Abstract
Aims: Non-valvular atrial fibrillation (NVAF) prevalence increases with age. Hence, evaluating the economic burden among older-aged patients is vital. This study aimed to compare healthcare resource utilization (HRU) and costs among newly-diagnosed older-aged NVAF patients treated with warfarin, rivaroxaban, or apixaban vs. dabigatran.
Materials and Methods: Newly-diagnosed older-aged (aged ≥65 years) NVAF patients initiating dabigatran, warfarin, rivaroxaban, or apixaban (first prescription date = index date) from 01JAN2010-31DEC2015 and with continuous enrollment for ≥12 months pre-index date were included from 100% Medicare database. Patient data were assessed until drug discontinuation/switch/dose change/death/disenrollment/study end (up to 12 months). Dabigatran initiators were 1:1 propensity score-matched (PSM) with warfarin, rivaroxaban, or apixaban initiators. Generalized linear models were used to compare all-cause HRU and costs per-patient-per-month (PPPM) between the matched cohorts.
Results: After PSM with dabigatran, 70,531 warfarin, 51,673 rivaroxaban, and 25,209 apixaban patients were identified. Dabigatran patients had significantly fewer generalized-linear-model-adjusted PPPM hospitalizations (0.114 vs. 0.123; 0.111 vs. 0.121), and outpatient visits (2.864 vs. 4.201; 2.839 vs. 2.949) than warfarin and rivaroxaban patients, respectively, but had significantly more PPPM hospitalizations (0.103 vs. 0.090) and outpatient visits (2.780 vs. 2.673) than apixaban patients (all p < .0001). Dabigatran patients incurred significantly lower adjusted total PPPM costs ($3,309 vs. $3,362; $3,285 vs. $3,474) than warfarin and rivaroxaban patients, respectively (all p < .01) but higher total PPPM costs ($3,192 vs. $2,986) than apixaban patients (all p < .0001).
Limitations: This study is subject to the inherent limitations of any claims dataset, including potential bias from coding errors and identification of medical conditions using diagnosis codes as opposed to clinical evidence. Medications filled over-the-counter or provided as samples by the physician are never captured in claims data.
Conclusions: Newly-diagnosed older-aged NVAF patients initiating dabigatran incurred significantly lower adjusted all-cause HRU and costs than warfarin and rivaroxaban patients but higher adjusted HRU and costs than apixaban patients.
Introduction
Atrial fibrillation (AF) is the most common arrhythmiaCitation1, affecting 2% of the world’s populationCitation2 and 2.7–6.1 million people in the United StatesCitation3,Citation4 Given the aging US population and increasing risk of AF with age, the estimated prevalence of AF is expected to double by 2030 and triple by 2050Citation5–7. AF is associated with poor quality of life and a substantially increased risk of stroke and other thromboembolic eventsCitation1–3 In the US, about 15% of all strokes and thromboembolic events are associated with AFCitation3. According to the American Heart Association, the total cost of stroke, including direct and indirect expenses is estimated to grow from $105.2 billion in 2012 to $240.7 billion by 2030, thus adding a substantial burden on the US economyCitation8. About 96% of AF patients in developed countries have non-valvular atrial fibrillation (NVAF), defined as AF without mitral stenosis or valvular prosthesesCitation2. Patients with NVAF exhibit symptoms such as chest pain, breathing difficulty, and have an increased risk of stroke or thromboembolic events compared to the non-NVAF populationCitation9–11.
Since the 1950s, the vitamin K antagonist warfarin has been the most common oral anticoagulant (OAC) prescribed for NVAF patientsCitation2,Citation3,Citation12. A meta-analysis of randomized trials showed that adjusted-dose warfarin reduced the risk of stroke by 64% when compared to placebo and by 37% compared to antiplatelet therapy such as aspirinCitation13. Despite warfarin being relatively inexpensive, it requires regular monitoring and dose modificationCitation14, due to interactions with food, other drugs, alcohol, factors such as liver function, age-related alterations, and certain genetic variations, some of which may predispose patients to unsafe bioavailabilityCitation2,Citation3.
In recent years, novel oral anticoagulants (NOACs) including dabigatran, rivaroxaban and apixaban have been approved by the US Food & Drug Administration for prevention of stroke in NVAF patientsCitation15,Citation16. These NOACs have been found to be safe and efficacious among NVAF patientsCitation17–21. Dabigatran, which is available in 2 dosages (110 mg and 150 mg), is associated with lower rates of stroke and major bleeding than warfarinCitation17. Rivaroxaban is associated with similar efficacy and lower rates of stroke and intracranial bleeding compared to warfarinCitation19. Apixaban is associated with lower rates of major bleeding, and lower all-cause mortality rates compared to warfarinCitation18. With NVAF’s definition and care management still evolving, current guidelines of care suggest that patients treated with NOACs may have an improved prognosisCitation1,Citation20–22. Although NOACs are more expensive than warfarin, they have wide therapeutic windows that allow for predictable dosing, thus reducing the need for periodic monitoringCitation3.
