Abstract
Aims: To evaluate the association of relapse and healthcare resource utilization in patients with schizophrenia (SZ), bipolar disorder (BD), or major depressive disorder (MDD) who switched antipsychotic medication versus those who did not.
Materials and methods: Medicaid claims from six US states spanning six years were retrospectively analyzed for antipsychotic switching versus non-switching. For all patients with SZ, BD, or MDD, and for the subset of patients who also had ≥1 extrapyramidal symptoms (EPS) diagnosis at baseline, times to the following outcomes were analyzed: underlying disease relapse, other psychiatric relapse, all-cause emergency room (ER) visit, all-cause inpatient (IP) admission, and EPS diagnosis.
Results: Switchers (N = 10,548) had a shorter time to disease relapse, other psychiatric relapse, IP admissions, ER visits, and EPS diagnosis (all, log-rank p < .001) than non-switchers (N = 31,644). Switchers reached the median for IP admission (21.50 months) vs non-switchers (not reached) and for ER visits (switchers, 9.07 months; non-switchers, 13.35 months). For disease relapse, other psychiatric relapse, and EPS diagnosis, <50% of patients had an event during the two-year study period. Subgroup analysis of those with ≥1 EPS diagnosis revealed similar associations.
Limitations: Only association, not causation, may be inferred, and there may be differences between the patient groups in parameters not evaluated.
Conclusions: These results show that disease and other psychiatric relapse, all-cause ER visits, IP admissions, and EPS diagnosis occurred earlier for patients who switched antipsychotics than for those who did not, suggesting that switching is associated with an increased risk of relapse in patients with SZ, BD, and MDD. This may be attributed to more-severely ill patients being less responsive than those with less-severe illness, which, in turn, may require more episodes of switching.
Introduction
Schizophrenia (SZ), bipolar disorder (BD), and major depressive disorder (MDD) are severe mental illnesses that can affect social and occupational functioningCitation1–5. Schizophrenia, a condition with symptoms that include hallucinations, delusions, reduced emotions, and movement disorders, affected 2.6 million Americans in 2014Citation6. Bipolar disorder, characterized by severe mood alterations that include manic and depressive episodes lasting from days to weeks or longer, affected 5.7 million AmericansCitation7,Citation8. For people with either SZ or BD, the mortality rate is two to three times higher than in the general populationCitation9,Citation10. Major depressive disorder, which manifests in persistent low mood and loss of interest in usual activities, was diagnosed in 16.1 million Americans in 2015 and is the leading cause of disability among the US population aged 15–44 yearsCitation11,Citation12.
While antipsychotics are commonly used as first-line treatment for schizophreniaCitation13,Citation14, several of them were recently approved and are increasingly used as augmentation agents for mood disordersCitation15,Citation16, such as BD and MDD, especially when therapies with mood stabilizers, antidepressants, and electroconvulsive treatments have failedCitation17. Antipsychotic drug therapy is further recommended as maintenance treatment for SZ, BD, and MDD to avoid exacerbation of symptoms and risk of relapseCitation18–20. Relapse leads to worsening of a patient’s quality of life, and it also creates a substantial economic burdenCitation21,Citation22. For example, one study estimated mental health treatment costs to be about two to five times higher for patients with SZ who experienced psychotic relapse than for those who did notCitation23.
