Abstract
Background: Both pembrolizumab (PEMBRO) and ipilimumab + nivolumab (IPI + NIVO) are FDA-approved immunotherapy regimens for advanced melanoma (AM). Each regimen has different toxicity profiles potentially impacting healthcare resource utilization (HCRU). This study compared real-world hospitalization and emergency department (ED) utilization within 12 months of therapy initiation of each regimen.
Methods: A retrospective cohort study was conducted in AM patients ≥18 years old initiating PEMBRO or IPI + NIVO between January 1, 2016–December 30, 2017. Patients were identified from 12 US-based academic and satellite centers. All-cause hospitalization ED visits were identified. These events were used to calculate rates per 1,000 patient months. Utilization between groups was compared using multivariate logistic regression.
Results: In total, 400 patients were included (200 PEMBRO, 200 IPI + NIVO). PEMBRO vs IPI + NIVO patients had poorer Eastern Cooperative Group (ECOG) performance status, 29% 2–4, vs 12% (p < .001); more diabetes, 21% vs 13% (p = .045); were more often PD-L1 expression positive, 77% vs 63% (p = .011); and less likely BRAF mutant, 35% vs 50% (p = .003). The proportion with more than one hospitalization over 12 months was 17% PEMBRO vs 24% IPI + NIVO. Less than 2% had more than one admission and none had more than two. Unadjusted mean (SD) hospitalizations per 1,000 patient-months were 16 (37) and 20 (38), PEMBRO and IPI + NIVO, respectively. Adjusted odds ratio for hospitalization was 0.6 (95% CI = 0.3–0.9; p = .027) for PEMBRO vs IPI + NIVO. ED visits occurred in 18% vs 21%, PEMBRO and IPI + NIVO, respectively, 0.7 (p = .186).
Conclusions: PEMBRO patients had a significantly lower probability of hospitalization through 12 months vs IPI + NIVO. The probability of ED visits did not differ.
Background
Approximately 96,480 new melanomas will be diagnosed in the US in 2019, with 7,230 expected deaths under the current treatment paradigmsCitation1. Prior to 2011, systemic treatments for advanced melanoma relied primarily on chemotherapy. These treatments were associated with poor overall survival and significant toxicityCitation2–4. Recent understanding of the role of regulatory pathways in T cells and the role in anti-tumor response has led to the revolution of immunotherapies to enhance anti-tumor responseCitation5. Programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) checkpoint inhibitors are the new standard of care for the treatment of patients with unresectable or metastatic melanoma, either as single agent therapy, or in combination with anti–CTLA-4Citation6,Citation7. Among checkpoint inhibitor-based regimens approved by the US Food and Drug Administration for advanced melanoma are pembrolizumab (PEMBRO) and ipilimumab with nivolumab (IPI + NIVO), both of which are associated with improved survival and lower toxicity vs chemotherapyCitation8–10.
Comparing PEMBRO with IPI in controlled trials, PEMBRO as a single agent demonstrated prolonged progression-free and overall survival with fewer high-grade adverse eventsCitation6, and NIVO administered in combination with IPI has better progression free survival and higher rates of objective response than with IPI aloneCitation7. Costs related to toxicity can be substantial, particularly with respect to inpatient management of AEsCitation11–13. In clinical trials, reported grade 3 or 4 treatment-related adverse events (AEs) are 59% with IPI + NIVO in combination and 17% with PEMBRO as a single agentCitation6,Citation7. Differences in the benefit-risk profiles between single agent and combination immunotherapy noted in trials may impact healthcare resource utilization (HCRU), including hospitalization and emergency department (ED) visitsCitation8,Citation9,Citation14–16. However, no real world studies have compared PEMBRO vs IPI + NIVO in terms of real-world hospitalization and emergency department utilization directly.
Given the focus of US payers to improve the cost effectiveness of oncology care through new payment incentives such as the Center for Medicare & Medicaid Innovation (CMS) Oncology Care Model (OCM), identifying treatment regimens that can minimize down-stream HCRU is important. The OCM represents a shift towards shared savings between payers and providers if financial and quality of care benchmarks are met. Primary OCM outcomes include reduction in total cost of care episodes, and reductions in risk-adjusted hospital admissions and ED visitsCitation17. Little real-world comparative data of immunotherapy melanoma treatment impact on hospitalizations or ED visits exists in the US to inform treatment selection to meet OCM goals, and no studies as of the completion of this study have compared PEMBRO with IPI + NIVO. Given restrictive trial entrance criteria and protocol-driven health resource use, real-world studies of immunotherapy impact on HCRU are needed. This is particularly relevant as patients with poorer performance status and comorbidity are typically excluded from controlled trials, yet may be at greater risk for HCRUCitation18.
