Abstract
Objectives: Colorectal cancer (CRC) screening programs have been reported to be cost-effective in many high-income countries. However, there was no such study in low- and middle-income countries. This study aimed to evaluate cost-effectiveness and budget impact of CRC screening modalities for average-risk persons in Thailand.
Methods: A decision tree coupled with a Markov model was used to estimate lifetime costs and health benefits of fecal immunochemical test (FIT) and colonoscopy using a societal perspective. The input parameters were obtained from a CRC screening project at a Thai tertiary care hospital, Thai health care costs and databases, and systematic literature review. Results were reported as incremental cost-effectiveness ratios (ICERs) in 2017 US Dollars (USD) per quality-adjusted life year (QALY) gained. Sensitivity analyses were performed to assess the influence of parameter uncertainty. Finally, budget impact analysis was conducted.
Results: At the Thai ceiling threshold of societal willingness-to-pay of 4,706 USD, the screening colonoscopy every 10 years and annual FIT, starting at age 50, was cost-effective, as compared to no screening resulting in 15.09 and 15.00 QALYs with the ICERs of 600.20 and 509.84 USD/QALY gained, respectively. Colonoscopy every 10 years and annual FIT could prevent 17.9% and 5.7% of early stage cancer and 27.8% and 9.2% of late stage cancer per 100,000 screening over lifetime when compared to no screening, respectively. The colonoscopy screening was cost-effective with the ICER of 646.53 USD/QALY gained when compared to FIT. The probabilities of being cost-effective for the colonoscopy and FIT were 75% and 25%, respectively. Budget impact analysis showed the colonoscopy screening required an 8-times higher budget than FIT.
Conclusions: Colonoscopy offers the best value for money of CRC screenings in Thailand. Annual FIT is potentially feasible since it requires less resources. Our findings can be used as part of evidence for informing policy decision making.
There was a lack of cost-effective study of colorectal cancer screening programs in low- and middle-income countries.
This study evaluated lifetime health outcomes and costs, and the cost-effectiveness of colorectal screening options for average-risk persons in Thailand.
Colonoscopy screening every 10 years is cost-effective with high probability of being cost-effective as compared with annual fecal immunochemical test.
Screening by annual fecal immunochemical test is more feasible in terms of human resource and budgetary burden.
Colorectal screening programs provides an opportunity for early diagnosis and treatments to prevent advance colorectal stages and avoid higher consequent costs.
This study contributes a new evidence-based knowledge for Thailand and can be used to support policy decision making process.
Key points for decision makers
1. Introduction
Colorectal cancer (CRC) is one of the major leading causes of cancer death worldwideCitation1. Rapid increases in both CRC incidence and mortality are now observed in many low- and middle-income countries (LMICs). In contrast, they have been stabilizing or declining in a number of developed countriesCitation2. This might be due to the increasing rate of early detection through screening programs coupled with proper therapeutic interventionsCitation2,Citation3. CRC screening has shown the benefit not only by decreasing cancer-related morbidity and mortality through retarding disease progression with an improving quality of life, but also lowering cost of careCitation4–10.
Several screening modalities, such as fecal immunochemical test (FIT) and colonoscopy, have been available with clear effectiveness on the reduction of CRC incidence and its associated mortalityCitation11–13. The US Preventive Services Task Force (USPSTF) 2016 recommendation concludes with high certainty that screening for CRC in average-risk, asymptomatic adults aged 50 to 75 years is substantially beneficial because CRC is most frequently diagnosed among adults aged 65 to 74 years and the median age of CRC death is 68 yearsCitation14. FIT is a noninvasive interventionCitation15,Citation16 with high sensitivity for CRC and colorectal adenomas detectionCitation17,Citation18. Patients with positive FIT tests require colonoscopy for confirmative diagnosisCitation16,Citation19. A CRC screening guideline recommends colonoscopy for higher adenomas and cancer detection rates, as compared to FITCitation20,Citation21. However, the colonoscopy requires bowel preparation and is more invasive and costlyCitation16.
In LMICs, health care financing sustainability is challenged in the long-run. Many countries face with disease burden coupled with aging population. Appropriate health care financing design is important to ensure availability of funding for the right services at the right time. Cost-effective solutions are needed especially in health promotion and preventionCitation5.
Despite a large number of studies demonstrating the value for money of CRC screenings in many western countriesCitation22, there has been an absence of cost-effectiveness study in LMICs. Thailand, a member of LMICs situated in Southeast Asia, has a high burden of CRC. With a half of the CRC detected at late stages, it is one of the leading causes of death in the countryCitation23. Recently, CRC population-based screening campaigns using either FIT or colonoscopy have been launched, indicating strong interest of policy makers in CRC prevention in Thailand. As Thailand is one of the leading countries advocating the use of health technology assessment (HTA) evidence to inform decision as part of Universal Health Coverage policy, there is a strong need to understand which screening intervention is the best buy option. This study aimed to determine cost-effectiveness of all relevant CRC screening options including FIT and colonoscopy in Thailand.
