Abstract
Aims: To describe the incidence and identify prognostic factors of central nervous system (CNS) adverse events (AEs) and any AEs (CNS, skin rash, or fracture) and evaluate the healthcare resource utilization (HCRU), direct medical costs, and therapy discontinuation associated with these AEs among non-metastatic prostate cancer (nmPC) patients who received secondary hormone therapies.
Methods and results: nmPC patients who had initiated secondary hormonal therapy with enzalutamide, bicalutamide, or abiraterone ≥1 year after androgen deprivation therapy (ADT) were identified in the MarketScan database. Survival analyses were used to describe the incidence of CNS or any AEs. Annual HCRU and costs were compared across patient groups (CNS AE vs no CNS AE; any AE vs no AE) using propensity score weighted generalized linear models. Multivariate Cox proportional hazards models were used to identify AE predictors and compare risks of discontinuation.
Results: The analysis included 532 patients who initiated secondary hormonal therapies, among whom 201 (38%) and 244 (46%) experienced a CNS AE and any AE, respectively. Median times to CNS AE and any AE from therapy initiation were 17.90 and 11.00 months, respectively. Predictors of any AE were any AE in the baseline period (≤6 months before starting therapy), Charlson Comorbidity Index (CCI) score (1 vs 0), surgical castration, and older age. Predictors of CNS AEs were CNS AE in the baseline period and CCI score (1 vs 0). CNS and any AEs were associated with significantly higher HCRU. CNS AEs were associated with significantly higher incremental total medical costs ($18,522). CNS AEs and any AEs significantly increased therapy discontinuation risk by 48% and 38%, respectively.
Conclusions: AEs increase the economic burden and therapy discontinuation among nmPC patients receiving secondary hormonal therapies subsequent to ADTs. These patients should be carefully evaluated for AEs to reduce therapy discontinuation, HCRU, and direct medical costs.
Background
Prostate cancer (PC) is the most common cancer and among the leading causes of cancer deaths in men in the United States (US)Citation1. PC patients who experience biochemical recurrence after definitive local therapies are often treated with androgen deprivation therapy (ADT), which includes medical castration (i.e. gonadotropin-releasing hormone [GnRH] agonists or antagonists) or surgical castration (orchiectomy)Citation2–4. These patients may eventually stop responding to ADT and experience rising prostate-specific antigen (PSA) levels despite castrate levels of serum testosterone (i.e. become castration-resistant). Castration-resistant PC (CRPC) patients who do not have radiographic manifestations of metastatic disease are defined as having non-metastatic CRPC (nmCRPC)Citation5–7.
One of the approaches used to manage nmCRPC patients is the addition of secondary hormonal therapies (i.e. additional hormonal therapies used after medical or surgical castration) to ADT. Secondary hormonal therapies for nmCRPC patients recommended by the National Comprehensive Cancer Network (NCCN) guidelines include first (bicalutamide, nilutamide, and flutamide) and second (enzalutamide, darolutamide, and apalutamide) generation antiandrogens as well as androgen receptor modulators (abiraterone acetate)Citation7–9.
Evidence shows that, although secondary hormonal therapies can delay the onset of metastasis, some may also increase the risk of experiencing adverse events (AEs), which may negatively impact the quality of survival for nmCRPC patientsCitation10,Citation11. The occurrence of treatment-related AEs is an especially important consideration in an otherwise largely asymptomatic disease state. Previous studies have shown that physicians, patients, and caregivers may be willing to trade survival to avoid AEs among non-metastatic PC (nmPC) patients, underscoring the importance of managing AEs in this populationCitation12,Citation13. Understanding the burden of AEs among nmPC patients treated with secondary hormonal therapies can help inform clinicians who prescribe and third-party payers who make coverage decisions about these therapies. Furthermore, quantifying the burden of AEs in this patient population assists in contextualizing the remaining unmet need for novel therapies with improved risk-benefit profiles. Evaluating the financial burden of AEs can also help better understand and communicate to payers the significance of reducing AEs in this population. A recent real-world analysis by Pilon et al.Citation14 showed that the 1-year probability of experiencing a central nervous system (CNS)-related AE was greater than 30% in patients with advanced PC using enzalutamide, bicalutamide, or abiraterone. However, this analysis was not specifically restricted to non-metastatic patients and did not assess the burden of AEs in terms of healthcare resource utilization (HCRU), medical costs, or therapy discontinuationCitation14. Other studies that described the economic burden of AEs in real-world settings have focused on patients with metastatic CRPC or hormone-sensitive (i.e. not castration-resistant) PCCitation15,Citation16. No studies have described the real-world burden associated with AEs among nmPC patients using secondary hormonal therapies. Given this gap, the specific objectives of the present study were to: (1) describe and understand the incidence of AEs and identify patient demographic and clinical characteristics prognostic of AEs, and (2) evaluate the burden associated with AEs in terms of HCRU, medical costs, and therapy discontinuation among nmPC patients who received secondary hormonal therapy prior to 2018.
Methods
The objectives, study population, study design, and statistical analyses were pre-specified in an approved study protocol (protocol ID: RD-OI-0214). The protocol was reviewed by expert clinicians, health economists, and epidemiologists at Bayer Healthcare Pharmaceuticals.