In addition to safety and effectiveness differences, real-world studies have also examined the economic burden associated with NOACs compared to warfarinCitation23–26. Although NOACs have higher prescription costs, they are associated with lower healthcare resource utilization (HRU) and costs compared to warfarinCitation5,Citation27. It has also been suggested that dabigatran and apixaban are cost-effective NOACsCitation28. A recent retrospective study using commercial database comparing OACs to dabigatran found that dabigatran users had lower all-cause HRU and costs compared to warfarin and rivaroxaban users but had all-cause HRU and costs similar to apixaban usersCitation5.
Given the lack of focus on Medicare populations and the increasing prevalence of NVAF with age, it would be informative to evaluate the economic burden among Medicare beneficiaries (aged ≥65 years) treated with OACs. Expanding on findings from prior retrospective studies in different populations, the current analysis compared all-cause HRU and costs of newly-diagnosed and newly-treated NVAF patients prescribed warfarin, rivaroxaban, or apixaban, vs. dabigatran. Dabigatran was the first approved NOAC, and it was therefore chosen as the reference drug for comparison with the other OACs in the present study. Additionally, our study provides the data to the payer with direct head-to-head comparison among NOACs that are needed in their formulary decision making.
Methods
Data source
This was a retrospective cohort study using 100% Medicare database from 01 Jan 2010–31 Dec 2015. Medicare provides health insurance coverage to 46 million persons aged ≥65 years in the USCitation29,Citation30. For insured beneficiaries, Medicare covers the costs of hospitalizations through Medicare Part A, the costs of physicians’ services through Medicare Part B, and the costs of prescription drugs through Medicare Part D. About a quarter of Medicare enrollees receive their insurance through Medicare Advantage (Part C). Neither patient identity nor medical records were disclosed for the purposes of this study. The study was exempt from Institutional Review Board approval as the data were de-identified and only aggregate results were reported. Compliance with all applicable laws and the Health Insurance Portability and Accountability Act regulations were maintained.
Subject selection
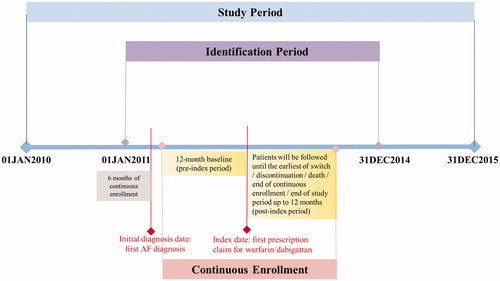
Patients were included in the study if they had ≥1 inpatient or outpatient claim for AF (International Classification of Diseases, 9th Revision, Clinical Modification: 427.31) between 01 Jan 2011 and 31 Dec 2014 (identification period). Patients were required to have been continuously enrolled (fee-for-service only) with medical (Parts A and B) and pharmacy (Part D) benefits for ≥6 months prior to the first observed AF diagnosis. Additionally, patients were required to have ≥1 outpatient pharmacy claim with a National Drug Code indicative of dabigatran, warfarin, rivaroxaban, or apixaban on or after the first observed AF diagnosis and between the launch date for each NOAC and 31 Dec 2014 (dabigatran vs. warfarin: 01 Jan 2011; dabigatran vs. rivaroxaban: 01 Nov 2011; dabigatran vs. apixaban: 01 Dec 2012). The first pharmacy claim for dabigatran, warfarin, rivaroxaban, or apixaban was designated as the index date. Patients were required to be ≥65 years of age on the index date and have continuous enrollment in Part A, B, and D health plans for ≥12 months prior to the index date (baseline period = 12 months prior to the index date). Patients were required to have no evidence of AF or cardiac surgery, hyperthyroidism, myocarditis, pericarditis, pregnancy, pulmonary embolism, valve replacement, chronic rheumatic heart disease, or valvular disease in the 6 months prior to the first observed AF diagnosis. Additionally, patients were excluded from the study if they had evidence of any anticoagulants use (dabigatran, rivaroxaban, warfarin, apixaban, argatroban, dalteparin, enoxaparin, fondaparinux, heparin, or tinzaparin) in the 6-month pre-index period or had evidence of >1 anticoagulant or >1 dose of the index NOAC on the index date. Patients’ data were assessed until the earliest of discontinuation of the index medication, switch to a non-index OAC, change in dosage of the index NOAC, death, end of continuous health plan enrollment, study end (31 Dec 2015), or end of the ≥12-month follow-up period (). Each NOAC captured had multiple dosages, and physicians are likely to change these dosages depending on the creatinine clearance level and various other patient factors. To make sure that the HRU and costs were only captured during the course of the index treatment, all patients were censored at the earliest of discontinuation of the index medication, switch to a non-index OAC, change in dosage of the index NOAC, death, end of continuous health plan enrollment, study end, or end of the ≥12-month follow-up period. Discontinuation was defined as a treatment gap of >90 days from the end of the last filled index prescription. Switching was defined as the initiation of a non-index OAC within 30 days of the last days’ supply of the index medication, provided the index medication was discontinued.5 Patients were categorized into 3 comparison groups based on their index OAC claim in the corresponding identification period, as follows: dabigatran vs. warfarin, dabigatran vs. rivaroxaban, and dabigatran vs. apixaban.