The objective of this analysis was to compare real-world risk of hospitalization and ED visits within 12 months of starting PEMBRO or IPI + NIVO in US-based academic and satellite centers.
Methods
Data source
This is an analysis of real-world hospitalization and emergency department utilization from an ongoing 3-year cohort study of advanced melanoma treatment and outcomes in patients treated in US-based academic and satellite centers. Twelve geographically dispersed centers (three from the Northeast, four from the Midwest, two from the South, three from the West) were recruited from a national database of oncology providers in the US. Physicians were recruited by telephone and underwent screening prior to acceptance as study investigators. Each physician/site was screened using a questionnaire to confirm eligibility prior to study enrollment. To contribute patients, academic centers needed to currently treat 15 advanced melanoma patients with PEMBRO and/or IPI + NIVO and satellite centers needed to treat at least seven. Physicians must have acknowledged that they had access to patient medical records sufficient to identify and collect all study parameters for the duration of the study. Qualifying physicians were enrolled into the study and signed the site agreement acknowledging and agreeing to study obligations. Sites were blinded to the sponsor and the sponsor was blinded to sites.
Study sample
Patients were selected for inclusion based upon birthdate using a random number table and were enrolled into two cohorts, with 200 each receiving either PEMBRO or IPI + NIVO in any line of therapy (LOT) for unresectable Stage III or Stage IV melanoma. Included were patients 18 years or older with histologically-confirmed diagnosis of unresectable Stage III or Stage IV cutaneous melanoma that received PEMBRO or IPI + NIVO in any LOT between January 1, 2016 through December 30, 2017. Patients receiving investigational agents in any LOT and those with uveal or ocular melanoma were excluded.
Data collection
Data were derived from retrospective chart review using a standardized data collection form. Sites received training on patient identification, data collection forms, and data dictionaries prior to study initiation. Data included in this analysis were baseline demographics (age, gender, race, ethnicity, marital status, insurance coverage, education level, income, employment), clinical characteristics at unresectable or metastatic melanoma diagnosis, including American Joint Committee on Cancer (AJCC) stage version 7, presence of brain metastases, PD-L1 expression testing prevalence and results (PD-L1 was recorded as positive if >0%), BRAF mutation testing prevalence and results, LDH (normal, 1–2 times upper limit of normal (ULN), 2 times ULN), comorbidities including the Charlson comorbidity indexCitation19, Eastern Cooperative Group (ECOG) performance status at treatment therapy initiation, line of therapy (LOT) IPI + NIVO or PEMBRO administered, hospitalizations, and ED visits through 1-year of treatment initiation.
Standard protocol approvals, patient privacy
Collected data were de-identified in compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Synthetic identifiers were created by investigators maintaining a patient-level crosswalk to protect the confidentiality of records. A central Institutional Review Board (IRB) and Compliance/Privacy approval was obtained from New England IRB prior to initiation of the study data.