However, several factors, such as costs, non-compliance, lack of efficacy, and presence of side effects may prompt a change in antipsychotic therapyCitation24,Citation25. Of concern are metabolic and neurological side effects that can develop from exposure to any class of antipsychotic drug. Among these, extrapyramidal symptoms (EPS), categorized in acute (akathisia, dystonia, pseudoparkinsonism), and tardive syndromes (dystonia and dyskinesia), are abnormally induced movement disorders that are sometimes irreversible or lethal, can worsen stigma of the underlying mental illness, and have been associated with relapse, rehospitalization, and reduced compliance to medicationCitation18,Citation26–30. When patients developed tardive dyskinesia (TD), withdrawal of the causative antipsychotic drug was the strategy of choice in the past based on eventual improvement of TD symptoms in 36–55% of patientsCitation31. However, a study showed that 53% of patients with SZ experienced psychotic relapse after withdrawal of the provoking drugCitation32. Similarly, reducing the antipsychotic dose as an alternative method of treating EPS still bears the risk of psychotic relapse, as evidenced by previous studies in patients with SZ, BD, and MDD that associated antipsychotic dose reductions with an increase in hospital utilization ratesCitation22,Citation33. Switching antipsychotics has also been considered as a strategy for managing emergent EPS. Three approaches (abrupt withdrawal, cross-tapering, and plateau cross-tapering) have been introduced for the procedure of switching antipsychotic medication, which may be suitable after considering several important factors, such as patient’s disposition, drug class, and drug interactionsCitation34,Citation35. However, patients may experience re-emergence of psychotic symptoms, anticholinergic discontinuation reactions, and rebound EPS during the transition period. Therefore, even though careful selection of an appropriate switching strategy reduces the risk of relapse and exacerbation of symptoms, a change of therapeutic agent does not guarantee alleviation from illness.
Limited real-world evidence exists on the risk of relapse in patients with SZ, BD, or MDD who switched antipsychotic treatment. To analyze the impact of antipsychotic medication switching on patients with SZ, BD, and MDD this retrospective study evaluated the risk of relapse, hospital-based resource utilization, and EPS diagnosis in patients who switched antipsychotic drugs versus those who did not switch. To assess the impact of EPS-related switching on patients’ outcomes, a subgroup of patients who had ≥1 EPS diagnosis at baseline was also analyzed.
Methods
Data source
A large retrospective cohort study was conducted using de-identified claims data for Medicaid-eligible beneficiaries. Based on availability, Medicaid data comprised complete medical claims (diagnoses, procedures, and paid amounts), pharmaceutical claims, enrollment history, and patient demographics of the most recent six years from six US states (Iowa, Kansas, Missouri, Mississippi, New Jersey, and Wisconsin). For all medical records, the study period ranged from 2008 to 2017. All data are de-identified and comply with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act (HIPAA); as such, institutional review was not required.
Sample selection
To compare the risk of relapse, inpatient admissions (IP), emergency room (ER) visits and EPS in patients with SZ, BD or MDD who switched antipsychotics versus those who did not switch antipsychotics, this study had the following eligibility criteria: ≥1 diagnosis of SZ, BD, or MDD and ≥2 pharmacy claims for an oral antipsychotic since diagnosis, as well as ≥1 antipsychotic monotherapy period of ≥90 days with a stable dose (up to 10% variation of the mean dose was allowed). Patients in the group that switched antipsychotics had a stable dose period of monotherapy of ≥90 days and then experienced a switch in antipsychotic treatment (the first prescription date for the new antipsychotic was defined as a potential index date). Patients in the group that did not switch antipsychotics had a stable dose period of monotherapy ≥91 days and did not experience a switch in antipsychotic treatment (the 91st day of the stable monotherapy period was defined as a potential index date). In both groups, patients had ≥6 months of continuous enrollment prior to ≥1 potential index date and were aged between 18 and 65 on ≥1 potential index date.
The impact of EPS-related switching was evaluated in a subgroup of patients who had ≥1 diagnosis of EPS during the baseline period, which included the six months before the index date. Patients were excluded if they used >1 antipsychotic concurrently during the study period. The index date was randomly selected from a given patient's potential index dates that met all sample selection criteria. Randomly selecting the index date increased the likelihood that the sample would reflect all stages of disease.