2. Methods
2.1. Overall description
A cost-effectiveness analysis was undertaken to estimate relevant costs and health outcomes of CRC screening programs, compared to no screening, in Thai average-risk population. Two screening programs were evaluated: (1) FIT every year (2) Colonoscopy every 10 years. Due to long-term outcomes of adenomatous polyps and CRC, the lifetime time horizon was chosen in this study. We undertook this study using a societal perspective recommended by the Thailand’s HTA guidelineCitation24. Our findings were presented by incremental cost-effectiveness ratios (ICERs) in US Dollars (USD) per quality-adjusted life year (QALY) gained. The cost-effectiveness threshold in this study was based on an official willingness-to-pay (WTP) of 160,000 Thai Baht/QALY (4,706 USD/QALY) used by the Health Economics Working Group of the National List of Essential Medicines (NLEM) committeeCitation25. Based on the Thailand’s HTA guideline, all future costs and health outcomes were discounted at the rate of 3% per annumCitation26. The study protocol was approved by Siriraj institutional review board (No. 302/2560).
2.2. Economic model
A hypothetical cohort of 100,000 individuals with Thai average-risk, asymptomatic population, following the criteria of National Cancer Institute of ThailandCitation27, was simulated in the study model. According to the USPSTF 2016 recommendation for the starting age of the CRC screeningCitation14, we used the age of 50 as the base case in this model. We also set a specific interval for repeating the screening test until 75 years of age, as recommended by the guidelineCitation27.
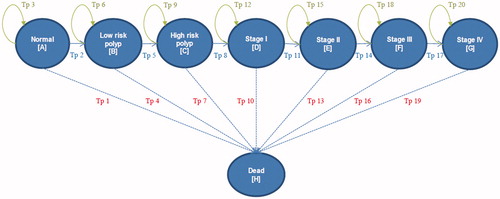
A hybrid economic model consisting of decision tree and Markov models was used in this study. A decision tree was constructed to divide patients into two groups including a screening group with therapeutic interventions and a no-screening group. As none of the screening methods are 100% accurate, the subjects in the screening group were then classified into four groups, including true positive, false positive, false negative, and true negative for validity of the models. The subjects, who underwent true positive and false negative arms, were considered having adenomatous polyp or CRC, others were normal. However, CRC is a slow progressive disease and screening programs also provides long-term effects, such as a decrease in cancer mortality that cannot be captured by only the decision tree model. Therefore, a Markov model was used in the analysis using a lifetime horizon with a one-year cycle length. According to the natural history of CRC progression from precancerous lesion or adenomatous polyp, the Markov model consisted of eight principal health stages including normal, low-risk polyp (LRP), high-risk polyp (HRP), CRC stage I-IV, and death. The hypothetical subjects, who involved in the screening test, would undergo therapeutic interventions if their screening results were positive. On the other hand, the no-screening group would be diagnosed and treated only after CRC symptoms developed. In the Markov model, the patients could remain in the same state or move to other states including death state during each cycle. Normal or no colonic lesions could progress to LRP. LRP could progress to HRP. In addition, HRP could progress to CRC stage I-IV stage by stage. Patients in every health state could not reverse to a previous health state, as shown in . We assumed that the stage-specific progression probability of the disease was constant over time in each cycle of each stage of cancer.
2.3. Model input parameters
For the colonoscopy screening model, the prevalence of LRP, HRP, and each stage of CRC was based on primary data from the CRC screening project of 1,594 cases that reported the adenomatous polyp detection rates in Thai people and evaluated the incidences of CRC during the colonoscopy screenings from 2007 to 2010 at the endoscopic center of Siriraj Hospital, one of the largest tertiary care centers in ThailandCitation28 and the secondary data from the prospective colonoscopy screening project of 1,404 cases at Chulabhorn Hospital, another tertiary care hospital in the countryCitation29. In this studyCitation29, the colonoscopy screenings were offered to 1,500 healthy volunteers, who were registered in the program between July 2009 and June 2010.
For FIT screening model, the prevalence of adenomatous polyps and each stage of CRC was based on the pilot population-based CRC screening program using annual FIT in Lampang Province, ThailandCitation30. Other input parameters (i.e. sensitivity and specificity of FIT and colonoscopy, transition probabilities, costs, and utilities) were obtained from literature including both local and international publications, as shown in .
Table 1. Input parameters used in the model.
2.3.1. Screening options and effectiveness
The sensitivity and specificity of FIT screening were obtained from a recent systematic review and meta-analysis of 19 studies (113,360 participants)Citation31. The overall sensitivity and specificity of the FIT screening for the CRC detection at cutoff values greater than 50 mcg/g were 67% (95% CI, 59–74%) and 96% (95% CI, 94–98%), respectively, as compared to the colonoscopy, the gold standard of CRC screening. We also calculated the participation to FIT screening, the compliance of long term annual screening, and the colonoscopy participation after positive FIT screening into the modelCitation30 to ensure that the life-time success rate and benefits of the FIT screening were not overestimated.