Statistical analysis
This is a multi-center study and the main unit of analysis was the patient. Analyses were conducted in a two-tailed fashion with alpha <0.05 using SAS® 9.4 (SAS Institute Inc., Cary, NC). Patient demographic and clinical characteristics were summarized descriptively within each cohort using means (standard deviations [SD]), medians (range), or proportions, and were compared using Pearson χ2 or student t-tests, depending upon data distribution. Utilization was presented as rates having any utilization within 12 months, and per 1,000 person months of follow-up (until death or loss to follow-up). Patients were censored at date of switch, if switched to another LOT prior to 12 months. Monthly utilization was summarized using means and SD, medians, and range. To test a hypothesis that any occurrence of hospitalization or ED occurrence is similar in the IPI + NIVO vs PEMBRO groups, the categorical variables of hospitalization and ED occurrence was examined by percentages and compared using logistic regression. Hospitalization or ED visit were examined as discrete endpoints and logistic regression was selected as the analytic approach, as hospitalizations within 1 year were anticipated to be binary yes/no, with few patients having two or more hospitalizations within 12 months. We controlled for factors specified in the a priori analysis plan and potential confounders unbalanced between groups as identified in univariate analysis. Included in initial models were the covariates ECOG performance status, BRAF mutation vs wild type, PD-L1 expression positive vs negative (each specified a priori), LDH status (normal, elevated 1–2 times upper limit of normal [ULN] or >2 times ULN), diabetes, or coronary artery disease comorbidity (added after examining potential confounders in univariate analysis). Covariates retained in regression models were those p < .1 (diabetes, coronary artery disease, and LDH). As sample size for comorbidities and clinical characteristics may impact tests of significance in χ2, standardized residuals were examined in cross-tabulationsCitation20. In the absence of statistical significance, covariates standardized residuals with an absolute value of 2.0 were also considered for inclusion as covariates in logistic regression models. We did not conduct statistical tests on data unadjusted for potential confounders. Unadjusted data were presented descriptively only. The sample size of 200 in each cohort was selected to detect a minimum 15% utilization rate of services ±5% with a 95% confidence interval.
Results
Demographic and clinical characteristics
Four hundred patient records were included, 200 each from patients receiving PEMBRO or IPI + NIVO. Detailed information on the methodology has been previously publishedCitation18. The patients were primarily Caucasian, aged 62 years on average at entry into the cohort, and half were male, with no differences between PEMBRO vs IPI + NIVO cohorts. The cohorts were similar with respect to marital status, education, employment, and income level. More than half of all patients were commercially insured regardless of cohort. Most had prescription drug coverage through a commercial insurer, or through a government payer ().
Table 1. Demographic characteristics.
There was no statistical difference in LOT of PEMBRO and IPI + NIVO administered, with each administered first line in 84.0% and 87.5% of patients, respectively. With respect to baseline clinical characteristics, PEMBRO and IPI + NIVO cohorts were similar in AJCC version 7 stage, M stage, LDH elevation among those tested. The PEMBRO cohort had significantly poorer ECOG performance status at treatment start, 29% ECOG 2–4, vs 12% (p < .001). A greater proportion of patients displayed BRAF wild-type tumor in the PEMBRO vs IPI + NIVO cohort (65.2% vs 50.3%, respectively) (p = .003). Patients receiving PEMBRO vs IPI + NIVO were more likely to be PD-L1 expression positive (-tumor proportion score >1%) (76.9% vs 63.1%, respectively) (p = .011) ().
Table 2. Clinical characteristics at treatment initiation.
The comorbidity index was similar between cohorts, however, the PEMBRO cohort had statistically more diabetes compared to IPI + NIVO (20.5% vs 13.0%, respectively) (p = .045). The PEMBRO cohort had a trend towards more CAD compared to IPI + NIVO (18.0% vs 11.5%, respectively) (p = .067). The PEMBRO cohort had statistically more mild liver disease compared to IPI + NIVO, however the prevalence was rare (6.0% vs 1.0%, respectively) (p = .011) ().
Table 3. Comorbidities at therapy initiation.
Hospital and emergency department utilization
Patients receiving PEMBRO vs IPI + NIVO were followed for a total of 2,270 and 2,264 months (median 16.3 and 17.5), respectively. The proportion with at least one hospitalization through 12 months was lower for patients treated with PEMBRO vs IPI + NIVO (17% vs 24%, respectively). Less than 2% of patients had more than one admission and none had more than two, regardless of cohort. The mean (SD) number of hospitalizations per 1,000 patient months for the two cohorts was 16 (37) for PEMBRO and 20 (38) for IPI + NIVO, and the mean (SD) hospital days per 1,000 patient days was 68 (180) vs 98 (21), respectively. ED visits were observed in 18.0% of visits in the PEMBRO cohort and 21.0% in IPI + NIVO; the mean (SD) number of ED visits per 1,000 patient months was 164 (36) vs 20 (40), respectively. ICU admissions were 2.0% vs 4.0%, for PEMBRO and IPI + NIVO, respectively; the mean (SD) ICU days per 1,000 patient months was 7 (5) vs 15 (10), for PEMBRO and IPI + NIVO, respectively ().
Table 4. Twelve-month hospitalizations, ICU admits and ER visits.