Demographic and baseline clinical characteristics
Patient demographics including age, sex, race, and geographic region as of the index date were captured. Individual comorbidities were identified based on International Classification of Diseases, 9th Revision, Clinical Modification codes (Online Table 1). Comorbidity burden during the baseline period was assessed using the Deyo-modified Charlson comorbidity index score and other clinical indices measuring stroke risk (including the CHADS2 Score [congestive heart failure, hypertension, age ≥75 years, diabetes, prior stroke] and CHA2DS2-VASc Score [congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke/TIA/thromboembolism, vascular disease, age 65–74 years, sex category]) and bleeding risk (including HAS-BLED [hypertension, abnormal renal/liver function, stroke, bleeding history/predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly] and ATRIA Score [anticoagulation and risk factors in atrial fibrillation]). Measures of overall health status (including the number of unique medications, the number of inpatients visits observed, the number of physician visits observed, home oxygen, and hip fracture) and concomitant medications identified by National Drug Codes were also assessed. Time to index NOAC was calculated as the number of days between the first NVAF diagnosis and the index date and the index dosage of the NOACs was reported.
Table 1. Sample size before and after propensity score matching.
Outcome measures
All-cause HRU and healthcare PPPM costs during the 12-month follow-up period were assessed. All-cause HRU was computed as the number of healthcare encounters, categorized as hospitalizations, outpatient visits, and pharmacy visits. Outpatient visits consisted of physician office, outpatient hospital, and emergency room (ER) visits. All-cause healthcare costs were categorized and assessed as hospitalization, outpatient, other (durable medical equipment, home health agency, skilled nursing facility, and hospice costs), pharmacy, total medical (hospitalization, outpatient, and other costs), and total costs (total medical and pharmacy costs). Costs included payments made by Medicare, the primary payer, patient copayments, deductibles, and coinsurance. All healthcare costs were adjusted for inflation to 2017 US dollars using the annual medical care and drug costs components of the Consumer Price IndexCitation31.
Statistical analysis
Descriptive statistics (means and standard deviations for continuous variables and numbers and percentages for categorical variables) were provided for all study variables, including demographics, baseline characteristics, and the outcome measures in the study cohorts. Statistical tests (chi-square for categorical and Student’s t-test for continuous variables) were conducted to assess differences between dabigatran and each of the other OACs individually.
Propensity score matching was used to achieve balance in patient characteristics. Dabigatran patients were 1:1 propensity score-matched (PSM) separately with warfarin, rivaroxaban, or apixaban patients based on a caliper width of 0.005. Logistic regression was used to calculate the propensity score for all patients, and each patient in the dabigatran cohort was matched to a patient in the respective comparison cohort with the closest propensity score. Variables used in the matching procedure included demographics, health status, comorbidity profile (Deyo–Charlson comorbidity index score, CHA2DS2-VASc score, HAS-BLED score, and individual comorbidities), time to index exposure, concomitant medications, and baseline log total healthcare costs. Multicollinearity of independent variables was assessed using the variance inflation factor. The adequacy of the matching procedure was assessed using standardized differences ([STDs], STD <10% is considered well-balanced) and L1 statisticsCitation32.
Generalized linear models with gamma distribution and log link function were used to estimate all-cause costs PPPM in PSM cohorts to account for the non-normal distribution of cost dataCitation33. Additionally HRU, including the number of visits PPPM, were estimated using generalized linear models with negative binomial distributionCitation33. Covariates in the generalized linear models included demographics (age, sex, race, and US region), CHA2DS2-VASc and HAS-BLED score. A critical value of p < .05 was specified a priori as a threshold of statistical significance for all analyses. All analyses were conducted using SAS software (version 9.4, SAS Institute, Cary, North Carolina, USA).
Sensitivity analysis
To ensure the robustness of the results, a sensitivity analysis was performed among the standard dosage patients, which included newly diagnosed NVAF patients on dabigatran 150 mg, rivaroxaban 20 mg, and apixaban 5 mg. The above-mentioned analysis was repeated in these patients, where dabigatran patients were 1:1 PSM separately with warfarin, rivaroxaban, or apixaban patients based on a caliper width of 0.005. All-cause HRU and health care costs were estimated using GLM between these matched cohorts.