To reduce differences between the groups, patients who did not switch were matched 3:1 to patients who switched, based on age category (i.e. ages 18–19, 20–24, 25–29…110–114 years), gender, state, index drug generation (first-generation antipsychotics (FGA) vs second-generation antipsychotics (SGA)), and underlying psychiatric disease (SZ, BD, or MDD). Two years of data following the index date were sampled from the patient’s record, unless treatment changed (i.e. a change in medication or a change in dose) or eligibility was lost. In the group of patients who switched antipsychotics, an increase in the dose of the new drug following a treatment switch did not count as a treatment change to allow for dose titration. The following data were collected for all patients during the baseline period: demographics (age, gender, state, and insurance plan type); underlying psychiatric diseases (SZ, BD, MDD, and all combinations thereof); index year; index drug class (FGA vs SGA); psychiatric-related comorbidity profile; Charlson Comorbidity Index (CCI) score, including individual comorbidities; psychotherapy; psychiatric medication; and observed disease duration, defined as the time from first observed SZ, BD, or MDD diagnosis to the index date.
Outcome measures
The following outcomes were assessed during the two-year study period: disease relapse (IP admission or ER visit for the underlying diagnosed psychiatric condition(s)), other psychiatric admission (IP admission or ER visit for psychiatric conditions other than the underlying diagnosed psychiatric condition), all-cause IP admission, and all-cause ER visit. These outcomes were compared between the two groups of patients (those who switched antipsychotics and those who did not) for the overall patient population and for the subgroup of patients with ≥1 diagnosis of EPS during the baseline period. Time to first EPS diagnosis was evaluated for the overall patient population and for the subgroup of patients who did not have an EPS diagnosis at baseline.
Statistical analysis
Characteristics of the two groups were compared using Wilcoxon signed-rank tests for continuous variables and McNemar’s tests for dichotomous variables. Kaplan–Meier analyses and log-rank tests were used to estimate and compare the time to each outcome of interest. Unadjusted and adjusted Cox proportional hazard models were used to compare outcomes of the two groups. Covariates in the adjusted model included age (continuous), disease duration, insurance plan, index year, baseline disease relapse, baseline psychiatric admission, baseline ER visits, baseline IP admissions, diagnoses of various psychiatric diseases, CCI score, psychotherapy use, and psychiatric medication use. To control for confounding, the covariates in the model were either identified during the baseline period or at the index date, both of which were before the study period.
Results
Baseline characteristics
Of 822,287 patients with ≥1 diagnosis of SZ, BD, or MDD, 11,035 patients who switched antipsychotics and 133,789 patients who did not switch met the selection criteria (). 31,644 patients who did not switch were matched 3:1 to 10,548 patients who switched antipsychotics, resulting in an identical distribution of age, gender, geography, and underlying disease ().
Figure 1. Patient selection flow diagram. 1Diagnoses for SZ were based on ICD-9 codes 295.xx; and ICD-10 codes F20.x and F25.x from the Medicaid claims database (the most recent 6 years for data of each state). 2Diagnoses for BD were based on ICD-9 codes 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.7x, or 296.8x; ICD-10 codes F30.x and F31.x. 3Diagnoses for MDD were based on ICD-9 codes 296.2x and 296.3x; ICD-10 codes F32.x and F33.x. Abbreviations. BD, bipolar disorder; ICD, International Classification of Diseases; MDD, major depressive disorder; SZ, schizophrenia.
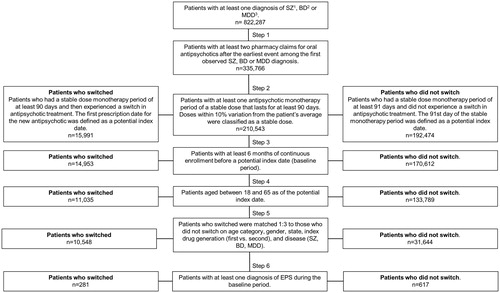
Table 1. Baseline characteristics of overall patients and patients with ≥1 EPS diagnosis.