The sensitivity of colonoscopy screening was obtained from a recent systematic review and meta-analysis of 49 studies (11,151 participants)Citation32. The overall sensitivity of the colonoscopy screening for the CRC detection was 94.7% (95% CI, 90.4–97.2%). The specificity of colonoscopy screening was obtained from a recent systematic review of 20 studies (79,551 participants)Citation33. The overall specificity of colonoscopy for the CRC detection was 99.8% (95% CI, 99.6–100%). For the result accuracy, we also calculated the participation to colonoscopy screening into the modelCitation30,Citation34.
The results from a recent network meta-analysis from 44 studies showed that colonoscopy was the most effective screening for preventing CRC deaths. Colonoscopy and FIT reduced CRC mortality by 61% (RR, 0.39; 95% CI, 0.31-0.50) and 59% (RR, 0.41; 95% CI, 0.29-0.59), compared to no screening, respectively. In addition, the colonoscopy screening reduced the overall incidence of CRC by 57% (RR, 0.43; 95% CI, 0.30-0.60) whereas FIT reduced the overall incidence of CRC by 21% (RR, 0.79; 95% CI, 0.69-0.92) when compared to no screeningCitation13.
2.3.2. Probability data
Based on a probability model, the annual incidence of LRP and the transition probabilities among all stages (LRP, HRP, and CRC stage I–IV) were calculated for estimating colorectal polyp progression ratesCitation35,Citation36. The probability of death among average-risk people was obtained from the age-specific mortality rate (ASMR) of Thai populationCitation37. The probability of death in each CRC stage was calculated from a meta-analysis of four studies in Thailand; two studies from Siriraj Hospital, Mahidol University (1,047 cases between January 2003 and December 2007Citation38 and 2,610 cases between January 2009 and December 2013 (unpublished data)), one study from 287 cases of Rajavithi Hospital between January 1995 and December 2003Citation39, and another study from 1,013 cases of Songklanagarind Hospital between January 2004 and December 2013Citation40.
2.3.3. Cost data
We included both direct medical costs and direct nonmedical costs. Indirect costs were not included since we assumed that lost or impaired ability to work or engage in leisure activities due to morbidity would be captured in the disutility of QALYCitation46. All costs data were obtained from published literature in Thailand. We estimated health care utilization using a micro-costing technique. We assumed that the average-risk people without colonic lesions would have either annual FIT screening or colonoscopy screening every 10 years. The patients with adenomatous polyps would have therapeutic removals following their colonoscopy screenings. In addition, they would repeat the colonoscopy screenings every five years. We estimated the total cost of colonoscopy considering complications based on the incidence of post-colonoscopy complications in a recent meta-analysisCitation42. The treatment costs of each CRC stage were obtained from a previous studyCitation40. In a follow up period, we estimated that the patients with CRC would seek outpatient visits four times per year and incur costs from laboratory tests. The standard unit costs for direct medical cost (i.e. FIT screening, colonoscopy screening, laboratory testing, and x-rays) and direct nonmedical costs (i.e. transportation, meals, accommodation, and facilities) were obtained from the standard cost list of Thailand HTACitation41. All costs were converted and reported in 2017 USD (1 USD = 34 Thai Baht) and using the consumer price index (CPI)Citation47.
2.3.4. Utility data
All utility values were obtained from published literature. The utility values of patients with or without adenomatous polyps were obtained from two studies. The first study is a population-based values for EQ-5D health states in Thai general populationCitation43. The second one is a large cohort study among 4,850 employees aged more than 45 years in Thailand using the Thai EQ-5D. A total of 1,409 respondents was interviewed in 2007 using the ranking, visual analogue scale, and time trade-off methodsCitation44. The utility values of patients with each CRC stage were obtained from a study of 81 participants in the US using standard gamble technique.Citation45.
2.4. Cost-effectiveness analysis
2.4.1. Base-case analysis
Based on the USPSTF 2016 recommendationCitation14, we used the starting age of screenings at 50 as the base case to demonstrate the screening benefits. The number of cancer cases prevented in both early and late stages was calculated. For the base-case analysis, we calculated the expected lifetime costs and outcomes, and ICERs for each screening program.
For model validation, we estimated the incidence of interval colorectal cancer (I-CRC), a CRC diagnosed within 5 years after a negative colonoscopy, from the model and compare it with the results of other studies.
2.4.2. Sensitivity analyses
A series of one-way sensitivity analyses were performed to examine the impact of changes in individual parameters on the ICERs. These parameters included all clinical outcomes, costs, utilities, and discount rates. A tornado diagram was developed to visualize the results of the analyses. We also varied the starting ages of the screenings from 40 to 80 years to evaluate the impact of the starting age on the results.