Results
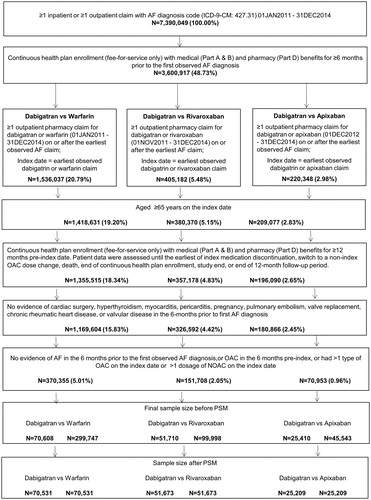
After applying the selection criteria, the study included a total of 370,355 patients in the dabigatran (70,608) vs. warfarin (299,747) comparison, 151,708 patients in the dabigatran (51,710) vs. rivaroxaban (99,998) comparison, and 70,953 patients in the dabigatran (25,410) vs. apixaban (45,543) comparison groups before propensity score matching. After propensity score matching, 70,531 patients each were included in the dabigatran vs. warfarin cohorts, 51,673 patients each for dabigatran vs. rivaroxaban, and 25,209 patients each for dabigatran vs. apixaban groups (, ).
Figure 2. Patient attrition. Abbreviations. AF, atrial fibrillation; NOAC, novel oral anticoagulant; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulant.
Patient characteristics
depicts the baseline demographic and clinical characteristics among the three comparison cohorts before propensity score matching. In the three comparison cohorts, the mean age was approximately 78 years, with a majority of patients female (about 55%) and White (about 91%). Patients in the dabigatran cohort had a significantly lower mean Deyo-Charlson comorbidity index score CHA2DS2-VASc score and HAS-BLED score and had lower comorbidity burden than warfarin, rivaroxaban, and apixaban respectively. Additionally, the dabigatran cohort had significantly fewer unique medications as compared to the warfarin, rivaroxaban, and apixaban cohorts, respectively, and the majority of these patients were on beta-blockers. Before PSM, most of the patients were on a higher dosage of NOACs on the index date which included approximately 80% patients on 150 mg dabigatran, 69% on 20 mg rivaroxaban and 71% on 5 mg apixaban on the index date. The HRU and costs in the baseline period are presented in Online Table 2. After propensity score matching, the variables in the matched comparison cohorts were well-balanced based on STDs <10% and a reduced L1 statistic. However, it should be noted that there were significant differences observed in the index NOAC dosage between the dabigatran and rivaroxaban or apixaban cohorts after PSM. Baseline characteristics after propensity score matching are displayed in .
Table 2. Baseline demographics and clinical characteristics (before propensity score matching).
Table 3. Baseline demographic, clinical characteristics, costs & HRU of newly-diagnosed and newly-treated NVAF patients after PSM.
Generalized-linear-model-adjusted HRU and healthcare costs in the 12-month follow-up period
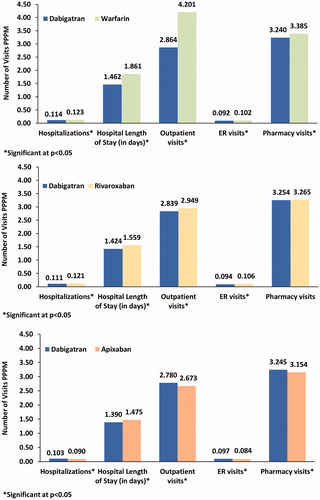
Multivariate analysis showed that compared to the warfarin and rivaroxaban cohorts, the dabigatran cohort had significantly fewer adjusted PPPM all-cause hospitalizations (0.114 vs. 0.123; 0.111 vs. 0.121), with shorter hospital length of stay (1.462 vs. 1.861 days; 1.424 vs. 1.559 days) respectively. Similarly, dabigatran cohort had significantly fewer PPPM outpatient visits (2.864 vs. 4.201; 2.839 vs. 2.949), physician office (2.13 vs. 2.88; 2.10 vs. 2.18), outpatient hospital (0.72 vs. 1.29; 0.72 vs. 0.75), and ER visits (0.092 vs. 0.102; 0.094 vs. 0.106), as compared to warfarin and rivaroxaban cohorts respectively (all p < .0001; ). However, compared to the apixaban cohort, the dabigatran cohort had significantly more adjusted PPPM all-cause hospitalizations (0.103 vs. 0.090), outpatient visits (2.780 vs. 2.673), ER visits (0.097 vs. 0.084), and pharmacy visits (3.245 vs. 3.154) (all p < .0001; ).
Figure 3. Adjusted all-cause HRU among patients with NVAF prescribed warfarin, rivaroxaban or apixaban vs. dabigatran. Abbreviations. ER, emergency room; PPPM, per patient per month. Covariates adjusted included age, sex, race, US region, CHA2Ds2-VASc, and HAS-BLED score.