In logistic regression analysis retaining covariates with p < .1, patients in the PEMBRO cohort had statistically significantly lower odds of hospitalization within 12 months compared to those receiving IPI + NIVO OR = 0.6 (95% CI = 0.3–0.9) (p = .027). The odds of hospitalization within 12 months were 2.0 (95% CI = 1.1, 3.8) (p = .033) times greater in patients with diabetes, 2.6 (95% CI = 1.3–5.2) (p = .005) times greater in patients with LDH elevated >2 times ULN normal, and 2.2 (95% CI = 1.2–4.0) (p = .009) times greater in patients with LDH elevated 1–2 times ULN normal ().
Figure 1. Logistic regression analysis: hospitalization within 12 months. Abbreviations. LDH, Lactate dehydrogenase; ULN, Upper limit of normal; CAD, coronary artery disease.
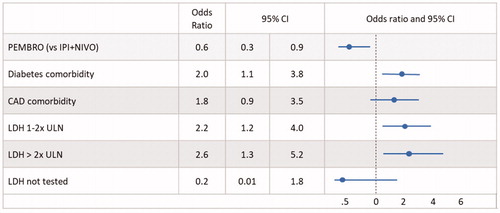
Figure 2. Logistic regression analysis: emergency department visits within 12 months. Abbreviations. LDH, Lactate dehydrogenase; ULN, Upper limit of normal; CAD, coronary artery disease.
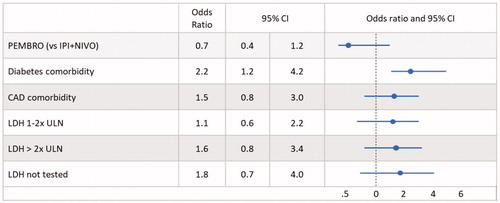
The point estimate indicated a lower risk for ED visits within 12 months for PEMBRO vs IPI + NIVO (p = .144), but this was not statistically significant in covariate adjusted analysis ().
Discussion
This is the first comparative study of which we are aware to assess real-world hospitalization and emergency department utilization in advanced melanoma patients prescribed either PEMBRO or IPI + NIVO in the real-world. Results from this chart review study suggest when controlling for relevant covariates, particularly those that differed at baseline including comorbidities and LDH, receiving PEMBRO for advanced melanoma was associated with a reduced odds of hospitalization, 0.6 (95% CI = 0.3–0.9) (p = .027) compared to receiving IPI + NIVO. This can be interpreted to mean that patients treated with PEMBRO were 40% less likely to be hospitalized. Poor performance status, a parameter in addition to comorbidity that may influence whether or not a patient might receive PEMBRO vs a more toxic IPI + NIVOCitation18, was not a significant predictor of utilization. Both PEMBRO and IPI + NIVO cohorts were comparable with no significant differences with respect to demographics, LOT of treatment, disease stage at advanced diagnosis, or prevalence of brain metastases. PEMBRO patients at baseline displayed higher rates of comorbidity including diabetes and a trend toward more heart disease, more prevalent elevated LDH, higher prevalence of BRAF wildtype and PD-L1 expression. Only the presence of diabetes and elevated LDH were also associated with increased odds of hospitalization, irrespective of treatment. There was a non-significant trend towards lower ED visits with PEMBRO vs IPI + NIVO. Diabetes was a significant predictor of hospitalizations and ED visits.
This lower probability of hospitalization with PEMBRO vs IPI + NIVO may be due to the differing toxicity profiles between the two treatments, however, as adverse event data are not reliably available retrospectively, AEs were not available for analysis. Previous clinical trials have demonstrated different benefit–risk profiles associated with both treatments with reported grade 3 or 4 AEs of 59% in IPI + NIVO and 17% for PEMBROCitation6–9. Our findings are supported by a recent study conducted in the US by Wang et al.Citation21. In this study rates of steroid-refractory toxicities and healthcare utilization were described in patients with melanoma treated with anti-PD-1 (nivolumab) with or without ipilimumab. Patients receiving IPI + NIVO were more likely to develop immune related AEs than those on monotherapy (72% vs 37%; p < .001). IPI + NIVO treated patients were more likely to be hospitalized (32% vs 7%; p < .001), have more than one hospitalizations for AEs (11% vs 3%; p = .001), and had a longer average time of hospitalization (mean = 1.92 vs 0.62 days; p = .002). Tarhini et al.Citation22 examined data from 273 stage IV patients receiving first‐line IPI. In this study, the greatest predictor of healthcare costs were grade 3/4 adverse events. Healthcare costs were 27 times higher during treatment intervals in which a grade 3/4 adverse event occurred compared with intervals without a grade 3/4 adverse event.