Secondary hormonal therapies
The secondary hormonal therapies included in this study were bicalutamide, abiraterone, and enzalutamide. These therapies were selected because Pilon et al.Citation14 showed that they may be associated with CNS AEs and that they were commonly used in CRPC settings during our study period (2012–2017). Additionally, bicalutamide demonstrated a favorable efficacy profile in terms of PSA progression among nmCRPC patientsCitation17. Abiraterone and enzalutamide were included in this study because they were available on the market for managing metastatic patients during our study period and their efficacy among CRPC patients in pre-chemotherapy and non-metastatic settings has been evaluated since 2012Citation18,Citation19. Their availability and ongoing evaluations could have resulted in the off-label use of abiraterone and enzalutamide as secondary hormonal therapies among nmPC patients during our study period. Apalutamide and darolutamide were not included in the study as they were introduced into the market in 2018 and 2019, respectively, which was outside the time frame of the study.
Data sources
We used the IBM Watson MarketScan database from 2012 through 2017 (inclusive), including the MarketScan Commercial Claims and Encounters, Medicare Supplemental and Coordination of Benefits, and Lab databasesCitation20. These databases cover all age groups and capture health plan enrollment history and claims for medical (i.e. provider and institutional) and pharmacy services among US patients with employer-sponsored and individual health plans. The study was conducted using the most recent 5-year data cut available from IBM Watson at the time of this analysis.
Study subjects
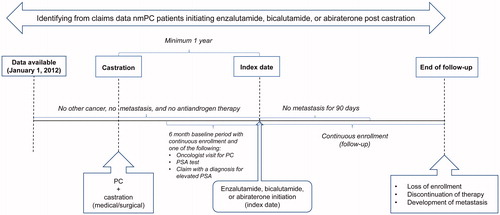
Adults (aged >18 years) were included if they had all of the following criteria: (1) healthcare claims from January 1, 2012, through December 31, 2017; (2) ≥1 claim for PC; (3) a medical or pharmacy claim for medical or surgical castration therapy (i.e. ADT); (4) ≥1 claim for secondary hormonal therapy with enzalutamide, bicalutamide, or abiraterone ≥1 year after first ADT claim; (5) a claim for a PSA test or an oncologist visit ≤6 months prior to first claim for enzalutamide, bicalutamide, or abiraterone therapy; and (6) continuous enrollment in a health plan for ≥6 months prior to and ≥3 months after their first claim for enzalutamide, bicalutamide, or abiraterone therapy.
The date of a patient’s first claim for a secondary hormonal therapy was defined as their index date, and the respective therapy (i.e. enzalutamide, bicalutamide, or abiraterone) was defined as their index therapy. Patients were excluded if they met any of the following criteria: (1) diagnosis of metastasis any time prior to receiving index therapy and within 90 days of index date, (2) diagnosis for any cancer other than PC prior to their index date, excluding non-melanoma skin cancer, or (3) any antiandrogen therapy prior to their index date.
Using a ≥1-year gap between a patient’s first castration claim and index date, evidence of PSA testing or oncologist visit within 6 months prior to index date, and the history of no prior antiandrogen use as inclusion/exclusion criteria helped ensure that the index therapy was not part of the patient’s ADT but rather was a secondary hormonal therapy, likely initiated due to failure of castration to control rising PSA levels. In the absence of laboratory test data, these selection criteria were applied to define our sample of nmPC patients who initiated secondary hormonal therapy post-ADT due to a possible rise in PSA levels. The diagnosis and National Drug Code (NDC) codes used to identify the outcomes, index therapies, and inclusion/exclusion criteria are presented in . Since the data were generated from insurance claims, there was no grading (i.e. grade 1–4 per Common Terminology Criteria for Adverse Events [CTCAE]) information available for any of the AEs.
Patient follow-up
Patient follow-up began on the index date and continued until the patients had a healthcare claim with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code for metastasis or until the end of continuous enrollment or discontinuation of index therapy, whichever occurred first. Study outcomes were assessed from the patient’s index date to the end of the follow-up and baseline characteristics were assessed within 6 months prior to the index date unless otherwise indicated. A detailed outline of the study subjects and follow-up is found in .
Outcomes by objective
Objective: Describe the incidence of AEs and explore patient demographic and clinical characteristics associated with them
Outcome: Adverse events
A patient was classified as having a CNS AE if they had a healthcare claim between their index date and end of follow-up that included a diagnosis code for ≥1 of the following conditions: amnesia or memory impairment, anxiety, ataxia, cognitive disorders, confusion, convulsions, disturbance in attention, dizziness, falls, fatigue/asthenia, hallucinations, headaches, insomnia, pain, paresthesia, seizures, weakness, and other CNS disorders. Conditions included as CNS AEs were selected based on a study by Pilon et al.Citation14 that also used the MarketScan database. A patient was classified as having any AE if they had a healthcare claim between their index date and end of follow-up that included a diagnosis code for a CNS AE, skin rash, or fracture. Patients could have both CNS AEs and any AEs, either category alone, or neither category. The incidence of CNS and any AEs were estimated separately, by following patients from initiation of index therapy to the first diagnosis code for the respective AE or end of follow-up, whichever occurred first.
Objective: Evaluate the burden associated with AEs in terms of HCRU, medical costs, and therapy discontinuation
Outcome: HCRU and medical costs
The HCRU (i.e. number of hospitalizations, emergency department [ED] visits, and outpatient visits) and total medical costs (i.e. hospitalization, outpatient, ED, and prescription costs) accrued by each patient during their entire follow-up were determined. Measures of direct medical costs were adjusted to 2017 US dollars using the Medical Care component of the US Consumer Price Index. To estimate the resource use and economic burden associated with CNS AEs and any AEs, patients were stratified by whether they experienced a CNS AE (≥1 CNS AE versus no CNS AE) and any AE (≥1 any AE versus no AE) during the follow-up period.
