIII exposure and initiating prophylaxis with emicizumab or FVIII. PwHA could develop inhibitors on accumulation of 20 FVIII exposure days. PwHA with inhibitors replaced FVIII with bypassing agents until inhibitors resolved spontaneously, following immune tolerance induction (ITI), or at the end of the time horizon. The primary model outcome was the difference in emicizumab versus FVIII treatment costs in 2019 USD. Sensitivity analyses were performed to test the robustness of results.
Results: Total incremental cost over 20 years was −$1,945,480 (emicizumab arm, $4,919,058; FVIII arm, $6,864,538). Prophylaxis costs (emicizumab arm, $4,096,105; FVIII arm, $6,290,919) comprised the majority of costs in both groups, followed by breakthrough bleed treatment for the FVIII arm ($342,652) and ITI costs for the emicizumab arm ($733,671). Higher costs in the FVIII group reflected earlier inhibitor development (FVIII, 4 months; emicizumab, 162 months) and switch to bypassing agents.
Limitations: The model design reflects a simplified treatment pathway for patients with severe HA who initiate FVIII or emicizumab prophylaxis. In the absence of clinical data, a key conservative assumption of the model is that patients receiving emicizumab and FVIII prophylaxis have the same risk of developing inhibitors.
Conclusions: This study suggests that prophylaxis with emicizumab results in cost savings compared to FVIII prophylaxis in HA.
Introduction
Hemophilia A (HA) is an X-linked bleeding disorder that affects approximately 20,000 males in the United StatesCitation1. People with hemophilia A (PwHA) have a chronic hemostatic defect due to deficient or defective FVIII and are susceptible to spontaneous and trauma-related bleedingCitation2. Although HA is rare, it is associated with significant healthcare resource use and costs: an observational study of 6 federal hemophilia treatment centers reported mean annual healthcare costs (2011 USD) of $185,257 per person for US PwHACitation3.
In the past decade, prophylactic FVIII replacement therapy has become the standard of care for patients with severe HA, defined as residual FVIII levels less than 1 international units per deciliter (IU/dL)Citation4. FVIII prophylaxis has been shown to reduce the incidence of bleeding events, decrease healthcare resource utilization, and improve health-related quality of life and functionality compared to episodic treatmentCitation5,Citation6. However, FVIII prophylaxis for US patients with severe disease is costly, with a mean annual clotting factor cost (2011 USD) of $289,172 per person, representing 94% of individuals’ total direct medical costsCitation3.
Approximately 30% of patients with severe HA develop inhibitory antibodies (inhibitors) to FVIII, typically early in life, that neutralize factor replacement therapy requiring often less effective treatmentsCitation7. Inhibitor development is considered the most severe treatment-related complication in hemophilia and is associated with cumulative exposure to FVIII, with the highest risk occurring after 20–50 daysCitation8,Citation9. Inhibitors can be eradicated in approximately 70% of patients undergoing immune tolerance induction (ITI)Citation10, enabling them to resume treatment with FVIII concentrates, but ITI can cost upwards of $1 million per patient due to the large doses of clotting factor required to induce tolerance, and involve frequent intravenous infusions leading to burdensome treatmentCitation11. If inhibitors persist, patients may require further treatment and prevention of bleeding events with bypassing agents that are less effective and more expensive than clotting factor concentratesCitation11. Primarily due to these specialty drug expendituresCitation12, average annual treatment costs for patients with inhibitors can be up to 3–5 times higher compared to those without inhibitorsCitation13–15. Furthermore, health-related quality of life, particularly in physical domains, is significantly worse in patients with inhibitorsCitation16,Citation17.
Emicizumab is a recombinant, humanized, bispecific antibody that restores hemostasis by bridging activated factor IX and factor X. Emicizumab replaces the hemostatic function of FVIII, but does not induce or enhance the development of FVIII inhibitors due to lack of FVIII structural homologyCitation18. Patients receiving emicizumab are still at risk for inhibitor development because they are exposed to FVIII used to treat breakthrough bleeds (BTBs), but the risk is offset by the slower accumulation of FVIII exposure days. Phase 3 randomized clinical trials have evaluated the use of emicizumab in the prevention of bleeding episodes in patients with inhibitors (HAVEN 1, NCT02622321; HAVEN 2, NCT02795767)Citation19,Citation20 and those who do not have inhibitors (HAVEN 3, NCT02847637)Citation21; emicizumab prophylaxis led to significantly lower annual bleed rates (ABRs) compared to no prophylaxis in the trials, and compared to bypassing agent and FVIII prophylaxis received by patients prior to the trials.
Although prophylaxis has improved outcomes for patients with severe HA, cost-effective strategies are needed to optimize disease management in patients for whom prophylaxis is indicated. The objectives of this study were to compare the total cost of FVIII versus emicizumab prophylaxis, accounting for FVIII inhibitor development, and to describe total treatment costs for patients who develop inhibitors to FVIII versus those who do not.