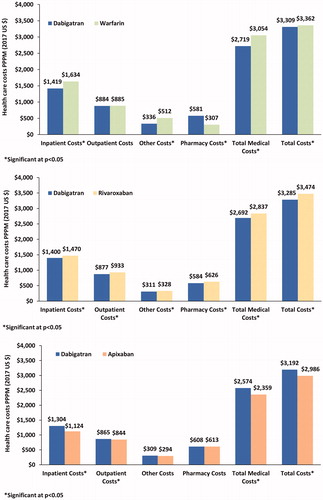
Similarly, the dabigatran cohort incurred significantly lower PPPM all-cause hospitalization ($1,419 vs. $1,634; $1,400 vs. $1,470), ER ($61 vs. $65; $62 vs. $74), other ($336 vs. $512; $311 vs. $328), total medical ($2,719 vs. $3,054; $2,692 vs. $2,837), and total costs ($3,309 vs. $3,362; $3,285 vs. $3,474) than the warfarin and rivaroxaban cohorts, respectively (all p < .05; ). However, the dabigatran cohort incurred significantly higher PPPM all-cause hospitalization ($1,304 vs. $1,124), outpatient ($865 vs. $844), physician office ($394 vs. $380), ER ($66 vs. $60), other ($309 vs. $294), total medical ($2,574 vs. $2,359), and total costs ($3,192 vs. $2,986) as compared to the apixaban cohort (all p < .05; ). Similar results were observed in the PSM cohorts, which are presented in Online Table 3.
Figure 4. Adjusted all-cause healthcare costs among patients with NVAF prescribed warfarin, rivaroxaban or apixaban vs. dabigatran. Abbreviations. ER, emergency room; PPPM, per patient per month. Covariates adjusted included age, sex, race, US region, CHA2Ds2-VASc, and HAS-BLED score.
Sensitivity analysis
After propensity score matching, 57,206 patients each were included in the dabigatran vs. warfarin cohorts, 40,815 patients each for the dabigatran vs. rivaroxaban cohorts, and 19,734 patients each for dabigatran vs. apixaban cohorts. Overall the results remained consistent with the main analysis showing that the dabigatran cohort had significantly lower total costs PPPM as compared to the warfarin ($3,102 vs. 3,193, p < .0001) and rivaroxaban ($3,045 vs. $3,225, p < .0001) cohorts but had significantly higher costs as compared to apixaban cohort ($2,964 vs. $2,758, p < .0001).
Discussion
This retrospective real-world study examined and compared the HRU and economic burden among newly-diagnosed, treatment-naïve older-aged (≥65 years) NVAF patients, who initiated treatment with either dabigatran or another OAC (warfarin, rivaroxaban, or apixaban). The current analysis is one of the largest real-world studies evaluating all-cause HRU and healthcare costs in the 100% Medicare patient population. This study demonstrated that older-aged NVAF patients treated with dabigatran had significantly lower all-cause HRU and healthcare costs on a PPPM basis, as compared to those treated with warfarin or rivaroxaban. However, HRU and healthcare costs were somewhat higher among dabigatran compared to apixaban patients.
Patients in the dabigatran cohort had significantly lower mean HRU than patients in the warfarin cohort. The dabigatran cohort had an average of 8% fewer hospitalizations, 32% fewer outpatient visits, and 26% fewer physician office visits PPPM. This may be driven by the need for frequent international normalized ratio monitoring and dose adjustments in the warfarin cohort, which is typically required every 3 days, weekly, or monthlyCitation34,Citation35. In addition, comorbidities including liver disease and gastrointestinal illnesses, which were more common among warfarin patients in the current study, may have influenced warfarin dosing and dose adjustments thereby contributing to the higher HRU and medical costs in this cohortCitation36. Since these may not be required for dabigatran, the results of our study showed that patients who initiated dabigatran had significantly lower all-cause PPPM healthcare costs, a finding which is consistent with previous retrospective studies among newly diagnosed NVAF patientsCitation5,Citation23,Citation24,Citation37. However, the index dose or the dose changes in warfarin were not captured in our study. Additionally, the higher HRU and costs in the warfarin cohort could also be attributed to its higher proportion of black patients. As per the Centers for Disease Control and Prevention, African Americans have a higher risk of comorbidities, including hypertension, diabetes, stroke, etc. This could be attributed to some social factors including unemployment, lower socioeconomic status, an unwillingness to see a physician because of higher costs, etcCitation38. Not surprisingly, our study showed that pharmacy costs were significantly higher for dabigatran users compared with warfarin users, which concurs with findings in prior studiesCitation23,Citation24. This could be attributed to the fact that dabigatran is available only as a branded medication, and has a higher unit cost relative to warfarin, which is available as a generic drugCitation37,39. Nevertheless, these higher pharmacy costs for dabigatran patients were offset by the significantly lower inpatient and medical costs, thus resulting in lower overall healthcare costs in patients treated with dabigatran. Due to these higher pharmacy costs, it was noticed that the difference in the total health care costs was only marginal (-$53). Although the medical costs were $355 lower for dabigatran as compared with warfarin, the magnitude of this difference is ∼50% lower than what was found in a prior study documented by Gilligan and coworkersCitation5.
Albeit differences in HRU were somewhat smaller compared to those observed with warfarin, the dabigatran cohort had on average 8% fewer hospitalizations, and 4% fewer outpatient visits PPPM than rivaroxaban users. These findings are also consistent with those of Gilligan et al. and other retrospective studies among newly-diagnosed NVAF patientsCitation5,Citation40. Notably, using MarketScan data, Tepper et al. documented less bleeding with dabigatran as compared to rivaroxaban patients, which could explain in part the lower HRU reported in this cohortCitation41. Further still, we found that all-cause healthcare costs were significantly lower for dabigatran-treated patients compared to rivaroxaban patients, which supports the observations of Gilligan and colleagues who noted lower inpatient, outpatient, outpatient pharmacy, and total healthcare costs for dabigatran patients compared to rivaroxaban patientsCitation5.