Differences between the two groups included the mean observed disease duration, which was 27.4 months for patients who switched antipsychotics and 15.0 months for patients who did not switch (p < .001), and mean duration of follow-up time, which was 5.2 months for patients who switched and 5.8 months for those who did not switch (p < .001). Patients who switched antipsychotics experienced higher rates of psychiatric comorbidities (including EPS, SZ spectrum disorders, substance-related and addictive disorders, anxiety disorders, personality disorders, p < .001; trauma- and stressor-related disorders, p < .01; relapses, ER visits, and IP admissions, p < .001) during the baseline period than those who did not switch. Furthermore, a greater proportion of patients who switched received psychotherapy (19.8% vs 15.6%, p < .001) and used psychiatric medications (antidepressant: 78.0% vs 73.3%, p < .001; mood stabilizer: 51.5% vs 45.9%, p < .001; anxiety medication: 47.4% vs 42.5%, p < .001; ADHD medication: 6.9% vs 6.2%, p < .05) during the baseline period. The CCI was also significantly greater for patients who switched than for those who did not (mean ± SD: 0.61 ± 1.2 vs 0.57 ± 1.16, p < .001).
In the subgroup of patients with ≥1 EPS diagnosis at baseline, 281 patients who switched antipsychotics were matched to 617 who did not switch, resulting in similar characteristics between the two subgroups (p > .05) during the baseline period, except for insurance type (FFS: 37.4% of patients who switched, 44.7% of patients who did not switch; p < .05) and disease duration (27.3 months for patients who switched, 15.8 months for patients who did not switch; p < .001).
Outcomes in the overall group and a subgroup of patients without an EPS diagnosis
In the overall group, patients who switched antipsychotics experienced relapse and healthcare resource utilization significantly earlier than did patients who remained on their antipsychotic medication (all, log-rank p < .001).
The median time to first underlying disease relapse and time to first psychiatric admission were not reached in either group during the two-year study period () However, patients who switched antipsychotics were more likely to have a disease relapse (adjusted HR = 1.36; 95% CI 1.28, 1.44; p < .001) and a psychiatric admission (adjusted HR = 1.20; 95% CI 1.13, 1.28; p < .001) than patients who did not switch.
Figure 2. Time to first disease relapse during the 2-year study period among all patients. Patient claims were analyzed for disease relapse after switching antipsychotic medication. Outcomes for both cohorts (those who switched, and those who did not) were assessed using Kaplan–Meier analysis and compared using a log-rank test. The number of patients at risk is represented for each time point. Abbreviations. CI, confidence interval; HR, hazard ratio.
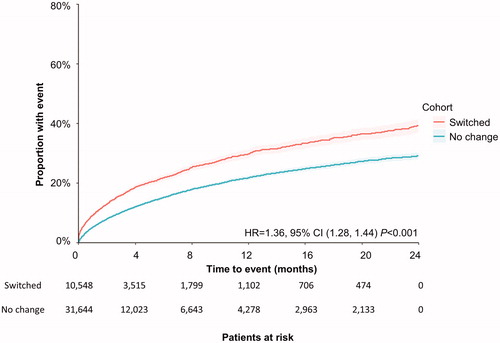
Figure 3. Time to first psychiatric admission during the 2-year study period among all patients. Patient claims were analyzed for first psychiatric admission following switching of antipsychotic medication. Outcomes for both cohorts (those who switched, and those who did not) were assessed using Kaplan–Meier analysis and compared using a log-rank test. The number of patients at risk is represented for each time point. Abbreviations. CI, confidence interval; HR, hazard ratio.
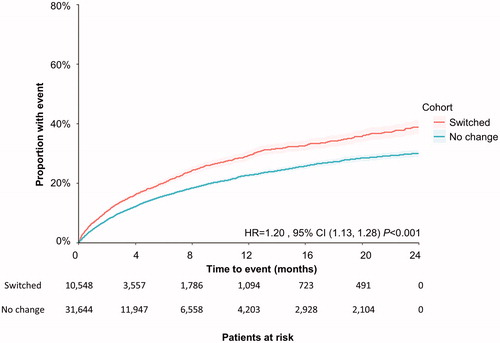
Patients who remained on their antipsychotic medication did not reach the median time to IP admission during the two-year study period. For patients who switched antipsychotics, the median time to IP admission was 21.50 months (log-rank p < .001). This event was more likely to happen during the study period in the group with patients who switched (adjusted HR = 1.26; 95% CI 1.20, 1.32; p < .001) than for those who did not switch ().