Outcome: Discontinuation
All-cause discontinuation of index therapy was defined as ≥90 days without refill of index therapy from the end date of the last prescription (i.e. the start date of enzalutamide, bicalutamide, or abiraterone prescription, plus days’ supply)Citation14. Patients who lost insurance enrollment or developed metastasis prior to discontinuation were considered censored for this analysis. The rate of all-cause discontinuation was compared between patients who had ≥1 CNS AE vs no CNS AE and ≥1 any AE vs no AE during the follow-up period.
Independent variables by objective
Objective: Describe the incidence of AEs and explore patient demographic and clinical characteristics associated with them
The multivariate models used to explore factors associated with experiencing a CNS AE and any AE were exploratory and did not have any prespecified independent variables of interest.
Objective: Evaluate the burden associated with AEs in terms of HCRU, medical costs, and therapy discontinuation
The primary independent variables in multivariate models assessing HCRU, medical costs, and rate of discontinuation of index therapy were the experience of a CNS AE (≥1 CNS AE vs no CNS AE) and any AE (≥1 any AE vs no AE) during the follow-up period.
Covariates
Key demographic, clinical, and therapy characteristics were identified for inclusion into all multivariate analyses. Data on patients’ insurance plan, geographic region, age, and index year were assessed at the index date. Charlson Comorbidity Index (CCI) score and the presence of AEs in the baseline period (≥1 CNS AE and ≥1 any AE) were assessed using the relevant ICD-9-CM and ICD-10-CM diagnosis codes. The presence of any castration therapy during the baseline period and treatment with surgical castration at index were obtained using the relevant ICD-9-CM and ICD-10-CM procedure code or NDC, Healthcare Common Procedure Coding System (HCPCS), and Current Procedural Therapy (CPT) codes. The covariates used to describe the sample were selected based on clinical opinion and recent studies of advanced PC patients using similar databasesCitation14,Citation21.
These covariates were used to assess which patient characteristics can be prognostic of future AEs independent of potential confounding factors such as index year, insurance, and region. This can help identify specific patient sub-groups with a higher baseline risk of such AEs. Hence, the covariates were included in multivariate analyses conducted to explore patient demographic and clinical characteristics associated with AEs. These covariates were also included in multivariate analyses that compared outcomes (i.e. HCRU, cost, discontinuation rates) among patients who experienced an AE vs those who did not. In these multivariate analyses, the covariates were included as potential confounders to control for differences at baseline between patients who experienced an AE vs those who did not, and hence their coefficients were not of interest and were not interpreted. Previous literature suggests that interpreting coefficients of confounders may be biasedCitation22.
Statistical analysis
Descriptive statistics for patient demographic and clinical characteristics, unadjusted measures of HCRU and total direct medical cost, and unadjusted measures of treatment discontinuation were reported for the full sample, and for patients who experienced an AE during the follow-up period vs those who did not (i.e. ≥1 CNS AE vs no CNS AE and ≥1 any AE vs no AE). The patient characteristics and unadjusted measures of the outcomes were presented by the manifestation of a CNS AE and any AE since these were the primary variables of interest in this study. Categorical variables were summarized using the number and percentage of patients in each category and compared using Pearson chi-square tests or Fisher’s exact tests, and continuous variables were summarized using mean, standard deviation, median, and interquartile range (IQR) and compared using t-tests or Mann-Whitney tests, as appropriate.
The incidence of AEs in nmPC patients receiving secondary hormonal therapies was estimated using time-to-event analyses which accounted for losses due to follow-up. Kaplan-Meier analyses were used to quantify the median time from therapy initiation to experiencing a CNS AE or any AE (i.e. CNS AE, rash, or fracture) and the incidence of CNS AEs and any AEs at 3, 6, and 12 months for the entire sample. Cox proportional hazards models were used to explore the association between patient covariates and the likelihood of experiencing an AE. Use of Cox proportional hazards models allowed us to study the effect of multiple variables simultaneously on the incidence of AEs. Studying multiple variables simultaneously is necessary when desiring to interpret the effect of each variable independent of the others. The patient covariates included in the Cox proportional hazards models are stated above.
Inverse probability weighted (IPW) multivariate generalized linear models (GLMs) were used to estimate HCRU (hospitalizations, ED visits, and outpatient visits) and total direct medical costs for patients with ≥1 CNS AE vs no CNS AE and for patients with ≥1 any AE vs no AE, while adjusting for baseline patient covariates.
To calculate the inverse probability weights, separate multivariable logistic regression models were used to estimate the probability of experiencing a CNS AE and any AE during follow-up, conditional on patients’ covariates and index therapy. The estimated probabilities were then used to compute separate inverse probability weights for each patient. To test whether weighting resulted in the balance of baseline covariates, the covariates were compared between patients with ≥1 CNS AE vs no CNS AE and patients with ≥1 any AE vs no AE using standardized differences. Variables for which the standardized difference exceeded 0.10 were included as covariates in the weighted multivariate GLMs to account for potential residual confounding.
The IPW GLMs were then used to estimate and compare the mean per patient per year (PPPY) HCRU (hospitalizations, ED visits, and outpatient visits) and total direct medical costs for patients with ≥1 CNS AE vs no CNS AE and for patients with ≥1 any AE vs no AE. Incorporation of the inverse probability weights in the GLMs ensured that baseline covariates were not likely to have any confounding effect on the observed association between experiencing AEs (CNS AEs and any AEs) and the outcomes (HCRU and costs).