In the current study, patients treated with dabigatran had significantly higher HRU and costs when compared to apixaban-treated patients. Mixed findings have been reported in the literature among real-world studies comparing HRU and cost outcomes for dabigatran and apixaban patientsCitation5,Citation14,Citation42,Citation43. Deitelzweig et al. and Amin et al. reported that older-aged NVAF patients treated with dabigatran incurred higher all-cause healthcare costs than apixaban patients which is consistent with our studyCitation42,Citation43. Gilligan et al. reported an increase of 9 and 12% in outpatient visits, and outpatient office visits, respectively, among NVAF patients treated with dabigatran as compared to apixaban. However, all-cause total costs were similar between dabigatran vs. apixaban users with no statistical significance observed in spite of the marginally lower costs in dabigatran usersCitation5. Similar results were observed in another retrospective analysis conducted using OptumInsight research databaseCitation14. Amin et al. and Deitelzweig et al. included only older-aged patients where the mean age in both studies ranged between 77 and 78 yearsCitation42,Citation43, whereas in the Gilligan et al. study, the mean age was about 68 years, which might explain the differing findings of these studiesCitation5. Additionally, Amin et al. and Deitelzweig et al. suggested that the lower HRU and costs in the apixaban as compared to dabigatran patients can be attributed to the lower risk of stroke or major bleeding-related hospitalizations in the older-aged NVAF patients treated with apixabanCitation42,Citation43. However, in a recently published study by Graham et al, it was observed that dabigatran and apixaban had similar risk for thromboembolic stroke and all-cause mortality, but dabigatran was associated with reduced risk for intracranial hemorrhage and increased risk for major extracranial bleeding, all of which suggested a more favorable benefit-harm profile in dabigatran as compared to apixabanCitation44. The diverse findings from these real-world studies have made it difficult to draw conclusions on the cost-effectiveness of dabigatran vs. apixaban in the older-aged NVAF patients. Therefore, extensive research is warranted in evaluating these findings, specifically clinical outcome-related resource utilization and costs in older-aged NVAF patients.
The economic results of our analyses provide insight into the substantial cost burden of treating NVAF patients. Particularly, inpatient costs accounted for a major portion of total healthcare costs, approximately 38% to 48%, in all treatment cohorts. Thus, comparisons of resource use and healthcare costs associated with various OACs may better inform clinicians and decision-makers regarding the optimal therapeutic options for managing NVAF patients. Our study suggests that the use of dabigatran over warfarin and rivaroxaban may reduce healthcare costs. However, as the total cost difference between dabigatran vs. the other OACs was marginal (despite costs being statistically significant), these results require additional validation. On this basis, further studies with a longer duration of follow-up are warranted to better understand the cost implications, especially for dabigatran compared with apixaban.
The potential strengths of our study include that, we evaluated a large nationally representative sample from 100% Medicare database, thus contributing to one of the largest real-world studies using data from the 100% Medicare database in the elderly patients with NVAF. Also, our study has followed the on-treatment approach, in which a patient was censored at discontinuation of the index drug or switch to a non-index OAC, thus resulting the economic outcomes to be more attributable to the index treatment. Additionally, the study used robust statistical methodologies like propensity score matching to generate comparable cohorts with similar demographic and clinical characteristics in the baseline.
Although NVAF is more prevalent in older-aged adults, findings of this study may not be generalizable to patients aged <65 years. However, it should be noted that the results from another claims-based study which did not restrict their population to those aged ≥65 years, were consistent with the results of our studyCitation5. Further still, it should be noted that the costs presented in our study are only crude costs and do not represent the weighted costs (i.e. cost-effectiveness analysis). Also, there may be residual confounding due to unobserved or unrecorded clinical characteristics, this would be an issue with other studies as well. Additionally, our study did not take into consideration the comparison with edoxaban due to its approval in January 2015, and it is unknown if our findings can be generalized to these patients. Finally, this study is subject to the inherent general limitations of any claims dataset, including potential bias from coding errors and identification of medical conditions using International Classification of Diseases, 9th Revision, Clinical Modification diagnosis codes as opposed to clinical evidence. While claims data are valuable for the effective examination of healthcare outcomes, treatment patterns, and costs, they are primarily collected for reimbursement purposes, and not researchCitation45. Additionally, the presence of a claim for a filled prescription does not indicate the medication was consumed or taken as prescribed. Furthermore, medications filled over-the-counter or provided as samples by the physician are never captured in claims data.
Conclusions
The results of this study compared the HRU and costs of OACs among newly-diagnosed newly-treated older-aged patients aged ≥65 years who presented with NVAF in a real-world setting. NVAF patients initiating treatment with dabigatran incurred significantly lower all-cause HRU and healthcare costs than patients treated with warfarin or rivaroxaban but incurred higher all-cause HRU and healthcare costs than patients treated with apixaban. However, forthcoming studies evaluating the clinical-event-related burden of NVAF are needed to help facilitate our understanding of dabigatran’s cost-effectiveness in older-aged Medicare patients.