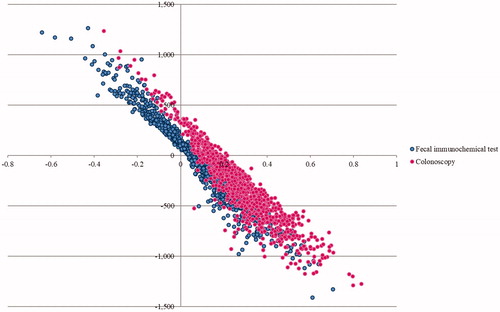
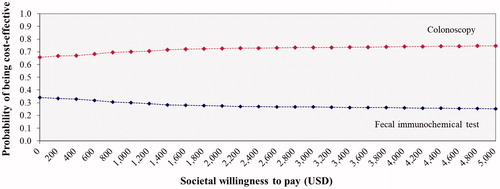
A probabilistic sensitivity analysis (PSA) was conducted to simultaneously examine the effects of all parameter uncertainties using a Monte Carlo simulation performed by Microsoft Excel 2003 (Microsoft Corp, Redmond, WA)Citation48. The distributions of each probability were assigned. Beta-distributions were used for the prevalence, effectiveness of CRC screening with therapeutic interventions, probability and utility parameters, in which their values ranged between 0 and 1. Gamma distributions were assumed for the costs, in which their values were above 0. A Monte Carlo simulation was run for 1,000 iterations to provide a range of values for total costs, outcomes, and ICERs. The results of the PSA were presented as cost-effectiveness acceptability curves. The expected net monetary benefit (NMB) was calculated for WTP threshold in Thailand to show the probability of each screening option being cost-effective for monetary values that a decision maker might be willing to pay.
2.4.3. Budget impact analysis
Budget impact analysis was conducted in order to evaluate financial impact from the colonoscopy screening every 10 years and annual FIT screening. The analysis considered a 5-year time horizon. The size of the target population was calculated using national epidemiological dataCitation49. The number of population, that was used in calculating the budget, included the whole Thai people aged between 50-75 years. Both the ideal and real-life situations were taken into consideration in this budget impact analysis.
3. Results
3.1. Base case analysis
Our base-case analysis of starting age at 50 years demonstrated that the CRC screening by annual FIT with therapeutic interventions could prevent the development of 1,049 (5.7%) early stage of cancer and 1,220 (9.2%) late stage of cancer per 100,000 screening over lifetime when compared to no screening according to the real-life participation rate. Moreover, the colonoscopy screening with therapeutic interventions every 10 years could prevent the development of 3,288 (17.9%) early stage of cancer and 3,695 (27.8%) late stage of cancer per 100,000 screening over lifetime when compared to no screening (Supplement file Tables 9(E) and 10(E)). Our findings were only a slightly lower than the results of recent systematic review and network meta-analysisCitation13 which showed that screening by colonoscopy and FIT reduced the overall incidence of CRC by 8–31% and 40–70%, respectively, compared with no screening. These findings may be explained by a lower participation rate.
As compared to no screening, the screenings by annual FIT and colonoscopy every 10 years were cost-effective with the ICERs of 509.84 and 600.20 USD/QALY gained, respectively. However, the colonoscopy screening every 10 years was cost-effective when compared to the annual FIT screening with the ICERs of 646.53 USD/QALY gained ().
Table 2. Lifetime costs and health outcomes of each colorectal cancer screening option.
For model validation, we used the estimated I-CRC within 5 years after normal colonoscopy screening to compare with the results of other studies. The incidence of I-CRC of both early and late stages in our model is about 3.1% per 5 years (Supplement file Table 14(E)) which is comparable with the range (2.6–3.0%) reported in previous published literaturesCitation50–52.
3.2. Sensitivity analyses
3.2.1. One-way sensitivity analyses
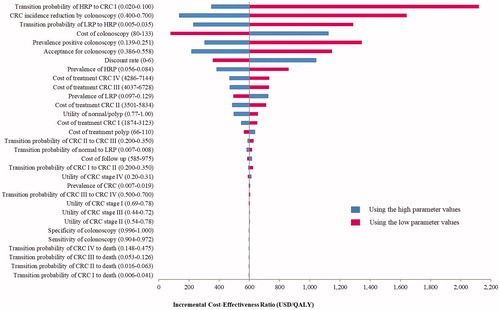
shows the tornado diagram as a result of the one-way sensitivity analyses of the colonoscopy screening, compared to no screening. Only the results from influencing parameters were included in the diagram. The ICER between the colonoscopy screening and no screening was sensitive most to the transition probability of HRP to CRC stage I, followed by the CRC incidence reduction by colonoscopy, the transition probability of LRP to HRP, the cost of colonoscopy, the prevalence of positive colonoscopy, and the participation to colonoscopy. Despite the ICER changes as a result of varying these parameters, the colonoscopy screening with the starting age at 50 years remained cost-effective. This impact was greater when the starting ages of screening were at 40 and 50 years than 60 and 70 years. Moreover, the results showed that screening age after 80 years was not cost-effective (Supplement file Table 11(E)).