The association between experiencing a CNS AE and any AE and the rate of all-cause therapy discontinuation was determined using Cox proportional hazards models. These models allowed us to interpret the effect of CNS AEs and any AEs on the rate of discontinuation independent of patient covariates. Separate models were used to estimate the association between rate of all-cause treatment discontinuation and CNS and any AEs. Each model included the baseline covariates stated above. The primary variables of interest (CNS AEs and any AEs) were incorporated in the models as time-varying covariates such that the time prior to experiencing the first AE was counted as time without AE and the time after experiencing the first AE was counted as time with AE. This was done to prevent misclassification of follow-up time pre- and post-AEs.
To estimate the marginal effect of CNS AE and any AE on probability of all-cause discontinuation at a given time point, the probability of all-cause discontinuation at each time point, conditional on prior CNS AE or any AE and patient covariates, was calculated for all patients using the coefficients from the Cox models. The mean predicted probability of all-cause discontinuation at each time point, conditional on prior CNS AE or any AE, was calculated as the mean of predicted probabilities for all patients who had contributed data at that time point. The predicted probability of all-cause therapy discontinuation at each time point was then plotted up to 30 months separately for patients with a CNS AE (or any AE) vs those without a CNS AE (or without any AE). Thus, the difference in the predicted probabilities of all-cause discontinuation at each time point was only conditional on whether a patient experienced a prior CNS AE or any AE, respectively. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc; Cary, NC).
Results
A total of 532 patients cumulatively followed for 504 person-years (mean [SD] follow-up per patient = 0.95 [0.72] years; median [IQR] = 0.74 [0.42–1.25] years) fulfilled the inclusion criteria, including 477 patients (89.66%) treated with bicalutamide, 32 (6.02%) with abiraterone, and 23 (4.32%) with enzalutamide. The sample selection is described in .
Figure 2. Patient selection flow chart. Abbreviations. AE, adverse event; CNS, central nervous system; GnRH, gonadotropin releasing hormone; PSA, prostate-specific antigen.
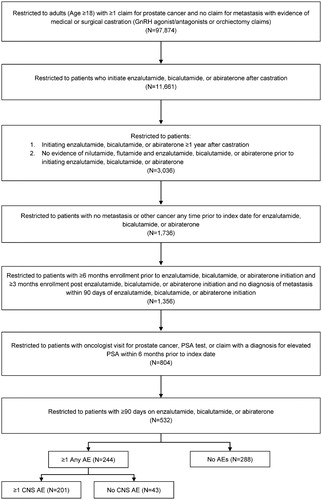
Patients with ≥1 CNS AE or ≥1 any AE during the follow-up period were significantly older at index date and were statistically significantly more likely to have a baseline CCI score > 0, be enrolled in Medicare Advantage than commercial plans, and have a CNS AE or any AE during the baseline period compared to patients with no CNS AEs or no AEs during follow-up (p < .05; ). The most common AEs in our patient population were fatigue/asthenia (15.60%), rashes (10.90%), insomnia (9.77%), fracture (8.27%), pain (6.58%), and weakness (5.83%). The proportions of patients with CNS AEs and any AEs among those treated with bicalutamide, abiraterone, and enzalutamide were 38.16% and 46.54%, 18.75% and 28.13%, and 56.52% and 56.52%, respectively. There was a statistically significant (p < .05) difference in the proportion of patients with CNS AEs and any AEs between those treated with abiraterone vs bicalutamide, and patients treated with abiraterone vs enzalutamide.
Table 1. Descriptive statistics for patients with vs without CNS AEs and with vs without any AEs.
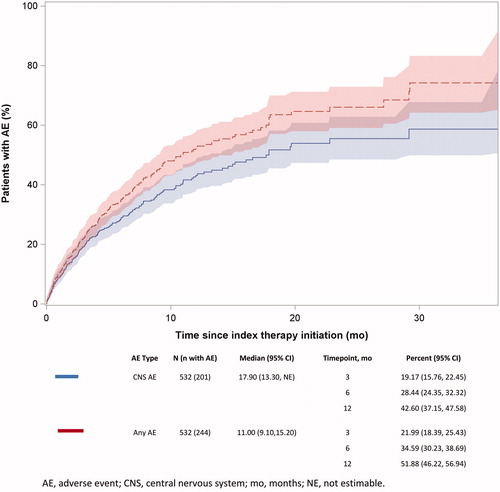
The probabilities of having a CNS AE within 3, 6, and 12 months of initiating index therapy were 19.17% (95% CI = 15.76–22.45%), 28.44% (95% CI = 24.35–32.32%), and 42.60% (95% CI = 37.15–47.58%), respectively (). The probabilities of having any AE within 3, 6, and 12 months of initiating index therapy were 21.99% (95% CI = 18.39–25.43%), 34.59% (95% CI = 30.23–38.69%), and 51.88% (95% CI = 46.22–56.94%), respectively (). The median times to first CNS AE and first any AE were 17.90 and 11.00 months, respectively.
Figure 3. Time from index date to first CNS AE and any AE. Abbreviations. AE, adverse event; CNS, central nervous system; mo, months.
Patient and clinical characteristics, CCI score (1 vs 0), and experiencing a CNS AE during the baseline period were associated with a significantly higher risk of experiencing a CNS AE during follow-up, whereas CCI score (1 vs 0), older age at baseline, surgical castration, and experiencing any AE during the baseline period were associated with a higher risk of experiencing any AE during follow-up (p < .05; ).