Transparency
Declaration of funding
This study was funded by Boehringer Ingelheim Pharmaceuticals.
Declaration of financial/other interests
RC had a Tufts Medical Center postdoctoral fellowship sponsored by Boehringer Ingelheim Pharmaceuticals. JFE, CW, and BOH are employees of Boehringer Ingelheim Pharmaceuticals, the study sponsor. LW is an employee of STATinMED Research, a paid consultant of the study sponsor. HY has no conflicts to disclose.
The peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
RC, JFE, CW, and BOH conceptualized and designed the study, contributed to the interpretation of the data, and substantially contributed to critical revisions of the intellectual content.
LW and HY conceptualized and designed the study, contributed to the acquisition and interpretation of the data, wrote the manuscript, and substantially contributed to critical revisions of the intellectual content.
Previous presentations
These study results have been presented at ACC.19.
Supplemental data for this article is available online at https://doi.org/10.1080/13696998.2019.1672698.
Download MS Excel (72.8 KB)Acknowledgements
The authors would like to thank Bonnie Donato, PhD, Swetha Palli, MS, and David A. Singer, PharmD, MS of Boehringer Ingelheim for their valuable contribution in preparation of this manuscript.
Data availability
The raw insurance claims data used for this study originate from Medicare data, which are available from the Centers for Medicare and Medicaid through ResDAC (https://www.resdac.org/).
References
- Fauchier L, Philippart R, Clementy N. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108(10):530–539.
- Silva R. Novel oral anticoagulants in non-valvular atrial fibrillation. CHAMC. 2014;12(1):3–8.
- Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, et al. Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with nonvalvular atrial fibrillation: a systematic review and meta-analysis of subgroups. Thrombosis. 2013;2013:1.
- Patel P, Pandya J, Goldberg M. NOACs vs warfarin for stroke prevention in nonvalvular atrial fibrillation. Cureus. 2017;9(6):e1395.
- Gilligan AM, Franchino-Elder J, Song X, et al. Comparison of all-cause costs and healthcare resource use among patients with newly diagnosed non-valvular atrial fibrillation newly treated with oral anticoagulants. Curr Med Res Opin. 2018;34(2):285–295.
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013;112(8):1142–1147.
- Marinigh R, Lip GY, Fiotti N, et al. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol. 2010;56(11):827–837.
- Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44(8):2361.
- Kim CK, Jung S, Yoon BW. Practical issues to prevent stroke associated with non-valvular atrial fibrillation. J Stroke. 2013;15(3):144–152.
- Gattellari M, Worthington JM, Zwar NA, et al. The management of non-valvular atrial fibrillation (NVAF) in Australian general practice: bridging the evidence-practice gap. A national, representative postal survey. BMC Fam Pract. 2008;9(1):62.
- Inoue H, Nozawa T, Hirai T, et al. Accumulation of risk factors increases risk of thromboembolic events in patients with nonvalvular atrial fibrillation. Circ J. 2006;70(6):651–656.
- Amin A, Keshishian A, Trocio J, et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr Med Res Opin. 2017;33(9):1595–1604.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation antithrombotic therapy in atrial fibrillation. Ann Intern Med. 2007;146(12):857–867.
- Amin A, Keshishian A, Vo L, et al. Real-world comparison of all-cause hospitalizations, hospitalizations due to stroke and major bleeding, and costs for non-valvular atrial fibrillation patients prescribed oral anticoagulants in a US health plan. J Med Econ. 2018;21(3):244–253.
- Lauffenburger JC, Farley JF, Gehi AK, et al. Effectiveness and safety of dabigatran and warfarin in real‐world US patients with non‐valvular atrial fibrillation: a retrospective cohort study. J Am Heart Assoc. 2015;4(4):e001798.
- Coleman CI, Antz M, Bowrin K, et al. Real-world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT-US study. Curr Med Res Opin. 2016;32(12):2047–2053.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- García-Lledó A, Mur JL, Recena JB, et al. Nonvalvular atrial fibrillation: the problem of an undefined definition. Rev Esp Cardiol (Engl Ed). 2014;67(08):670–671.
- Martins RP, Galand V, Colette E, et al. Defining nonvalvular atrial fibrillation: a quest for clarification. Am Heart J. 2016;178:161–167.
- Molteni M, Polo Friz H, Primitz L, et al. The definition of valvular and non-valvular atrial fibrillation: results of a physicians' survey. Europace. 2014;16(12):1720–1725.
- Francis K, Yu C, Alvrtsyan H, et al. Healthcare utilization and costs associated with dabigatran compared to warfarin treatment in newly diagnosed patients with non-valvular atrial fibrillation. Curr Med Res Opin. 2015;31(12):2189–2195.