Methods
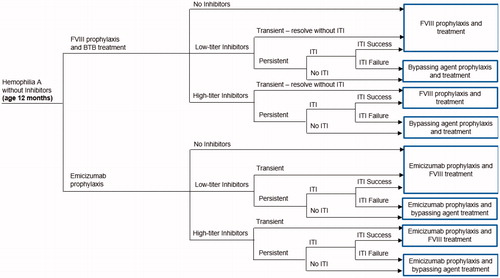
A decision tree model was developed using Microsoft Excel (Microsoft Corporation, Redmond, WA) applying the US healthcare payer perspective. The model population included hypothetical previously untreated patients (PUPs), one-year-old, male patients with severe HA who have no inhibitors or exposure to FVIII, and are initiating prophylaxis with either emicizumab or FVIII (). The primary model outcomes were the differences in treatment costs, number of BTBs and time to inhibitor development between the 2 treatment arms. A 20-year time horizon was chosen to capture the full treatment pathways during childhood including the development of an inhibitor.
Figure 1. Model structure. Abbreviations. BTB, breakthrough bleeds; FVIII, factor VIII; ITI, immune tolerance induction.
Model inputs and assumptions
A list of model parameters is provided in , and medication dosing is outlined in . The model assumes that these parameters are unchanged over the 20-year time horizon, with the exception of age-specific body weight, which was used to calculate medication doses.
Table 1. Model inputs for 12 month old previously untreated patients with severe hemophilia A receiving emicizumab or FVIII prophylaxis.
Table 2. Medication dosing model inputs for 12 month old previously untreated patients with severe hemophilia A receiving emicizumab or FVIII prophylaxis.
Prophylaxis
Emicizumab dosing was consistent with the HAVEN 3 trial and consistent with the product information packageCitation18,Citation21, using a loading dose of 3 mg/kg weekly for 4 weeks followed by a maintenance dose of 1.5 mg/kg weekly. Based on expert opinion, dosing for FVIII prophylaxis started at 30 IU/kg once weekly for 3 months and then increased to twice weekly for the next 3 months, followed by a maintenance dose of 30 IU/kg every other day for the remainder of the model, as indicated in the product label and reflecting general standard practiceCitation25. Patients in the FVIII arm who developed transient inhibitors received an increased FVIII dose of 50 IU/kg every other day for 6 months until their inhibitor resolved and then reverted to the previous dose. Patients in the FVIII arm who failed ITI (see inhibitor development section) were assumed to switch to activated prothrombin complex concentrate (aPCC) prophylaxis using a dose of 85 IU/kg every other dayCitation26.
Breakthrough bleed treatment
Patients in both arms initially received FVIII for the treatment of BTBs prior to the development of an inhibitor. Patients without an inhibitor received a single 50 IU/kg infusion while those with a low-titer inhibitor received 2 infusionsCitation2. Recombinant factor VIIa (rFVIIa) and aPCC were used to treat BTBs among patients with high-titer inhibitors or those in whom ITI failed; it was assumed that 80% of bleeds were treated with rFVIIa and 20% were treated with aPCC based on reported US population usage in childhoodCitation27. Patients treated with rFVIIa received a single 110 mcg/kg infusion while those treated with aPCC received two 85 IU/kg infusions, conservative estimates for bleeding event treatmentCitation26,Citation28. Dosing for aPCC did not exceed 100 IU/kg due to the risk of thrombotic microangiopathy and thrombotic events in patients receiving aPCC doses greater than 100 IU/kg over 24 hours while on emicizumabCitation18.
Annual bleed rate
The ABRs for emicizumab and FVIII prophylaxis was sourced from the HAVEN 3 trial, a randomized, multicenter, open-label, phase III study that evaluated the efficacy, safety, and pharmacokinetics of emicizumab prophylaxis versus no prophylaxis (episodic/on-demand FVIII treatment) in people with HA at least 12 years old without inhibitors to FVIIICitation21. ABR in HAVEN 3 was 1.5 in patients randomized to emicizumab at the labeled dose, and 4.8 during FVIII prophylaxis received by the same patients prior to study enrollment. The ABR for patients on aPCC prophylaxis (10.5) was calculated using the underlying ABR for on-demand treated patients from the HAVEN 3 trial (38.2) and applying a reduction of 72.5%, sourced from a randomized trial comparing aPCC prophylaxis and on-demand treatment in patients with inhibitorsCitation23.