Figure 4. Time to first IP admission during the 2-year study period among all patients. Patient claims were analyzed for IP admission following switching of antipsychotic medication. Outcomes for both cohorts (those who switched, and those who did not) were assessed using Kaplan–Meier analysis and compared using a log-rank test. The number of patients at risk is represented for each time point. Abbreviations. CI, confidence interval; HR, hazard ratio; IP: inpatient.
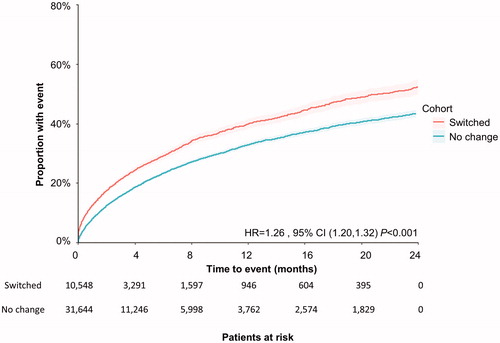
Similarly, for patients who switched antipsychotics, the median time to first ER visit occurred earlier (9.07 months vs 13.35 months; log-rank p < .001) and was more likely to happen during the study period (adjusted HR = 1.11; 95% CI 1.07, 1.16; p < .001) than for those who remained on their antipsychotic drug.
For patients who switched antipsychotics in the overall group, the first EPS diagnosis occurred earlier (log-rank p < .01) and was more likely to happen during the study period (adjusted HR = 1.28; 95% CI 1.06, 1.55; p < .05) than for those who did not switch. However, the median time to this event was not reached in either group. A similar outcome was observed for a subgroup of patients without an EPS diagnosis at baseline (log-rank p < .001).
Outcomes in the subgroup of patients with ≥1 EPS diagnosis
In the subgroup of patients with ≥1 EPS diagnosis, a similar trend related to switching antipsychotics was observed for all outcomes (excluding EPS diagnosis, which was not assessed) as in the overall patient group. Among those who switched, the median was reached for time to disease relapse (21.60 months vs not reached for patients who did not switch) and for time to IP admission (10.36 months vs not reached for patients who did not switch). The median time to ER visit was lower for those who switched (5.82 months) compared to those who did not change (10.68 months). Similarly, the likelihood of experiencing the first underlying disease relapse (adjusted HR = 1.35; 95% CI 0.97, 1.88), psychiatric admission (adjusted HR = 1.29; 95% CI 0.88, 1.89), IP admission (adjusted HR = 1.34; 95% CI 1.01, 1.78), and ER admission (adjusted HR = 1.18; 95% CI 0.90, 1.54) during the study period was increased for patients who switched antipsychotics compared with those who did not switch. However, only IP admission was statistically significant (p < .05).
Discussion
The results presented in this study show that patients with SZ, BD, or MDD who switched antipsychotic medications were at higher risk for disease relapse, psychiatric admission, all-cause IP admission, all-cause ER visits, and EPS diagnosis than those who continued the antipsychotic medication. Analysis in a subgroup of patients with ≥1 EPS at baseline showed that the presence of EPS among patients who did not switch antipsychotics was associated with shorter time to first relapse and healthcare resource utilization (IP admission and ER visits), and that switching antipsychotics was associated with a quicker onset of assessed outcomes. Hospital re-admissions of patients with SZ, BD, or MDD may be indicative of treatment failure after antipsychotic switching. All three conditions are severe mental illnesses that contribute substantially to the economic burden for patients and the healthcare system. The costs for treating SZ were estimated at $38 billion USD in 2013; in addition, costs for patients who experienced relapse have been estimated to be two to five times higher than for those who did not relapseCitation23,Citation36. The economic burden of BD in the US was approximately $151 billion in 2009Citation37, and for MDD it was $210.5 billion USD in 2013Citation38. Therefore, risks associated with antipsychotic drug switching should be weighed carefully by healthcare professionals and patients when considering a change of the therapy plan.