- Gilligan AM, Gandhi P, Song X, et al. All- cause, stroke-, and bleed-specific healthcare costs: comparison among patients with non-valvular atrial fibrillation (NVAF) newly treated with dabigatran or warfarin. Am J Cardiovasc Drugs. 2017;17(6):481–492.
- Deitelzweig S, Luo X, Gupta K, et al. Effect of apixaban versus warfarin use on health care resource utilization and costs among elderly patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23(11):1191–1201.
- Laliberté F, Cloutier M, Crivera C, et al. Effect of rivaroxaban versus warfarin on health care costs among nonvalvular atrial fibrillation patients: observations from rivaroxaban users and matched warfarin users. Adv Ther. 2015;32(3):216–227.
- Amin A, Keshishian A, Xie L, et al. Comparison of short-term bleeding-related health care utilization and costs among treatment-naïve nonvalvular atrial fibrillation patients initiating apixaban, dabigatran, rivaroxaban, or warfarin. Chest. 2015;148(4):65A.
- Wisløff T, Hagen G, Klemp M. Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. Pharmacoeconomics. 2014;32(6):601–612.
- Cubanski J, Swoope C, Boccuti C, et al. kff.org [Internet]. A primer on Medicare: key facts about the Medicare program and the people it covers. San Francisco (CA): The Henry J. Kaiser Family Foundation; c1948–2019; 2015 [cited 2019 Mar 13]. Available from: http://files.kff.org/attachment/report-a-primer-on-medicare-key-facts-about-the-medicare-program-and-the-people-it-covers
- cms.gov [Internet]. On its 50th anniversary, more than 55 million Americans covered by Medicare; Baltimore (MD): Centers for Medicare and Medicaid Services, c1965–2019; 28 Jul 2015 [cited 2019 Mar 13]. Available from: https://www.cms.gov/newsroom/press-releases/its-50th-anniversary-more-55-million-americans-covered-medicare
- bls.gov [Internet]. Inflation and prices calculators. Washington (DC): US Department of Labor Bureau of Labor Statistics; c1884–2019. [cited 2019 Mar 13]. Available from: http://www.bls.gov/data/#prices
- Verzillo S, Berta P, Bossi M. CEM: a SAS macro to perform coarsened exact matching. J Stat Comput Simul. 2017;87(2):227–238.
- Seiter K, Latremouille-Viau D, Guerin A, et al. Burden of infections among chronic myeloid leukemia patients receiving dasatinib or nilotinib: a real-world retrospective healthcare claims study in the United States. Adv Ther. 2018;35(10):1671–1685.
- Kılıç S, Kemal HS, Yüce Eİ, et al. Comparison of warfarin use in terms of efficacy and safety in two different polyclinics. Anatolian J Cardiol. 2017;18(5):328.
- Khan TI, Kamali F, Kesteven P, et al. The value of education and self‐monitoring in the management of warfarin therapy in older patients with unstable control of anticoagulation. Br J Haematol. 2004;126(4):557–564.
- Shikdar S, Bhattacharya PT. International normalized ratio (INR). StatPearls Publishing; 2019 [cited 2019 Mar 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507707/
- Bancroft T, Lim J, Wang C, et al. Health care resource utilization, costs, and persistence in patients newly diagnosed as having nonvalvular atrial fibrillation and newly treated with dabigatran versus warfarin in the United States. Clin Ther. 2016;38(3):545–556.
- Centers for Disease Control and Prevention. Vital signs. African American health [cited 2019 Aug 15]. Available from: https://www.cdc.gov/vitalsigns/pdf/2017-05-vitalsigns.pdf.
- Jain R, Fu AC, Lim J, et al. Health care resource utilization and costs among newly diagnosed and oral anticoagulant-naive nonvalvular atrial fibrillation patients treated with dabigatran or warfarin in the United States. J Manag Care Spec Pharm. 2018;24(1):73–82.
- Gilligan AM, Franchino-Elder J, Song X, et al. Comparison of stroke- and bleed-related healthcare resource utilization and costs among patients with newly diagnosed non-valvular atrial fibrillation and newly treated with dabigatran, rivaroxaban, or warfarin. Expert Rev Pharmacoecon Outcomes Res. 2019;19(2):203–212.
- Tepper PG, Mardekian J, Masseria C, et al. Real-world comparison of bleeding risks among non-valvular atrial fibrillation patients prescribed apixaban, dabigatran, or rivaroxaban. PLOS One. 2018;13(11):e0205989.
- Amin A, Keshishian A, Trocio J, et al. A real-world observational study of hospitalization and health care costs among nonvalvular atrial fibrillation patients prescribed oral anticoagulants in the US Medicare population. JMCP. 2018;24(9):911–920.
- Deitelzweig S, Luo X, Gupta K, et al. All-Cause, stroke/systemic embolism–, and major bleeding-related health-care costs among elderly patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Clin Appl Thromb Hemost. 2018;24(4):602–611.
- Graham DJ, Baro E, Zhang R, et al. Comparative stroke, bleeding, and mortality risks in older Medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596–604.e11.
- Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. CLEP. 2017;9:267.