Inhibitor development, treatment, and ITI
Patients in the model could develop low-titer (≤5 Bethesda units [BU]/mL) or high-titer (>5 BU/mL) inhibitors after ∼20 cumulative days (range, 20–50 days) of FVIII exposure, whether received for prophylaxis or episodic treatment in either armCitation9,Citation22. The probability of inhibitor development in patients with severe HA was obtained from the Survey of Inhibitors in Plasma-Product Exposed Toddlers (SIPPET) studyCitation22, which found a significantly higher incidence of inhibitors among patients receiving recombinant FVIII prophylaxis compared to those receiving plasma-derived FVIII. A weighted average risk across these two FVIII product categories was calculated for the model using market share assumptions of 6% for plasma-derived FVIII and 94% for recombinant FVIII, reflecting current US practice. Due to a lack of data on inhibitor development in patients receiving emicizumab prophylaxis, the probability of and number of exposure days required for inhibitor development were assumed to be the same in both treatment arms. However, inhibitor development was assumed to be delayed in the emicizumab arm due to less frequent FVIII exposure.
Inhibitors could be transient (i.e. spontaneously resolve within 6 months) or persistent. It was assumed that once patients had resolution of their inhibitors (spontaneously or via ITI), they did not return. In the FVIII arm, patients with low-titer transient inhibitors were assumed to receive 6 months of increased FVIII dosing and switch back to their previous dose upon resolution of their inhibitors. While, patients with high-titer transient inhibitors were assumed to receive 6 months of bypassing agents (85 IU/kg dose of aPCC prophylaxis every other day)Citation26 and switch back to FVIII treatment upon resolution of inhibitors. Patients in the emicizumab arm who developed inhibitors continued emicizumab prophylaxis but switched to high-dose FVIII (low-titer inhibitors) or bypassing agents (high-titer inhibitors) for BTB treatment.
The model assumed that a proportion of patients in each arm with persistent inhibitors would undergo ITI, which was assumed to start 3 months after identification of inhibitors and result in either success (eradication) or failure (chronic inhibitor). It was assumed that patients would undergo only 1 round of ITI using either a low-dose (50 mg/kg dose 3 times per week) or high-dose (200 mg/kg dose daily) regimen, the duration of which was reflective of the time to inhibitor resolution in the International ITI Study by Hay et al. (38 months and 22 months for the low-dose and high-dose regimens, respectively)Citation10. During ITI, patients received bypassing agents for BTB treatment only; no additional prophylaxis was given. Assumptions for the model’s ITI utilization and efficacy inputs were based on clinical opinion and the results of the International ITI study, and were assumed to be the same for both arms due to lack of such data for emicizumab at the time of the studyCitation10.
Costs
Medication doses were calculated using age-specific body weights obtained from US growth chartsCitation29,Citation30 and subsequently converted to costs using wholesale acquisition costs obtained from IBM Micromedex in January 2019Citation24. As medication costs comprise more than 90% of total direct costs in patients with severe HACitation3,Citation27, costs considered in the model were limited to FVIII, emicizumab, and bypassing agents costs and excluded healthcare resource use such as office and emergency visits and hospitalizations. It was assumed that no patients required joint surgery during the model’s time horizon, which was a reasonable assumption given that the starting population was 1 year of age and ended 20 years later.
Total costs are reported in 2019 US dollars for each treatment arm and disaggregated for prophylaxis, BTBs, ITI, and bypassing agents. In addition, costs were calculated for patients with and without inhibitors in each treatment arm, without consideration for the likelihood of each outcome. The costs do not reflect any product wastage or rounding of doses to the nearest vial size. Costs were discounted at 3% per year.
Sensitivity and scenario analyses
One-way sensitivity analyses were conducted for incremental costs and incremental number of cumulative BTBs over the 20-year time horizon. Model parameter values were varied by ±20% to generate an upper and lower bound for these analyses.
In addition, scenario analyses were conducted varying the model time horizon, age at initiation of prophylaxis, ABR for aPCC prophylaxis, and treatment pathway for patients who develop inhibitors in the FVIII arm. Model results were calculated for time horizons from 1 to 20 years to assess the short- and long-term economic impact of emicizumab prophylaxis compared to FVIII prophylaxis. Because PwHA may initiate prophylaxis before age 12 months, age at initiation of FVIII or emicizumab prophylaxis was reduced to 6 months to identify any impact on model outcomes. Multiple data sources were available for the underlying ABR in patients receiving aPCC. HAVEN 1 compared emicizumab prophylaxis to no prophylaxis in patients with inhibitors; in patients treated with bypassing agent prophylaxis prior to trial enrollment, ABR was 15.7 (compared to 10.5 used in the base case). Additional scenario analysis were done using recent data from the Atlanta protocol on the use of ITI in patients who develop inhibitors during emicizumab prophylaxis, consisting of FVIII given at 100 IU 3 times weekly, but due to incomplete efficacy data was assumed to be equally effective to high-dose and low-dose ITICitation31. Finally, because emicizumab is also indicated for prophylaxis in patients with inhibitors, is associated with a lower bleed rateCitation19,Citation20, and is less expensive than aPCC, it may be an economically attractive alternative. As such, the model also evaluated a scenario in which patients in the FVIII arm who develop high-titer inhibitors or fail ITI for low-titer inhibitors switch to emicizumab prophylaxis instead of aPCC prophylaxis.