Table 2. Full Cox proportional hazards model of time to CNS AEs and any AEs.
Patients with ≥1 CNS AE or ≥1 any AE during follow-up had significantly higher unadjusted HCRU and total direct medical costs compared to patients without CNS AEs or any AEs (p < .05; ). These results were consistent for the three most commonly observed AEs in our population. Patients with fatigue/asthenia had a significantly (p < .05) higher number of inpatient (0.45 vs 0.16), outpatient (32.27 vs 22.88), and ED visits (2.33 vs 0.41) as well as higher direct medical costs ($25,393.40 vs $13,470.20) compared to patients without fatigue/asthenia (). Insomnia was also associated with significantly (p < .05) higher HCRU and costs (inpatient visits = 0.40 vs 0.18; outpatient visits = 39.24 vs 22.73; direct medical costs = $33,170.10 vs $13,474.60; ), whereas rashes did not have a statistically significant impact on healthcare costs and were associated with higher number of outpatient visits (29.78 vs 23.67) ().
Using the weighted GLMs, we found that patients with ≥1 CNS AE as well those with ≥1 any AE had significantly (p < .05) higher PPPY HCRU compared to patients without CNS and any AEs (). Patients with ≥1 CNS AE also had significantly higher (p < .05) PPPY total medical costs ($18,522.28) compared to patients without CNS AEs ().
Table 3. Per person per year adjusted HCRU and costs among patients with any AEs and patients with CNS AEs during follow-up.
After accounting for CNS AEs and any AEs as time-varying covariates and adjusting for baseline characteristics, the multivariate analysis showed that both CNS AEs (hazard ratio [HR] = 1.48; 95% CI = 1.12–1.95) and any AEs (HR = 1.38; 95% CI = 1.05–1.82) increased the hazard of all-cause therapy discontinuation (). The predicted probabilities of all-cause discontinuation obtained from the multivariate analysis showed that CNS AEs and any AEs during follow-up increased the probability of all-cause discontinuation at 3, 6, and 12 months. The probabilities (%) of discontinuation of therapy within 3, 6, and 12 months of initiating index therapy if patients experienced a CNS AE vs no CNS AE were 5.68% (95% CI = 2.49–8.75%) vs 3.90% (95% CI = 1.76–5.98%); 38.67% (95% CI = 25.06–49.41%) vs 28.55% (95% CI = 18.40–37.18%); and 71.36% (95% CI = 53.63–81.57%) vs 58.15% (95% CI = 41.83–69.16%), respectively, while holding all other covariates constant (). The probabilities of discontinuation of therapy within 3, 6, and 12 months of initiating index therapy if patients experienced any AE vs no any AE were 5.35% (95% CI = 2.35–8.26%) vs 3.93% (95% CI = 1.78–6.03%); 36.89% (95% CI = 23.83–47.32%) vs 28.69% (95% CI = 18.45–37.38%) and 69.13% (95% CI = 51.58–79.56%) vs 58.21% (95% CI = 41.72–69.30%), respectively, while holding all other covariates constant ().
Figure 4. Time from initiation of enzalutamide, bicalutamide, or abiraterone to discontinuation due to (a) CNS AE and (b) any AE. Abbreviations. AE, adverse event; CNS, central nervous system; mo, months.
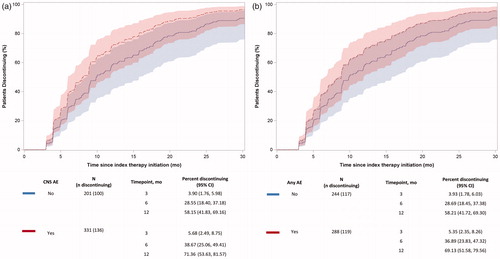
Table 4. Full Cox proportional hazards model for the time to discontinuation of therapy due to CNS AEs and any AEs.
Discussion
This is the first real-world study to examine the incidence and burden of AEs among nmPC patients treated with secondary hormonal therapies after receiving ADT. The study sought to address the gaps in the existing literature by specifically studying nmPC patients treated with secondary hormonal therapies and evaluating their burden of AEs in terms of incidence, HCRU, costs, and discontinuation. Our study findings further contextualize and highlight the importance of identifying and developing novel therapies with improved safety profiles for this patient population. Although we cannot strictly identify who among this patient population may have had castration resistance at the time of enzalutamide, bicalutamide, or abiraterone initiation due to a lack of serial PSA testing information for these patients, the fact that they received additional hormonal therapy after receiving ADT prior to any coded diagnosis of metastatic disease clinically suggests that many may have developed resistance to the ongoing castration therapy. The definitions of nmCRPC and CRPC patients used in our study are similar to definitions that have been previously validated using comparable claims databasesCitation14,Citation23.
We found a high incidence rate of CNS AEs as well as any AEs in our sample, with 1-year probabilities greater than 40%. Additionally, we found that AEs (CNS or any) were associated with higher HCRU and costs. The annual direct medical costs of patients with ≥1 CNS AE significantly exceeded those of patients without CNS AEs by more than $18,500. The annual direct medical costs of patients with ≥1 any AE exceeded those of patients without any AEs by more than $11,000, but the difference was not statistically significant. Lastly, our data also showed that patients who experienced ≥1 CNS or any AE while on index therapy were significantly more likely to discontinue the index therapy after the AE compared with those who did not experience similar AEs. These findings substantiate the importance of preventing and reducing AEs among nmPC patients who receive secondary hormonal therapies post-receipt of ADTs.