Our study is unique in that we used chart review data, which may be richer in detailed clinical information than claims dataCitation23. The majority of published real-world HCRU and cost studies conducted in the US have been retrospective analyses of claims data which is lacking data such as genetic testing and LDH results and patients’ performance status. Toy et al.Citation23 examined HCRU in patients via administrative claims data of metastatic melanoma patients receiving ipilimumab, vemurafenib, paclitaxel (alone and in combination), interleukin-2, dacarbazine (alone and in combination), or temozolomide as the first line of therapy. HCRU between IPI and vemurafenib groups were compared. Analysis did not identify significant differences in resource utilization between IPI and vemurafenib, with the exception of IPI patients having fewer non-treatment related outpatient visits. Klink et al.Citation24 conducted a study in US claims data calculated per patient per month (PPPM) HCRU by first-line treatment drug class including PD-1 inhibitors, CTLA-4 inhibitors, CTLA-4 + PD-1 combination, BRAF monotherapy, BRAF + MEK combination, and chemotherapy. The prevalence of hospitalizations during first-line treatment was lower in PD-1-treated patients (25.9%) compared with 34.7–45.5% of all other groups (p < .05). Neither of these claims-based studies had the benefit of LDH laboratory testing, an important covariate in our analysis. No known current studies using claims have directly compared PEMBRO with IPI + NIVO.
There are several strengths of this study. There is little data on HCRU related to PD-1 inhibitor agents in the real world. Understanding differences in HCRU taking into account differences in other risk factors is important, as oncology care in the US is moving towards including pay for performance incentives. CMS OCM evaluations include measuring reductions in risk-adjusted hospitalizations and ED visits. This study helps to fill a HCRU evidence gap by using real-world chart review data outside a controlled trial setting to quantify the HCRU burden of melanoma treatments and may help identify approaches that are associated with less costly utilization.
Limitations
This study data collection methodology was a retrospective medical chart review and patients were not randomized to therapy. As such, results are limited by the completeness of information that was recorded in those charts and data that were collected. Although investigators were required to have had access to hospitalization and ED data, if patients traveled outside of their immediate area for treatment and this was not reported to their oncologist, this data may not have been included in the analysis. This study did not capture the occurrence of adverse events, as these events may be unreliably captured outside of a clinical trial setting. Study parameters important in predicting HCRU such as ECOG performance status are not always assessed or recorded in routine medical practice. However, as an assessment of performance status can be made using clinical judgment and patient objective presentation during a clinical consultation, this may or may not be a limitation in extrapolating our findings to routine practice. This study also did not capture reasons for hospitalization. Our study only examined utilization associated with PEMBRO as a single agent PD-1 inhibitor, although nivolumab was also approved for monotherapy in this indication; as such, no data are available on hospitalizations and ED visits for patients treated with nivolumab as a monotherapy as these treatments were not a part of this study. Finally, this analysis did not include some clinical characteristics, like tumor disease bulk or aggressiveness that may have impacted utilization.
Further research is warranted to overcome these limitations through methods including prospective randomized pragmatic trials. Pragmatic trials are more naturalistic, yet patients could be randomized to either treatment modality, which would minimize differences at baseline, possibly confounding the HCRU outcomes. Data are also prospectively captured in pragmatic trials, which would facilitate capturing reasons for hospitalizations and AEs in a manner consistent with clinical trials. Prospective follow-up would also minimize the chance of under-reporting of down-stream hospitalizations and emergency room visits. Analyses of electronic medical record (EMR) data are growing in use to answer key questions of HCRU. As existing limitations associated with EMR data are addressed, this may be a viable approach to future research. As adverse events typically occur within 6 months of initiation of immuno-oncology agents, different results may have been obtained had we selected different timepoints for analysis.
Conclusion
Patients receiving PEMBRO had a significantly lower probability of hospitalization compared with IPI + NIVO in the real-world through 12 months controlling for relevant baseline characteristics. PEMBRO for advanced melanoma reduced the odds of hospitalization to 0.6 (95% CI = 0.3–0.9) (p = .027) compared to receiving IPI + NIVO. These findings support treatment with PEMBRO may improve performance benchmarks for reduced HCRU consistent with OCM initiatives.