Switching antipsychotics has become a widespread practice but may not be appropriate in some cases. For example, switching antipsychotics is not recommended for patients who experience a recent recovery from a psychotic episode unless they have been stable for at least three monthsCitation39. Furthermore, some problems, such as drug efficacy or minor side effects, may resolve with simple adjustments, such as dosage changes, and some may be alleviated once the treatment is stabilizedCitation34. Switching antipsychotic medication is also contraindicated if patients are at risk of becoming a danger to themselves or others, as they may experience worsening of symptoms during the transition period. In some cases, however, switching the antipsychotic drug may be necessary for a variety of reasons that include unacceptable side effects and relapse despite complianceCitation25. In a prospective observational study that analyzed leading physicians’ reasons for switching antipsychotics, lack of tolerability was the main factor for 65% of their patients, followed by lack of efficacy in 44% of their patientsCitation24. Several studies showed that switching from conventional first-generation agents to oral second-generation agents improved symptoms of mental illness with long-term effectivenessCitation25. Thus, in some cases the benefits of a different medication may outweigh the risks associated with antipsychotic switching.
Strengths
This study has several strengths, including the use of claims-based data from multiple US state data sets with a two-year follow-up period. Furthermore, to minimize confounding effects, patients who continued the current antipsychotic drug were matched 3:1 to patients who switched the medication (including, e.g. baseline relapse rate, psychiatric comorbidities, psychiatric medications, and healthcare resource utilization) resulting in similar demographic and comorbidity profiles. In addition, the inclusion criterion of at least 90 days of a stable monotherapy dose prior to the index date further reduced the possibility that outcomes described in this study have been affected by changes other than antipsychotic switching.
Limitations
Limitations of this study include those inherent to claims-based analyses. Given the observational design of this study, only association, not causation, may be inferred. Furthermore, there may be differences between the patient groups in parameters that were not captured in the available data set. In addition, reasons for antipsychotic switching could not be determined from the available data. Another limitation is the use of diagnosis codes for identifying comorbidities, as it is likely that EPS diagnoses are underreported in the claims databaseCitation40. Thus, patients with early signs of EPS, among other comorbidities, may be underestimated in the current analyses. This study cannot rule out that the higher risk of relapse and healthcare resource utilization among patients who switched antipsychotics is in part due to the characteristics of the group. Longer duration of underlying mental illness, more comorbidities, and more psychiatric medications at baseline can indicate a more-severe or chronically ill population. However, more-severely ill patients were excluded from the analysis when they met criteria indicative of more-resistant disease, such as receiving multiple antipsychotics and substantial changes in dosing. In addition, outcome measures were controlled for multiple covariates in the Cox model that may be reflective of illness severity, such as psychiatric, substance use and medical comorbidities; illness duration; and concomitant medication use.
Nonadherence to medication often leads to relapse and hospitalization and thus has the potential to affect the outcomes in this study. However, enrollment criteria were designed to screen for nonadherent patients by excluding those who had fewer than 90 days of stable prescription monotherapy with antipsychotic medication prior to the index study date. A scenario in which increased risk of hospitalization among patients who switched antipsychotics is a result of nonadherence after the index date still provides evidence for switching-related, unfavorable treatment effects. The attrition of patients during the two-year study period, including the different attrition rates between the study groups, was a result of group definitions, differences in outcomes, and possibly nonadherence. Patients were censored at any change in antipsychotic use not covered by the original inclusion criteria or after an outcome event occurred. Because they are designed to handle censored or truncated time-to-event dataCitation41, the Kaplan–Meier analyses and the Cox models accounted for attrition and censoring of patients.