Included within our definitions of CNS AEs (amnesia or memory impairment, anxiety, ataxia, cognitive disorders, confusion, convulsions, disturbance in attention, dizziness, falls, fatigue/asthenia, hallucinations, headaches, insomnia, pain, paresthesia, seizures, weakness, and other CNS disorders) and any AEs (CNS AE, skin rash, and fracture), the most prevalent AEs in our patient population were fatigue/asthenia (15.60%), rashes (10.90%), insomnia (9.77%), fracture (8.27%), pain (6.58%), and weakness (5.83%). Previous studies in patients with PC have shown that non-steroidal antiandrogens, including bicalutamide, are independently associated with increased risk of fractures and anemia, which can manifest as pain, fatigue, and weakness in cancer patientsCitation24–26. Prior clinical trials of enzalutamide and abiraterone in metastatic as well as nmPC populations show that patients using these drugs have elevated rates of AEs, specifically fatigue, weakness, and fracturesCitation27,Citation28. Similar rates of CNS AEs were reported in a previous study by Pilon et al.Citation14 using real-world data from patients with advanced PC, including those with metastatic disease. We found that patients initiating enzalutamide had the highest likelihood of experiencing a CNS AE, which is also consistent with the Pilon et al.Citation14 study. The Pilon et al.Citation14 study reported 1-year probabilities of CNS AEs ranging from 34–46% for patients on enzalutamide, bicalutamide, or abiraterone, with enzalutamide particularly associated with a significantly higher risk of CNS AEs. In contrast to Pilon et al.Citation14, our study specifically evaluated nmPC patients newly initiating enzalutamide, bicalutamide, or abiraterone therapy post-castration; this disease state is becoming increasingly relevant from a treatment perspective given the recent approval of several second-generation hormonal agents in nmCRPCCitation10,Citation11,Citation29. In addition to providing information about the incidence of AEs in the nmPC patient population, our results showed that prior AEs and a CCI score of 1 (vs 0) in the baseline period increased the likelihood of experiencing an AE after initiating enzalutamide, bicalutamide, or abiraterone therapy, consistent with what has previously been reported among patients with advanced PCCitation14. Although we did not observe an association between a CCI score ≥2 and having a CNS AE and any AE during follow-up, sensitivity analyses conducted after collapsing the CCI score as 0 vs ≥1 showed that CCI remained a statistically significant predictor of future AEs (results available upon request), indicating that a lack of association between a CCI score ≥2 and risk of future AEs could be attributable to a smaller number of patients in this category.
The high proportion of patients seen in this study with baseline AEs and a CCI score ≥1, which were prognostic of future AEs, points to the importance of clinicians educating PC patients about the possible risks associated with initiating hormonal therapies after ADT and prior to the diagnosis of metastatic disease. These associations also help clinicians identify patient sub-groups (those with prior AEs and comorbid conditions) who may be at a higher risk of experiencing AEs in the future and hence may need more careful monitoring while on secondary hormonal therapies.
Various studies have shown significant cost burden among patients with advanced PCCitation30. Other studies have reported on the impact of AEs on HCRU among patients on ADT, with results showing that AEs increased patient costs by 100–265%Citation31. However, our study is one of the first to quantify the economic burden of AEs among patients who initiated additional hormonal therapies after ADT. Our analysis found that AEs further increase HCRU and cost burden beyond the costs associated with management of nmPC patients on secondary hormonal therapies after ADT. These findings are important from a payer’s perspective since they inform payers of the financial implications of AEs in these patients.
Our study results also show that PC patients in the studied cohort who experience AEs have a higher likelihood of all-cause treatment discontinuation compared to patients who do not experience AEs. These findings further emphasize the importance of managing and reducing AEs in the nmPC population since therapy discontinuation may increase the risk of disease progression/metastasis. Other covariates that were associated with treatment discontinuation included treatment with surgical castration, use of castration therapy during the baseline period, and index year. However, we were not able to corroborate these findings with previous literature or plausible clinical explanations. Future research should look into these aspects further, especially in studies where a larger sample size is available, which may help better clarify some of these observed associations.
Our study provides evidence that AEs are independently associated with increased HCRU and medical costs and a higher likelihood of treatment discontinuation. To a first approximation, it seems plausible that this association may prevail irrespective of therapy. In this regard the current study provides important and relevant evidence, as the treatment landscape of nm(CR)PC continues to evolve. Reducing the burden of AEs in the largely asymptomatic nmPC and nmCRPC population is paramount to maintaining patients’ quality of survival and reducing the economic burden associated with nmCRPC management.
Strengths and limitations
The primary strength of this analysis is that it is among the first to evaluate the economic impact of AEs, as well as the discontinuation rates associated with them, among nmPC patients on secondary hormonal therapies post-ADT in the real-world setting. However, this study has some limitations. First, the reliance on diagnosis codes for defining AEs and the lack of clinical details (e.g. grading of AEs) suggest that, although relevant AEs are captured, it is not possible to determine AE severity. Furthermore, in the absence of physician notes, it is uncertain whether the manifestation of conditions classified as CNS AEs were indeed CNS-related. Nevertheless, the use of administrative databases to estimate the incidence and costs of chemotherapy-related AEs and use of ICD-9-CM and ICD-10-CM codes for the identification of AEs are well accepted for retrospective database studies, and our classification of CNS AEs was based on a review of previous studiesCitation14,Citation32–35. The absence of physician notes also prevents us from understanding the rationale for initiating or discontinuing specific secondary hormonal therapies. However, understanding prescribing patterns was beyond the scope of this analysis.