Extrapolation to US population
The patient-level model outcomes were extrapolated to the US population to estimate the difference in total costs to the US health system when all previously untreated patients initiate emicizumab prophylaxis instead of FVIII prophylaxis over a 20-year period. An estimated 400 new cases of HA are diagnosed each yearCitation32, of which 50% are severeCitation33. Of these, an estimated 80.7% receive prophylaxisCitation34. The model assumes that these parameters are unchanged over the 20-year model time horizon.
Results
Base case (per-patient level)
presents the total and incremental results over a 20-year time horizon for emicizumab and FVIII prophylaxis. The total discounted costs for patients treated with emicizumab were $4,919,058 compared to $6,864,538 for patients treated with FVIII (difference, −$1,945,480). Prophylaxis costs comprised the majority of costs in both groups: 83.3% for emicizumab and 91.6% for FVIII. Bleed treatment accounted for 5.0% of total costs in FVIII arm, compared to 1.8% in the emicizumab arm. Total costs were higher in the FVIII arm, both in patients who did and did not develop inhibitors; in the emicizumab arm, however, cost savings were substantially greater in patients who developed inhibitors due to avoidance of aPCC prophylaxis.
Table 3. Base case results: modeled 20-year costs and outcomes in 12 month old previously untreated patients with severe hemophilia A receiving emicizumab or FVIII prophylaxis.
There were 77 fewer bleeds for patients treated with emicizumab over the modeled time horizon, with time to inhibitor development being approximately 13 years later for emicizumab. Patients treated with emicizumab compared to FVIII prophylaxis had higher ITI costs both in absolute terms and as a percentage of total costs: $733,671 (14.9%) for emicizumab versus $230,967 (3.4%) for FVIII. The higher ITI costs for emicizumab resulted from later inhibitor development (162 months, or 13.5 years vs 4 months), at which time patients are older, weigh more, and thus require a higher total dose. Conversely, the higher total costs and bleed treatment costs in the FVIII group result from earlier inhibitor development and switch to bypassing agents.
Sensitivity analysis
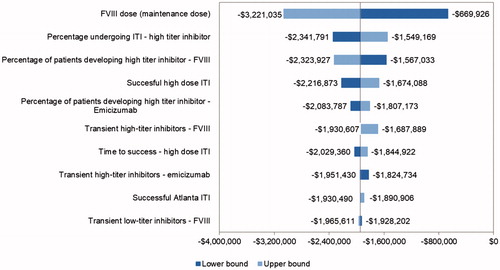
presents the one-way sensitivity analysis on incremental costs over a 20-year time horizon. Individualization of FVIII prophylaxis is common based on pharmacokinetic FVIII studiesCitation35–39. The FVIII prophylaxis maintenance dose was the biggest driver in the model: If the dose was reduced to 20 IU/kg, the cost savings fell to about $670,000; if the dose was increased to 40 IU/kg, the cost savings increased to more than $3 million. The percentage of patients developing inhibitors and ITI parameters, including the percentage of patients undergoing ITI, success rates, and duration of ITI, were also key drivers of model results.
Scenario analyses
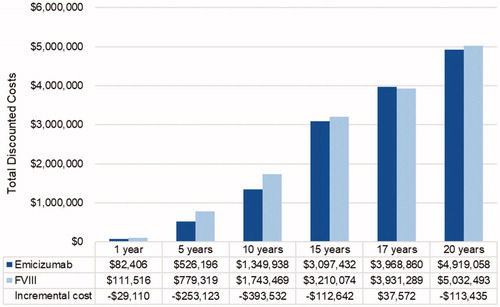
We compared total costs at 1, 5, 10, 15, 17 and 20-year time horizons. Costs in the FVIII arm were consistently higher than in the emicizumab arm, with cost savings starting at −$47,814 in year 1 and increasing to −$1,945,480 in year 20 ().
Figure 3. Scenario analysis: total discounted cost by time horizon. Costs presented in 2019 US dollars. Abbreviation. FVIII: factor VIII.
Initiating FVIII or emicizumab prophylaxis at 6 months of age rather than 12 months had minimal impact on the results; Incremental discounted costs decreased from −$1,945,480 in the base case to −$1,868,888 in this scenario. Prophylaxis and ITI costs decreased slightly in both groups due to lower patient weight at the time ITI was given.