Transparency
Declaration of funding
This study was funded by Merck & Company.
Declaration of financial/other relationships
Shillington and Harshaw are employees of EPI-Q Inc. Shillington is a shareholder of EPI-Q Inc. Liu, Diede, and Scherre are employees and shareholders of Merck and Company. Lee is a consultant to Merck & Company. Macahilig and Dave are employees of Medical Data Analytics. Joseph has been an advisor to Merck & Company, Bristol-Myers Squibb, Exelixis, Incyte, Insys, Merck, and Novartis. Lee is a consultant to Merck & Company.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they have received research, speaking and/or consulting support from companies including Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate, and National Psoriasis Foundation. They also consult for others through Guidepoint Global, Gerson Lehrman, and other consulting organizations. They are a founder and majority owner of www.DrScore.com. They are a founder and part owner of Causa Research. Another reviewer has disclosed that they participate as an investigator in different melanoma trials using PEMBRO and IPI + NIVO. The reviewers have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
None reported.
References
- American Cancer Society. https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html.
- Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: Current status and future prospects. Oncologist. 2011;16(1):5–24.
- Lens MB, Eisen TG. Systemic chemotherapy in the treatment of malignant melanoma. Expert Opin Pharmacother. 2003;4(12):2205–2211.
- Ives NJ, Stowe RL, Lorigan P, et al. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol. 2007;25(34):5426–5434.
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61.
- Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546.
- Keytruda [package insert]. Whitehouse Station, NJ: Merck & Co; 2019. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed October 16, 2019.
- Opdivo [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2019. https://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed October 16, 2019.
- Dobry AS, Zogg CK, Hodi FS, et al. Management of metastatic melanoma: improved survival in a national cohort following the approvals of checkpoint blockade immunotherapies and targeted therapies. Cancer Immunol Immunother. 2018;67(12):1833–1844.
- Vouk K, Benter U, Amonkar MM, et al. Cost and economic burden of adverse events associated with metastatic melanoma treatments in five countries. J Med Econ. 2016;19(9):900–912.
- Wehler E, Zhao Z, Pinar Bilir S, et al. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur J Health Econ. 2017;18(1):49–58.
- Bilir SP, Ma Q, Zhao Z, et al. Economic burden of toxicities associated with treating metastatic melanoma in the United States. Am Health Drug Benefits. 2016;9(4):203–213.
- Ghate SR, Z Li Z, Tang J, et al. Economic burden of adverse events associated with immunotherapy and targeted therapy for metastatic melanoma in the elderly. Am Health Drug Benefits. 2018;11(7):334–343.
- Vouk K, Benter U, Amonkar MM. Cost and economic burden of adverse events associated with metastatic melanoma treatments in five countries.
- Arondekar B, Curkendall S, Monberg M, et al. Economic burden associated with adverse events in patients with metastatic melanoma. J Manag Care Spec Pharm. 2015;21(2):158–164.
- “What Is The Oncology Care Model, And Why Is The Evaluation Important?, “Health Affairs Blog, February 14, 2019.
- Joseph RW, Shillington AC, Macahilig C, et al. Factors associated with immunotherapy selection in patients with advanced melanoma. Immunotherapy. 2018;10(16):1361–1369.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Agresti A. 2002. Categorical data analysis. (2nd Ed.). New York (NY): Wiley.
- Wang Lx Quach HT, Moodabigil NV. Health care utilization and steroid-refractory toxicities from immune checkpoint inhibitors. Cancer. 2019.
- Tarhini A, Corman SL, Rao S, et al. Healthcare resource utilization and associated costs in patients with advanced melanoma receiving First‐Line ipilimumab. Journal of Cancer Therapy. 2015;06(10):833–840.
- Toy EL, Vekeman F, Lewis MC, et al. Costs, resource utilization, and treatment patterns for patients with metastatic melanoma in a commercially insured setting. Curr Med Res Opin. 2015;31(8):1561–1572.
- Klink AJ, Chmielowski B, Feinberg B. Health care resource utilization and costs in First-Line treatments for patients with metastatic Melanoma in the United States. J Manag Care Spec Pharm. 2019;25(8):869–877.