Second, certain clinical variables such as Eastern Cooperative Oncology Group (ECOG) scores, cancer stage, hormone sensitivity, grade, and tumor size are not available in claims databases. The absence of relevant clinical covariates in our model could have contributed to residual confounding. Although we used analytic methods such as propensity weighting and covariate adjustment to minimize the impact of selection bias and observed confounding, our study findings remain subject to unobserved confounding. Future studies should be conducted using data sources that better capture patient clinical data in order to address the availability bias that is prevalent in claims data. Studies conducted using rich sources of clinical data, such as patient medical charts, electronic medical records (EMR), and registries, can address some of the limitations of our study.
Third, analyses were performed by treating the initiation of one of the index therapies post-castration as a proxy for developing resistance to castration therapies. As already noted above, although our definition was guided by clinical opinion, there is some uncertainty on whether patients were truly castration-resistant. Furthermore, our definition did not allow the inclusion of CRPC patients being managed expectantly without intervention with hormonal therapies or those who had initiated combined androgen blockade. Fourth, this study focused only on three antiandrogens as secondary hormonal therapies; corticosteroids, estrogens, and other antiandrogens, including nilutamide, flutamide, apalutamide, and darolutamide, were not included in this study. Since this study aimed to understand the role of secondary hormonal therapies in the context of potential AEs and cost burden among non-metastatic PC patients using the Pilon et al.Citation14 work as a model, we restricted our study to the same therapies (with the exception of chemotherapy) included in the Pilon et al.Citation14 study. In addition, only four patients in our database initiated nilutamide or flutamide, which is consistent with recent literature that suggests that nilutamide and flutamide are not commonly used agents among nmPC patientsCitation36. Similarly, corticosteroids and estrogens are also not frequently used as secondary hormonal therapies. Hence, we believe that exclusion of these drugs is unlikely to impact our findings. Apalutamide and darolutamide were not included in our study since they were introduced into the market in 2018 and 2019, respectively, which is beyond the time frame of the data cut used to conduct this analysis. Future research including apalutamide and darolutamide may clarify their relative role and impact on some of the parameters evaluated in the current study.
The MarketScan database does not capture patients’ insurance claims activity outside of the included plans and patients were only followed until development of metastasis, end of enrollment, or discontinuation of therapy (whichever occurred first), thus any long-term implications of AEs on HCRU and costs might have been missed and may have resulted in underestimating the true healthcare costs associated with AEs. We annualized the HCRU and cost outcomes for our analysis and did not account for duration of follow-up. However, since the median length of follow-up differed by less than 3 months for patients with ≥1 CNS AE vs no CNS AE and less than 4 months for patients with ≥1 any AE vs no AE, we believe it is unlikely to bias the HCRU and cost estimates obtained in our analysis. It was not possible to conduct multivariate analyses to compare outcomes (HCRU, cost, and treatment discontinuation rates) between patients initiating different secondary hormonal treatment agents due to the low sample size of patients treated with enzalutamide and abiraterone. However, our objective was not to examine outcomes of interest stratified by treatment agent. Instead, our study sought to evaluate the burden of AEs among nmPC patients receiving secondary hormonal therapies from 2012 through 2017. Similarly, we were also unable to study the incidence or burden associated with specific AEs since the sample sizes for individual AEs were too small to be studied separately or compared. Hence, we only compared patient groups stratified by manifestation of ≥1 CNS AEs and ≥1 any AEs, similar to the work by Pilon et al.Citation14. Lastly, in the absence of imaging data, we relied on diagnosis codes to adjudicate whether a patient’s disease was metastatic or not, which may be subject to undercoding.
Despite the limitations described above, some of which are inherent to observational studies, our findings are potentially generalizable to non-elderly, commercially insured (i.e. primary or supplemental) nmPC patients and Medicare beneficiaries with commercial coverage (i.e. Medicare Advantage) diagnosed with nmPC who initiate secondary hormonal therapy after castration with ADT and have no evidence of cancers other than PC.
Conclusion
This study provides evidence that AEs are independently associated with treatment discontinuation and resource use in nmPC patients who are treated with secondary hormonal therapies. While the treatment landscape of nm(CR)PC continues to evolve, our study highlights the importance of considering the AE profiles of secondary hormonal therapies prior to their initiation among nmPC patients. Lastly, this study informs payers of the importance of downstream health economic consequences related to therapy discontinuation, HCRU, and associated direct medical costs of AEs of interest in nm(CR)PC patients.
Transparency
Declaration of funding
This research study was funded by Bayer Healthcare Pharmaceuticals.
Acknowledgements
The authors would like to thank Zhiyong Chen, formerly of Pharmerit International, for his helpful comments on the statistical analysis; Catherine Mirvis, of Pharmerit International, for medical writing assistance; and Shelly Ikeme, formerly of Bayer, for contributions to earlier versions of this study.
Additional information
Funding
References
- American Cancer Society. Key statistics for prostate cancer; 2019 [cited 2019 Jun 6]. Available from: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html#references
- Connolly RM, Carducci MA, Antonarakis ES. Use of androgen deprivation therapy in prostate cancer: indications and prevalence. Asian J Androl. 2012;14(2):177–186.
- Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200.
- American Urological Association. Castration-resistant prostate cancer; 2018. [cited 2019 Apr 25]. Available from: https://www.auanet.org/guidelines/prostate-cancer-castration-resistant-guideline#x1929
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422.