When the ABR in patients receiving aPCC prophylaxis was increased from 10.5 to 15.7, the cost of treating BTBs increased in both arms, but more so in the FVIII arm because patients develop inhibitors earlier and receive aPCC prophylaxis over a longer time period. As a result, cost savings with emicizumab were greater in this scenario (−$2,021,420) compared to the base case (−$1,945,480).
Use of the Atlanta protocol rather than low- or high-dose ITI regimens resulted in lower costs in the emicizumab arm compared to the base case ($4,727,808 vs. $4,919,058), due to lower FVIII doses used. In addition, if patients in the emicizumab arm did not undergo ITI upon development of inhibitors (i.e. continued treatment of breakthrough bleeds with higher-dose FVIII for low-titer inhibitors and bypassing agents for high-titer inhibitors) total costs were further reduced compared to the base case ($4,192,021 vs. $4,919,058). The avoidance of ITI saved $733,671 and was only partially offset by an increase in the cost of bleed treatment compared to the base case ($95,916 vs. $89,282), due to higher use of bypassing agents for BTBs.
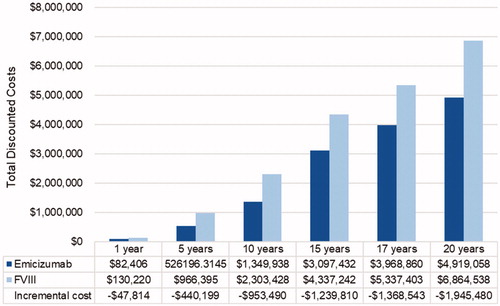
If patients in the FVIII arm who develop high-titer inhibitors or fail ITI for low-titer inhibitors switched to emicizumab prophylaxis instead of aPCC prophylaxis, costs were lower in the emicizumab arm for the first 15 years (). Between 15 and 17 years, patients in the emicizumab arm begin to undergo ITI, and thus cumulative costs exceed those in the FVIII arm at year 17 ($37,572). By year 20, accumulation of costs in the FVIII group again outpaces that in the emicizumab group, and total costs are $113,435 lower in the emicizumab group.
Extrapolation to US population
The model estimated that if all previously untreated persons with severe HA in the US start prophylaxis with emicizumab instead of FVIII over the next 20 years, a total of 131,273 bleeds could be prevented and $3.10 billion in healthcare costs could be avoided using the US payer perspective.
Discussion
The model results estimate emicizumab prophylaxis to be a cost-effective treatment for severe HA PUPs when compared to the current standard of care, FVIII prophylaxis. Prophylaxis comprised the majority of costs in both treatment arms and bleed treatment costs were a higher proportion of total costs in the FVIII prophylaxis arm (5.1% vs 1.8%).
The model results complement the clinical data supporting the use of emicizumab in patients without inhibitors, which showed a reduction in ABR from 4.8 to 1.5 in patients switching from FVIII to emicizumab prophylaxisCitation21. Additionally, the modeled emicizumab prophylaxis delayed the development of inhibitors by more than 13 years compared to FVIII prophylaxis.
Costs of FVIII prophylaxis in our model were aligned with previous models and studies. In a model comparing costs of prophylaxis with recombinant and plasma-derived FVIII for previously untreated patients, Neufeld and colleagues found 5-year costs of $834,621 and $1,237,163, respectively, compared to $754,259 in our modelCitation27. The differences are most likely due to inclusion of hospitalization costs and assumption that all patients undergo ITI in the previous analysis. In a real-world analysis using data from the Hemophilia Utilization Group Study part-Va, (HUGS-Va), mean annual clotting factor costs for patients with severe hemophilia receiving FVIII prophylaxis were $289,172 in 2011 USD ($353,992 in 2018 USD), compared to $328,967 in our modelCitation3. Mean age of patients in the HUGS-Va study was 21.1 years, reflecting a greater patient weight and higher FVIII doses than patients in our model. Finally, a budget impact model estimated cost for FVIII prophylaxis per patient at $606,913 over 2 years (excluding recombinant FVIII Fc) without ITICitation40, similar to our estimate of $317,269 per year averaged over the 20-year model time horizon.
The sensitivity analysis results consistently showed cost savings for emicizumab when FVIII doses were varied by 20% in each direction, although there was a large difference in incremental cost savings. The sensitivity analysis also consistently showed a lower number of BTBs for emicizumab-treated patients, potentially leading to improved quality of life and additional hospital and emergency cost savings which were not captured.
The results of the scenario in which patients with inhibitors in the FVIII arm switch to emicizumab prophylaxis are of particular interest, as this is an approved indication for emicizumab. The real-world cost difference between a strategy of emicizumab prophylaxis in previously untreated patients (emicizumab arm) and the current standard of practice, i.e. initiating FVIII prophylaxis and switching to emicizumab (scenario) or bypassing agents, likely lies somewhere between the model’s base case cost savings of −$1.95 million and the scenario cost savings of $113,435. There is no standard of care but general sense from physicians is that there still is a role for inhibitor eradication using FVIII ITI. There is emerging data to support a combined approach of ITI while on emicizumab prophylaxis.