Figure 2. Tornado diagram illustrating the one-way sensitivity analysis results of screening by colonoscopy every 10 years compared to no screening.
The results revealed cost-effective findings if the participation to FIT screening and colonoscopy screening were more than 21% and 17%, respectively. The FIT screening was not cost-effective if its cost was higher than 12 USD whereas the colonoscopy screening was not cost-effective if it was more expensive than 310 USD. In addition, FIT was cost-effective when compared to colonoscopy if participation rate of colonoscopy screening was less than 29% or cost of colonoscopy was higher than 275 USD.
3.2.2. Multivariate probabilistic sensitivity analyses
The results of the PSA were based on 1000 Monte Carlo simulations. The PSA results of both screening tests were illustrated by the cost-effectiveness plane in and the acceptability curves in . The cost-effectiveness acceptability curves showed the superiority of the colonoscopy screening every 10 years over the annual FIT for all WTP values. At the WTP in Thailand, the probability for being cost-effective for colonoscopy every 10 years was 75% and for the probability of annual FIT was 25%.
3.3. Budget impact analysis
The results of budget impact analysis were based on the target population, Thai average-risk, asymptomatic adults aged 50 to 75 years including 17.6 million people as an ideal scenario. Then the real-life screening participation rate was calculated using Thailand data. Based on 62.9% participation rate, the number of annual FIT screening was 11,548,958 people per year and for those who need further colonoscopy was 92,496 people. The budget impact was 25.5 million USD per year. On the other hand, for colonoscopy screening as a first option, the number of colonoscopy was 1,857,108 people per year at participation rate of 47.2%, and the budget impact was 198.1 million USD per year. It caused large budget impact to the health plan as shown in .
Table 3. Budget impact analysis.
4. Discussions
Our findings demonstrated that the CRC screening by annual FIT and colonoscopy every 10 years accompanied with therapeutic interventions among Thai average-risk population could prevent the development of CRC. This study showed that either annual FIT screening or colonoscopy screening every 10 years was cost-effective, as compared to no screening, in Thailand. Even though the participation and compliance of the annual FIT screening were relatively higher than those for the colonoscopy screening since the FIT screening was not invasive, as compared to the colonoscopy screeningCitation19,Citation53, the colonoscopy screening every 10 years was cost-effective when compared to the annual FIT screening. One possible reason is that the colonoscopy screening has a higher sensitivity and specificity. The FIT screening has to be more frequently repeated and its positive result has to be confirmed by the colonoscopyCitation14.
Our results were consistent with the study findings in other countriesCitation53,Citation54. The reasons may be associated with higher incidence and mortality reduction benefits of colonoscopy screening as reported in a recent systematic review and network meta-analysisCitation13. In addition, our results were consistent with a recent large community-based organized CRC screening in the US which showed the great effect of screening on CRC incidence and mortality due to greater detection of early-stage cancersCitation55. CRC screening significantly increased the up-to-date status of screening, from 38.9% in 2000 to 82.7% in 2015. Higher rates of screening were associated with a 25.5% and 52.4% reduction in CRC incidence and mortality, respectively, between 2000 and 2015.
However, the CRC prevented by colonoscopy every 10 years and annual FIT were 1,049 and 3,288 cases of early cancer and 1,220 and 3,695 cases of late cancer per 100,000 screening over lifetime when compared to no screening once considering the real-life participation rate. The small difference between early and late stage CRC incidence prevention may be caused by the data from the first-year pilot population-based CRC screening project in ThailandCitation30 which lead to the detection of both early and late stages of cancer. The late stage detection should be lower in the long term after campaign is well established as national policy, which is similar to the results from recent published studyCitation56.
Both FIT and colonoscopy screening strategies had more impact if they started at the ages of 40 to 50 years rather than 60–70 years. Moreover, the results showed that the screenings after the age of 80 years were not cost-effective. These results were consistent with the USPSTF 2016 that recommended the screening should start at the age of 50 yearsCitation14 and with the Cancer Intervention and Surveillance Modeling Network (CISNET) that suggested the starting at the age of 45 years would offer a better increase in life-years (LYs) gainedCitation57. In addition, the 2018 guideline from the American Cancer Society suggested the age 45 years as the starting age for the CRC screening and clinicians discourage any individuals older than 85 years from continuing the CRC screeningCitation58. In other words, the 50–75 years of age seem to be the most optimal range for the CRC screening. Otherwise, it might be too late to detect the CRC and the treatment might be less effective.