Conclusions
This study shows that antipsychotic switching is associated with an increased risk of relapse, healthcare resource utilization, and EPS diagnosis in patients with SZ, BD, or MDD, which suggests that switching antipsychotic medication may result in exacerbation of the patient’s health status. It is important to carefully and individually assess the risks of switching antipsychotic drugs when considering a change for patients who require maintenance antipsychotic treatment. Together with previous reports that demonstrated similar risks for antipsychotic withdrawal and dose reduction, this study highlights the unmet need for alternative strategies to manage troublesome side effects and to achieve effective maintenance therapies for patients with SZ, BD, or MDD.
Transparency
Declaration of funding
This study was funded by Teva Pharmaceuticals, Petach Tikva, Israel.
Declaration of financial/other relationships
Benjamin Carroll: Employee of Teva Branded Pharmaceutical Products R&D, Inc.
Rajeev Ayyagari: Employee of Analysis Group, Inc.
Darren Thomason: Employee of Analysis Group, Inc.
Fan Mu: Employee of Analysis Group, Inc.
Michael Philbin: Employee of Teva Pharmaceuticals, Field Medical.
Author contributions
BC: Conception and design; analysis and interpretation of the data; drafting of the paper and revising it critically for intellectual content; final approval of the version to be published
RA: Conception and design; analysis and interpretation of the data; drafting of the paper and revising it critically for intellectual content; final approval of the version to be published
DT: Conception and design; analysis and interpretation of the data; drafting of the paper and revising it critically for intellectual content; final approval of the version to be published
FM: Conception and design; analysis and interpretation of the data; drafting of the paper and revising it critically for intellectual content; final approval of the version to be published
MP: Conception and design; revising manuscript critically for intellectual content; final approval of the version to be published
Acknowledgements
We thank Dana Meyen, PhD (Chameleon Communications International with funding from Teva Pharmaceuticals) for editorial assistance in the preparation of this report.
References
- Dean BB, Gerner D, Gerner RH. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in bipolar disorder. Curr Med Res Opin. 2004;20(2):139–154.
- Hammer-Helmich L, Haro JM, Jonsson B, et al. Functional impairment in patients with major depressive disorder: the 2-year PERFORM study. NDT. 2018;14:239–249.
- IsHak WW, James DM, Mirocha J, et al. Patient-reported functioning in major depressive disorder. Ther Adv Chronic Dis. 2016;7(3):160–169.
- Tohen M, Hennen J, Zarate CM Jr, et al. Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. Am J Psychiatry. 2000;157(2):220–228.
- Barrios M, Gomez-Benito J, Pino O, et al. Functioning in patients with schizophrenia: a multicentre study evaluating the clinical perspective. Psychiatry Res. 2018;270:1092–1098.
- Treatment Advocacy Center. Schizophrenia fact sheet. [cited 2018 Nov 14]. Available from: https://www.treatmentadvocacycenter.org/evidence-and-research/learn-more-about/25-schizophrenia-fact-sheet.
- NIH: Mental health information on bipolar disorder. [cited 2018 Nov 14]. Available from: https://www.nimh.nih.gov/health/topics/bipolar-disorder/index.shtml.
- DBSA. Bipolar disorder statistics. [cited 2018 Nov 14]. Available from: https://secure2.convio.net/dabsa/site/SPageServer/?pagename=education_statistics_bipolar_disorder.
- Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. PS. 2009;60(2):147–156.
- Brown S, Kim M, Mitchell C, et al. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196(2):116–121.
- NIH: Mental health information on major depression. [cited 2018 Nov 14]. Available from: https://www.nimh.nih.gov/health/statistics/major-depression.shtml.
- ADAA: Understand the facts: depression. [cited 2018 Nov 14]. Available from: https://adaa.org/understanding-anxiety/depression.
- Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56.
- Leo RJ, Regno PD. Atypical antipsychotic use in the treatment of psychosis in primary care. Prim Care Companion J Clin Psychiatry. 2000;02(06):194–204.