To our knowledge, this is the first study to evaluate the costs and outcomes of emicizumab prophylaxis, compared to FVIII prophylaxis, in PUPs with severe HA. The modeled treatment pathway is clinically realistic and flexible, including low- and high-titer inhibitors, spontaneous resolution of transient inhibitors, and options to pursue ITI or maintain higher doses of FVIII for low-titer and bypassing agents for high-titer inhibitors. The ABRs used in the model are sourced from well-designed, randomized clinical trials. Finally, the model considered a number of alternative scenarios regarding the time horizon, age at initiation of prophylaxis, and ABR on bypassing agents.
Forthcoming studies will allow for further elucidation of the effectiveness of emicizumab use in various clinical scenarios. Data on the use of emicizumab prophylaxis in infants as young as 2 months old with inhibitors were recently published, with all 11 patients experiencing no spontaneous bleeds and marked reduction in bypassing agent utilization over a median of 36 weeks of follow-upCitation41. An observational study (MOTIVATE; NCT04023019) has been designed to compare ITI with FVIII, bypassing agent prophylaxis and/or breakthrough bleed treatment; ITI with FVIII, emicizumab prophylaxis, and bypassing agents for breakthrough bleeds; and emicizumab or bypassing agent prophylaxis without ITI and with bypassing agents for breakthrough bleeds. An additional concern in the hemophilia community is the lack of emicizumab safety and efficacy data in very young patientsCitation42. As such, a study (NCT04030052) has been initiated to prospectively investigate the safety, immunogenicity and hemostatic efficacy of prophylactic emicizumab given with a concomitant low dose rFVIII in infants and children <3 years old with hemophilia A who have had little to no previous exposure to FVIII. While we await these important data, our model provides a clinically realistic simulation of costs and outcomes based on the best evidence currently available.
Limitations
The model design reflects a simplified treatment pathway for patients with severe HA who initiate FVIII or emicizumab prophylaxis. Clinical events such as partial ITI success (i.e. from high-titer to low-titer inhibitors) and the return of inhibitors in patients who undergo successful ITI were not included in the model. Infections resulting from the presence of central venous access devices, which are often used in FVIII prophylaxis patients due to frequent infusionsCitation43,Citation44, were not included in the model, nor were joint surgeries such as synovectomies and arthroplasties. These limitations are likely to bias the results against emicizumab by underestimating costs in the FVIII arm, given the higher ABR with FVIII prophylaxis. Except for treatment changes resulting from inhibitor development and resolution, the model assumes that treatment is stable over the 20-year time horizon. Prophylaxis discontinuation, changes in prophylaxis frequency, and switching between treatments (e.g. between recombinant and plasma-derived FVIII products) are not incorporated in the model. It is possible that costs are over-estimated in both arms due to discontinuation of prophylaxis in adulthood in some patients, but this is not a current standard of practiceCitation45,Citation46.
Clinical data for the model’s emicizumab arm are limited to the HAVEN 1, 2, and 3 trials. The model’s target population is PUPs 12 months of age, but HAVEN 1 and HAVEN 3 were conducted in patients aged 12 years or older at enrollment. Because ABR may increase with ageCitation47, use of ABR from adult trials my over-estimate ABR in children. None of the HAVEN trials included ITI, thus the optimal ITI regimen for patients receiving emicizumab is unknown. Data supporting the Atlanta protocol are encouraging, but immature. It is possible that patients on emicizumab who develop inhibitors and are successfully treated with ITI may require low-dose regimen ITI or for shorter duration but conversely, these patients may also require a small maintenance dose of FVIII to maintain tolerization, the costs of which are not included in the model.
In the absence of clinical data, a key conservative assumption of the model is that patients receiving emicizumab prophylaxis have the same risk of developing inhibitors as those receiving FVIII prophylaxis. Although no de novo inhibitors developed during HAVEN 3, this is likely due to the fact that patients were at least 12 years old, had substantial previous FVIII exposure, and had no documented inhibitor in the last 5 yearsCitation21. Studies with longer follow-up are needed to determine whether the risk of inhibitor development is impacted by the prophylaxis regimen used. Lower risk of inhibitor development with emicizumab would result in additional cost savings compared to those estimated by the model.
Expert opinion was sought to fill gaps in parameter estimates for some model inputs due to a lack of data. These inputs included the proportion of patients with low- and high-titer inhibitors who undergo ITI, the distribution of low- and high-dose regimens among patients undergoing ITI, and the proportion of BTBs treated with rFVIIa and aPCC in patients with inhibitors. Expert opinion was also used to supplement published data on the number of FVIII exposure days after which inhibitors develop, and the dose of aPCC used for the treatment of BTBs.