Although the colonoscopy screening provided the best value for money in this study, the results of budget impact analysis showed that 8-times higher budget was required for the colonoscopy screening. In addition, supply constraint is also a limitation. Colonoscopy is no longer an option for prevention program of public health insurance except for individual preferences who would like to self-finance. In other words, the annual FIT screening was more affordable when compared to the colonoscopy screening. Therefore, FIT is only the best option for benefit package of the national health insurance in Thailand. Clinicians and policy decision makers may consider our results as a part for including the CRC screening program into national health benefit package. However, the country must also consider other factors, aside from economic factors, including socio-cultural values and ethical issuesCitation59.
In addition, the suboptimal rate of screeningCitation19, patient complianceCitation53, and lack of health awarenessCitation34 are also the challenges of the cost-effectiveness of the CRC screenings. The data from Lampang province showed that the participation rate of the FIT screening in Thailand was about 63% and about one-fourth (28%) of patients with positive FIT did not further perform any colonoscopyCitation30. The diagnosis of CRC among these patients could be missed. In addition, the cost-effectiveness results depended on the levels of patients’ participation and compliance and the costs of screening options. According to our results, the higher percentage of participation yielded the more cost-effective (Supplement file Table 13(E)). The previous study also showed the same trend that the benefits of screening would increase if the levels of participation and compliance are higherCitation19,Citation53. New strategies enhancing the participation and preference levels are therefore essential for the successful screening policy.
To our knowledge, this is the first economic evaluation of CRC screenings in LMICs using Thailand as an example. Our findings are highly valid and contextually relevant due to three main reasons. First, various gastroenterologists were involved throughout our cost-effectiveness analysis. Second, the local data were used in our analysis when possible. We also adjusted the mortality rates of these patients by incorporating Thai ASMR to reflect Thai population contextCitation37. The LRP, HRP, and each CRC stage prevalence was also obtained from previous studies in Thailand. The meta-analysis of the annual probabilities of CRC mortality in each stage was performed using the data from the large four studies in the countryCitation38–40. All cost data were retrieved from reliable local sourcesCitation41. Moreover, we had used the costs of treatments of each CRC stage from previous studies in Thailand. Third, other input parameters used in the model were collected from the most recent systematic review, meta-analysis, and large randomized controlled trials. In addition, we comprehensively searched literature to identify relevant probabilities, costs, and utilities, such that all estimates in the model incorporated the majority of data that were currently available for CRC. Our results should be robust and had good quality for policy decisions. Last, our models were quite valid because the results showed that colonoscopy and FIT could prevent the development of both early and late stages of cancer compared with no screening consistent with the previous studyCitation13. Moreover, the incidence of I-CRC in our model is similar to what reported in other studiesCitation50–52.
There are several limitations in our study. First, other screening strategies in the international guidelines, such as FIT-fecal DNA test, flexible sigmoidoscopy (FS) and CT colonography were not included in this study because they were not considered practical test options in Thailand as usual screening testsCitation14,Citation27,Citation54,Citation60,Citation61. FS has advantages over colonoscopy such as less bowel preparation, lower cost, and less complicationsCitation14,Citation60. Nonetheless it reduces only distal colon and rectum cancer incidence and mortality. It provides lower effectiveness in the protection of right-sided colon cancerCitation60. The CT colonography requires an expensive machine, a specific software, and a complicated bowel preparation similar to what required in colonoscopy. In addition, the patients have to expose with contrast media. Second, according to the findings, the colonoscopy screening every 10 years was cost-effective when compared to the FIT screening. The annual FIT screening is a simpler test and also more practical in reality. The colonoscopy may not be implemented in some areas of Thailand due to the lack of endoscopists in case of applying colonoscopy as a first screening method. Last, it is important to discuss the generalizability of our findings for any applications in other countries. It is widely known that any cost-effectiveness findings are not directly transferable to other countries because of the differences in healthcare system, decision-making criteria, and costs dataCitation62. Recently, it has been advised that the potential transferability should focus on model structure, study design approach, and parameter values, especially those related to natural history of disease rather than on findings. Our study provided the most up-to-date and comprehensive information, which can be used or applied to the conduct of cost-effectiveness analysis of the CRC screenings in other countries.
5. Conclusions
Our study showed that CRC screening can prevent the development of both early and late stages of CRC, especially in the starting age before 50 years. The colonoscopy screening every 10 years was a cost-effective strategy, as compared to the annual FIT screening and no screening, in Thailand. It provides an opportunity for early diagnosis and treatments to prevent advance CRC stages and avoid higher costs of treatments. However, the annual FIT screening is more affordable, as compared to the colonoscopy screening. In addition, the transferability and practicability should also be considered for real-world applications. Health policy makers and clinicians may consider our study results for including either the annual FIT or colonoscopy screening in the CRC screening program in Thailand.
Transparency
Declaration of funding
The authors received no funding for this work.