- Zhang TY, Agarwal N, Sonpavde G, et al. Management of castrate resistant prostate cancer-recent advances and optimal sequence of treatments. Curr Urol Rep. 2013;14(3):174–183.
- Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17(Suppl 2):S72–S79.
- Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73(2):178–211.
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–1418.
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–2474.
- Srinivas S, Mohamed AF, Appukkuttan S, et al. Physician benefit risk preferences for non-metastatic castration-resistant prostate cancer treatment (nmCRPC). J Clin Oncol. 2019;37(Suppl 15):e16610.
- Sandy Srinivas AFM, Appukkuttan S, Botteman M, et al. Patient and caregiver benefit-risk preferences for non-metastatic castration-resistant prostate cancer treatment (nmCRPC). J Clin Oncol. 2019;37(Suppl 27):196.
- Pilon D, Behl AS, Ellis LA, et al. Assessment of real-world central nervous system events in patients with advanced prostate cancer using abiraterone acetate, bicalutamide, enzalutamide, or chemotherapy. Am Health Drug Benefits. 2017;10(3):143–153.
- Behl AS, Ellis LA, Pilon D, et al. Medication adherence, treatment patterns, and dose reduction in patients with metastatic castration-resistant prostate cancer receiving abiraterone acetate or enzalutamide. Am Health Drug Benefits. 2017;10(6):296–303.
- Uemura H, DiBonaventura M, Wang E, et al. The treatment patterns of castration-resistant prostate cancer in Japan, including symptomatic skeletal events and associated treatment and healthcare resource use. Expert Rev Pharmacoecon Outcomes Res. 2017;17(5):511–517.
- Lodde M, Lacombe L, Fradet Y. Salvage therapy with bicalutamide 150 mg in nonmetastatic castration-resistant prostate cancer. Urology. 2010;76(5):1189–1193.
- Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098–2106.
- Ryan CJ, Crawford ED, Shore ND, et al. The IMAAGEN study: effect of abiraterone acetate and prednisone on prostate specific antigen and radiographic disease progression in patients with nonmetastatic castration resistant prostate cancer. J Urol. 2018;200(2):344–352.
- IBM Watson Health. MarketScan [cited 2019 June 28]. Available from: https://truvenhealth.com/your-healthcare-focus/government/analytic-research/marketscan
- Zhong Y, Valderrama A, Yao J, et al. Economic evaluation of treating skeletal-related events among prostate cancer patients. Value Health. 2018;21(3):304–309.
- Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298.
- Alemayehu B, Buysman E, Parry D, et al. Economic burden and healthcare utilization associated with castration-resistant prostate cancer in a commercial and Medicare Advantage US patient population. J Med Econ. 2010;13(2):351–361.
- McLeod DG. Tolerability of nonsteroidal antiandrogens in the treatment of advanced prostate cancer. Oncologist. 1997;2(1):18–27.
- Cella D. The functional assessment of cancer therapy-anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–19.
- Melton LJ, 3rd, Alothman KI, Khosla S, et al. Fracture risk following bilateral orchiectomy. J Urol. 2003;169(5):1747–1750.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197.
- Ryan CJ, Crawford ED, Shore ND, et al. IMAAGEN trial safety and efficacy update: effect of abiraterone acetate and low-dose prednisone on prostate-specific antigen and radiographic disease progression in patients with nonmetastatic castration-resistant prostate cancer. J Clin Oncol. 2016;34(15_suppl):5061–5061.
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–1246.
- Satoh T, Ledesma DA, Yoshihara N. Hospital resource use in metastatic castration resistant prostate cancer (MCRPC) in national university hospitals in Japan. Value Health. 2017;20(9):A446.
- Krahn MD, Bremner KE, Luo J, et al. Health care costs for prostate cancer patients receiving androgen deprivation therapy: treatment and adverse events. Curr Oncol. 2014;21(3):e457–65.
- Hansen RN, Ramsey SD, Lalla D, et al. Identification and cost of adverse events in metastatic breast cancer in taxane and capecitabine based regimens. Springerplus. 2014;3(1):259.
- Hansen RN, Hackshaw MD, Nagar SP, et al. Health care costs among renal cancer patients using pazopanib and sunitinib. J Manag Care Pharm. 2015;21(1):37–44.
- Hassett MJ, O'Malley AJ, Pakes JR, et al. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98(16):1108–1117.
- Schultz NM, Penson DF, Wilson S, et al. Adverse events associated with cumulative corticosteroid use in patients with castration-resistant prostate cancer: an administrative claims analysis. Drug Saf. 2019:1–11. DOI:10.1007/s40264-019-00867-6.
- Shah R, Ikeme S, Botteman M, et al. PCN156 characteristics and treatment patterns for non-metastatic castration resistant prostate cancer (nmCRPC) patients in Germany, France, and United Kingdom (UK). Value Health. 2019;22:S85.
Appendices
Table A1. ICD-9-CM and ICD-10-CM diagnosis codes.
Table A2. ICD-9-CM, ICD-10-CM, CPT, HCPCS, and procedure codes.
Table A3. NDC, ICD-9, and ICD-10 drug codes.
Table A4. Unadjusted healthcare resource utilization and costs among patients with and without fatigue/asthenia.
Table A5. Unadjusted healthcare resource utilization and costs among patients with and without insomnia.
Table A6. Unadjusted healthcare resource utilization and costs among patients with and without rashes.