Conclusions
Assuming the same rate of inhibitor development, prophylaxis with emicizumab is associated with cost savings of nearly $2 million per patient over 20 years compared to FVIII prophylaxis in PUPs with severe HA. These findings were robust across various time horizons (1 to 20 years), ABRs, probabilities of inhibitor development, medication dosing and cost ranges, and ITI scenarios. In addition, emicizumab prophylaxis delayed the development of inhibitors by more than 13 years compared to FVIII prophylaxis. Cost savings of more than $3 billion could be potentially realized if all previously untreated patients with severe HA initiated prophylaxis with emicizumab instead of FVIII over the next 20 years.
Transparency
Declaration of funding
Research and analysis were funded by Genentech, Inc.
Declaration of financial/other interests
AP and KR are employees of Genentech, Inc. SCorman and SChaplin are employees of Pharmerit International and served as consultants to Genentech, Inc. during this study. RS has research funded by Genentech, Octapharma, Shire, Grifols and Kedrion and participated in advisory boards with the above and CSL Behring, Bayer, Roche, Uniqure, Biomarin and Spark. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors were involved in the conception and design of the model, interpretation of the results, drafting the paper and revising it critically for intellectual content, and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Previous presentations
Presented at AMCP Nexus 2018, Orlando, FL, October 22–25, 2018 and American Society of Hematology 2018 Annual Meeting, December 1–4, 2018.
Acknowledgements
Editorial support was provided by Catherine Mirvis of Pharmerit International.
References
- Centers for Disease Control and Prevention. Data & statistics: hemophilia. 2016 [cited 2018 Aug 28]. Available from: https://www.cdc.gov/ncbddd/hemophilia/data.html.
- Valentino LA, Mamonov V, Hellmann A, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on‐demand and prophylaxis treatments in hemophilia A management. J Thrombosis Haemostasis. 2012;10(3):359–367.
- Zhou ZY, Koerper MA, Johnson KA, et al. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ. 2015;18(6):457–465.
- Fischer K, Ljung R. Primary prophylaxis in haemophilia care: guideline update 2016. Blood Cells Mol Dis. 2017;67:81–85.
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544.
- O'Hara J, Sima CS, Frimpter J, et al. Long-term outcomes from prophylactic or episodic treatment of haemophilia A: a systematic review. Haemophilia. 2018;0(0):1–11.
- Rota M, Cortesi PA, Steinitz-Trost KN, et al. Meta-analysis on incidence of inhibitors in patients with haemophilia A treated with recombinant factor VIII products. Blood Coag Fibr. 2017;28(8):627–637.
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47.
- Gouw SC, van der Bom JG, Marijke van den Berg H. Marijke van den Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648–4654.
- Hay CRM, DiMichele DM. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119(6):1335–1344.
- Colowick AB, Bohn RL, Avorn J, et al. Immune tolerance induction in hemophilia patients with inhibitors: costly can be cheaper. Blood. 2000;96(5):1698–1702.
- Chen SL. Economic costs of hemophilia and the impact of prophylactic treatment on patient management. Am J Manag Care. 2016;22(5 Suppl):S126–S133.
- Guh S, Grosse SD, McALISTER S, et al. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. 2012;18(2):268–275.
- Guh S, Grosse SD, McAlister S, et al. Health care expenditures for Medicaid-covered males with haemophilia in the United States, 2008. Haemophilia. 2012;18(2):276–283.
- Armstrong EP, Malone DC, Krishnan S, et al. Costs and utilization of hemophilia A and B patients with and without inhibitors. J Med Econ. 2014;17(11):798–802.
- Morfini M, Haya S, Tagariello G, et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia. 2007;13(5):606–612.
- McLaughlin JM, Munn JE, Anderson TL, et al. Predictors of quality of life among adolescents and young adults with a bleeding disorder. Health Qual Life Outcomes. 2017;15(1):67.
- HEMLIBRA [prescribing information]. Genentech Inc. South San Francisco, CA. November. 2018.
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–818.
- Young G, Sidonio RF, Liesner R, et al. HAVEN 2 updated analysis: multicenter, open-label, phase 3 study to evaluate efficacy, safety and pharmacokinetics of subcutaneous administration of emicizumab prophylaxis in pediatric patients with hemophilia a with inhibitors. Blood J. 2017;130:85.
- Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–822.
- Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054–2064.
- Antunes SV, Tangada S, Stasyshyn O, et al. Randomized comparison of prophylaxis and on-demand regimens with FEIBA NF in the treatment of haemophilia A and B with inhibitors. Haemophilia. 2014;20(1):65–72.