Declaration of financial/other relationships
Pochamana Phisalprapa (PP), Siripen Supakankunti (SS), and Nathorn Chaiyakunapruk (NC) declare no potential conflicts of interest with respect to the research, organization, and publication of this work. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
PP participated in the study concept and design, data acquisition, data analysis, data interpretation, manuscript drafting, critical revision of the manuscript, and the final review of the manuscript. SS and NC participated in the study concept and design, data interpretation, manuscript drafting, critical revision of the manuscript, and the final review of the manuscript.
Previous presentations
This work was presented at 24th Eurasia Business and Economics Society Conference in Bangkok on January 11, 2018.
7._Appendix_CEA_CRC_screening_JME_20-9-2019_no_highlight.docx
Download MS Word (187.9 KB)Acknowledgements
The authors would like to thank Ph.D. program in Economics, Faculty of Economics, Chulalongkorn University for academic supports, Chayanis Kositamongkol, research assistant, for collecting the data. We would also like to express our gratitude to Associate Professor Surachat Ngorsuraches, Ph.D., Harrison School of Pharmacy, Auburn University, USA, for his careful review and suggestions to improve our reporting of this study.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E86.
- Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomark Prev. 2009;18(6):1688–1694.
- Murphy CC, Harlan LC, Lund JL, et al. Patterns of colorectal cancer care in the United States: 1990–2010. J Natl Cancer Inst. 2015;107(10):djv198.
- Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696.
- Jakovljevic M, Jakab M, Gerdtham U, et al. Comparative financing analysis and political economy of noncommunicable diseases. J Med Econ. 2019;22(8):722–727.
- Vekic B, Dragojevic-Simic V, Jakovljevic MM, et al. Medical cost of colorectal cancer services in Serbia Between 2014 and 2017: national data report. Front Pharmacol. 2019;10:526.
- Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553–1568.
- Jakovljevic M, Malmose-Stapelfeldt C, Milovanovic O, et al. Disability, Work absenteeism, sickness Benefits, and cancer in selected european OecD countries—forecasts to 2020. Front Public Health. 2017;5:23.
- Jakovljevic M, Fernandes PO, Teixeira JP, et al. Underlying differences in health spending within the World Health Organisation Europe Region—comparing EU15, EU post-2004, CIS, EU candidate, and CARINFONET countries. IJERPH. 2019;16(17):3043.
- Dagovic A, Walstra KM, Gutzwiller FS, et al. Resource use and costs of newly diagnosed cancer initial medical care. Eur J Oncol. 2014;19(3):166–184.
- Qaseem A, Denberg TD, Hopkins RH, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156(5):378–386.
- von Karsa L, Patnick J, Segnan N, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45(1):51–59.
- Zhang J, Cheng Z, Ma Y, et al. Effectiveness of screening modalities in colorectal cancer: a network meta-analysis. Clin Colorect Cancer. 2017;16(4):252–263.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564–2575.
- Sharp L, Tilson L, Whyte S, et al. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer. 2012;106(5):805–816.
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595.
- Duffy MJ, van Rossum LG, van Turenhout ST, et al. Use of faecal markers in screening for colorectal neoplasia: a European group on tumor markers position paper. Int J Cancer. 2011;128(1):3–11.
- Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer. 2013;49(14):3049–3054.
- Sano Y, Byeon JS, Li XB, et al. Colorectal cancer screening of the general population in East Asia. Dig Endosc. 2016;28(3):243–249.
- Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104(3):739.
- Burt RW, Cannon JA, David DS, et al. Colorectal cancer screening. J Natl Compr Canc Netw. 2013;11(12):1538–1575.
- Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33(1):88–100.
- Leite NC, Salles GF, Araujo AL, et al. Prevalence and associated factors of non‐alcoholic fatty liver disease in patients with type‐2 diabetes mellitus. Liver Int. 2009;29(1):113–119.
- Thai Health Technology Assessment Guideline Working Group. Thai health technology assessment guideline: Bangkok: Chulalongkorn University Press; 2008.
- Teerawattananon Y, Tritasavit N, Suchonwanich N, et al. The use of economic evaluation for guiding the pharmaceutical reimbursement list in Thailand. Z Evid Fortbild Qual Gesundhwes. 2014;108(7):397–404.
- Permsuwan U, Kansinee G, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thailand. 2014;97(5):S50–S58.
- National Cancer Institute Thailand. Guideline on screening, diagnosis and treatment of colorectal cancer. Bangkok: National Cancer Institute Thailand; 2015.
- Aswakul P, Prachayakul V, Lohsiriwat V, et al. Screening colonoscopy from a large single center of Thailand-something needs to be changed?. Asian Pac J Cancer Prev. 2012;13(4):1361–1364.
- Siripongpreeda B, Mahidol C, Dusitanond N, et al. High prevalence of advanced colorectal neoplasia in the Thai population: a prospective screening colonoscopy of 1,404 cases. BMC Gastroenterol. 2016;16(1):101.