- Kemp DE. Managing the side effects associated with commonly used treatments for bipolar depression. J Affect Disord. 2014;169 (Suppl 1):S34–S44.
- Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. AJP. 2009;166(9):980–991.
- Patkar AA, Pae CU. Atypical antipsychotic augmentation strategies in the context of guideline-based care for the treatment of major depressive disorder. CNS Drugs. 2013;27(S1):29–37.
- Lerner PP, Miodownik C, Lerner V. Tardive dyskinesia (syndrome): current concept and modern approaches to its management. Psychiatry Clin Neurosci. 2015;69(6):321–334.
- Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93.
- Practice guideline for the treatment of patients with major depressive disorder: Third edition: American Psychiatric Association; 2010. [cited 2018 Nov 14]. Available from: https://www.guidelinecentral.com/summaries/practice-guideline-for-the-treatment-of-patients-with-major-depressive-disorder-third-edition/#section-society.
- Almond S, Knapp M, Francois C, et al. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry. 2004;184(4):346–351.
- Caroff SN, Mu F, Ayyagari R, et al. Hospital utilization rates following antipsychotic dose reductions: implications for tardive dyskinesia. BMC Psychiatry. 2018;18(1):306.
- Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10(1):2.
- Roussidis A, Kalkavoura C, Dimelis D, et al. Reasons and clinical outcomes of antipsychotic treatment switch in outpatients with schizophrenia in real-life clinical settings: the ETOS observational study. Ann Gen Psychiatry. 2013;12(1):42.
- Masand PS. A review of pharmacologic strategies for switching to atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;07 (03):121–129.
- Correll CU, Detraux J, De Lepeleire J, et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119–136.
- Youssef HA, Waddington JL. Morbidity and mortality in tardive dyskinesia: associations in chronic schizophrenia. Acta Psychiatr Scand. 1987;75(1):74–77.
- Ballesteros J, Gonzalez-Pinto A, Bulbena A. Tardive dyskinesia associated with higher mortality in psychiatric patients: results of a meta-analysis of seven independent studies. J Clin Psychopharmacol. 2000;20(2):188–194.
- Zutshi D, Cloud LJ, Factor SA. Tardive syndromes are rarely reversible after discontinuing dopamine receptor blocking agents: experience from a university-based movement disorder clinic. Tremor Other Hyperkinet Mov (NY). 2014;4:266.
- Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21(2):151–156.
- Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127–148.
- Gilbert PL, Harris M, McAdams L, et al. Neuroleptic withdrawal in schizophrenic patients: a review of the literature. Arch Gen Psychiatry. 1995;52(3):173–188.
- Carroll B, Mu F, Ayyagari R, et al. Hospital utilization rates following antipsychotic dose reductions among patients with bipolar and major depressive disorders; 2017. [cited 2018 Nov 14]. Available from: https://www.psychcongress.com/posters/hospital-utilization-rates-following-antipsychotic-dose-reductions-among-patients-bipolar.
- Burns T, Chabannes JP, Demyttenaere K. Switching antipsychotic medications: general recommendations and switching to amisulpride. Curr Med Res Opin. 2002;18(4):201–208.
- Grande I, Bernardo M, Bobes J, et al. Antipsychotic switching in bipolar disorders: a systematic review. Int J Neuropsychopharm. 2014;17(03):497–507.
- Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(06):764–771.
- Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness-1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213–219.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(02):155–162.
- Ganguli R. Rationale and strategies for switching antipsychotics. Am J Health Syst Pharm. 2002;59(suppl_8):S22–S26.
- Cortese L, Jog M, McAuley TJ, et al. Assessing and monitoring antipsychotic-induced movement disorders in hospitalized patients: a cautionary study. Can J Psychiatry. 2004;49(1):31–36.
- Kleinbaum D, Klein M. Survival analysis: a self-learning text. 3rd ed. New York (NY): Springer; 2012.