- IBM Micromedex. Red book online [cited 7 Jan 2019]. Available from: http://www.micromedexsolutions.com/micromedex2/librarian/ (subscription required).
- ADVATE (antihemophilic factor [recombinant] package insert). Westlake Village, CA: Baxalta US Inc. 2016.
- FEIBA (anti-inhibitor coagulant complex) package insert. 2018. Available from: https://www.shirecontent.com/PI/PDFs/FEIBA_USA_ENG.pdf.
- Neufeld EJ, Sidonio RF, O’Day K, et al. Cost analysis of plasma-derived factor VIII/von Willebrand factor versus recombinant factor VIII for treatment of previously untreated patients with severe hemophilia A in the United States. J Med Econ. 2018;21(8):762–769.
- NOVOSEVEN (Coagulation - Factor VIIa [Recombinant]) package insert. Accessed 7 January 2018.
- Centers for Disease Control and Prevention. Data table for boys length-for-age and weight-for-age charts [cited 23 Jul 2018]. Available from: https://www.cdc.gov/growthcharts/who/boys_length_weight.htm.
- World Health Organization. Data table for boys length-for-age and weight-for-age charts [cited 23 Jul 2018]. Available from: https://www.cdc.gov/growthcharts/data/zscore/wtage.xls.
- Batsuli G, Zimowski KL, Tickle K, et al. Immune tolerance induction in paediatric patients with haemophilia A and inhibitors receiving emicizumab prophylaxis. Haemophilia. 2019. doi: 10.1111/hae.13819.
- Centers for Disease Control and Prevention. Data & statistics: hemophilia [cited 23 Jul 2018]. Available from: https://www.cdc.gov/ncbddd/hemophilia/data.html.
- Centers for Disease Control and Prevention. Report on the Universal Data Collection Program, 2005–2009 [cited 6 Aug 2018]. Available from: https://www.cdc.gov/ncbddd/blooddisorders/udc/documents/report-udcprogram_january2005-december-2009_jan-2014.pdf.
- American Thrombosis & Hemostasis Network. ATHN Research Report Brief - September 30, 2017 [cited 28 Aug 2018]. Available from: https://athn.org/documents/document_file/302.
- Nagao A, Yeung CHT, Germini F, et al. Clinical outcomes in hemophilia A patients undergoing tailoring of prophylaxis based on population-based pharmacokinetic dosing. Thromb Res. 2019;173:79–84.
- Megias-Vericat JE, Bonanad S, Haya S, et al. Bayesian pharmacokinetic-guided prophylaxis with recombinant factor VIII in severe or moderate haemophilia A. Thromb Res. 2019;174:151–162.
- Mingot-Castellano ME, Parra R, Nunez R, et al. Improvement in clinical outcomes and replacement factor VIII use in patients with haemophilia A after factor VIII pharmacokinetic-guided prophylaxis based on Bayesian models with myPKFiT((R)). Haemophilia. 2018;24(5):e338–e343.
- Iorio A. Using pharmacokinetics to individualize hemophilia therapy. Hematology Am Soc Hematol Educ Program. 2017;2017(1):595–604.
- Iorio A, Edginton AN, Blanchette V, et al. Performing and interpreting individual pharmacokinetic profiles in patients with Hemophilia A or B: Rationale and general considerations. Res Pract Thromb Haemost. 2018;2(3):535–548.
- McMullen S, Buckley B, Hall E, et al. Budget impact analysis of prolonged half-life recombinant FVIII therapy for hemophilia in the United States. Value Health. 2017;20(1):93–99.
- Barg AA, Avishai E, Budnik I, et al. Emicizumab prophylaxis among infants and toddlers with severe hemophilia A and inhibitors-a single-center cohort. Pediatr Blood Cancer. 2019;66:e27886.
- Pierce GF, Hart DP, Kaczmarek R. Safety and efficacy of emicizumab and other novel agents in newborns and infants. Haemophilia. 2019. doi: 10.1111/hae.13822.
- Komvilaisak P, Connolly B, Naqvi A, et al. Overview of the use of implantable venous access devices in the management of children with inherited bleeding disorders. Haemophilia. 2006;12(s6):87–93.
- Santagostino E, Mancuso ME. Venous access in haemophilic children: choice and management. Haemophilia. 2010;16(s1):20–24.
- Walsh CE, Valentino LA. Factor VIII prophylaxis for adult patients with severe haemophilia A: results of a US survey of attitudes and practices. Haemophilia. 2009;15(5):1014–1021.
- Ljung R, Gretenkort Andersson N. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169(6):777–786.
- Scott MJ, Xiang H, Hart DP, et al. Treatment regimens and outcomes in severe and moderate haemophilia A in the UK: The THUNDER study. Haemophilia. 2018;25(2):205–212.