- Khuhaprema T, Sangrajrang S, Lalitwongsa S, et al. Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme. BMJ open. 2014;4(1):e003671.
- Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171–81.
- Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal cancer: CT colonography and colonoscopy for detection—systematic review and meta-analysis. Radiology. 2011;259(2):393–405.
- Allameh Z, Davari M, Emami M. Sensitivity and specificity of colorectal cancer mass screening methods: a systematic review of the literature. Iran J Cancer Prev. 2011;4(2):88–105.
- Saengow U, Chongsuwiwatvong V, Geater A, et al. Preferences and acceptance of colorectal cancer screening in Thailand. Asian Pac J Cancer Prev. 2015;16(6):2269–2276.
- Gopalappa C, Aydogan-Cremaschi S, Das TK, et al. Probability model for estimating colorectal polyp progression rates. Health care Manag Sci. 2011;14(1):1–21.
- Leshno M, Halpern Z, Arber N. Cost-effectiveness of colorectal cancer screening in the average risk population. Health Care Manage Sci. 2003;6(3):165–174.
- World Health Organization. Life tables by country: Thailand; 2016. [cited 2018 Oct 18]. http://apps.who.int/gho/data/view.main.61640?lang=en.
- Techawathanawanna S, Nimmannit A, Akewanlop C. Clinical characteristics and disease outcome of UICC stages I-III colorectal cancer patients at Siriraj Hospital. J Med Assoc Thai. 2012;95(2):S189–S98.
- Laohavinij S, Maneechavakajorn J, Techatanol P. Prognostic factors for survival in colorectal cancer patients. J Med Assoc Thai. 2010;93(10):1156–1166.
- Sermsri N, Boonpipattanapong T, Prechawittayakul P, et al. Influence of payer source on treatment and outcomes in colorectal cancer patients in a university hospital in Thailand. Asian Pac J Cancer Prev. 2014;15(20):9015–9019.
- Riewpaiboon A. Standard cost lists for health technology assessment. Nonthaburi: Health Intervention and Technology Assessment Program (HITAP); 2011.
- Reumkens A, Rondagh EJ, Bakker CM, et al. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016;111(8):1092–1101.
- Tongsiri S, Cairns J. Estimating population-based values for EQ-5D health states in Thailand. Value Health. 2011;14(8):1142–1145.
- Kimman M, Vathesatogkit P, Woodward M, et al. Validity of the Thai EQ-5D in an occupational population in Thailand. Qual Life Res. 2013;22(6):1499–1506.
- Ness RM, Holmes AM, Klein R, et al. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94(6):1650–1657.
- Riewpaiboon A. Measurement of costs. J Med Assoc Thailand Chotmaihet thangphaet. 2008;91:S28–S37.
- Bank of Thailand. Foreign Exchange Rates; 2017. [cited 2018 Oct 18]. http://www2.bot.or.th/statistics/ReportPage.aspx?reportID=123&language=eng
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation: Oup Oxford; 2006.
- Population of Thailand Classified by Age; 2018. [cited 2019 May 15]. http://stat.dopa.go.th/stat/statnew/upstat_age_disp.php
- Richter JM, Campbell EJ, Chung DC. Interval colorectal cancer after colonoscopy. Clin Colorectal Cancer. 2015;14(1):46–51.
- Le Clercq CMC, Bouwens MWE, Rondagh EJA, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63(6):957–963.
- Erichsen R, Baron JA, Stoffel EM, et al. Characteristics and survival of interval and sporadic colorectal cancer patients: a nationwide population-based cohort study. Am J Gastroenterol. 2013;108(8):1332–1340.
- Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening – an overview. Best Pract Res Clin Gastroenterol. 2010;24(4):439–449.
- Patel SS, Kilgore ML. Cost effectiveness of colorectal cancer screening strategies. Cancer Control. 2015;22(2):248–258.
- Levin TR, Corley DA, Jensen CD, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155(5):1383–1391.e5.
- Vicentini M, Zorzi M, Bovo E, et al. Impact of screening programme using the faecal immunochemical test on stage of colorectal cancer: results from the IMPATTO study. Int J Cancer. 2019;145(1):110–121.
- Zauber A, Knudsen A, Rutter C, et al. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach AHRQ Publication 14-05203-EF-2 2015. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- Wolf AM, Fontham ET, Church TR, et al. Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. Cancer J Clin. 2018;68(4):250–281.
- Aggarwal A, Ginsburg O, Fojo T. Cancer economics, policy and politics: what informs the debate? Perspectives from the EU, Canada and US. J Cancer Policy. 2014;2(1):1–11.
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: Recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Am J Gastroenterol. 2017;112(7):1016–1030.
- Pox CP. Controversies in colorectal cancer screening. Digestion. 2014;89(4):274–281.
- Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions. International Society for Pharmacoeconomic and Outcomes Research (ISPOR)—13th International Meeting, Toronto; 2008.