Abstract
Aims: This analysis evaluates the cost-effectiveness of first-line treatment with FOLFIRI + cetuximab vs FOLFIRI + bevacizumab for patients with RAS wild-type (wt) metastatic colorectal cancer (mCRC) in Germany based on the randomized phase 3 FIRE-3 trial. For patients with RAS wt mCRC, FOLFIRI + cetuximab yielded statistically significant median overall survival gains over FOLFIRI + bevacizumab.
Materials and methods: A standard 3-state partitioned survival cost-utility model was developed to compare the health benefits and costs of treatment from a German social health insurance perspective using individual patient–level trial data. Health outcomes were reported in life-years (LYs) and quality-adjusted life-years (QALYs) gained. Survival was estimated based on Kaplan-Meier (KM) curves supplemented with best-fitting parametric survival model extrapolations. Subgroup analyses of patients with a left-sided primary tumor location or patients with metastases confined to the liver were performed.
Results: In the modified intention-to-treat analysis, FOLFIRI + cetuximab, providing 0.68 additional LYs (0.53 QALYs), yielded incremental cost-effectiveness ratios (ICERs) of €36,360/LY and €47,250/QALY. In subgroup analyses, patients experienced improved survival gains without a corresponding increase in costs, resulting in lower ICERs. Our model was most sensitive to changes in treatment duration across all lines of therapy, utility of progressive disease, as well as patients’ weight and body surface area.
Limitations: This cost-effectiveness analysis was based on patient-level data from the FIRE-3 trial. Trial outcomes may not adequately reflect those in the real-world setting. Additionally, resource use and costs were obtained from tariff lists, which do not account for differences in treatment practice. These considerations limit generalizability of outcomes to other countries, or within the German healthcare setting.
Conclusions: Based on our analyses, FOLFIRI + cetuximab is cost-effective compared with FOLFIRI + bevacizumab in patients with RAS wt mCRC, with ICERs well below willingness-to-pay thresholds for diseases with a high burden.
Introduction
Colorectal cancer (CRC) is the second most frequent cause of cancer-related deaths in both men and women, with more than 25,000 deaths in Germany annuallyCitation1–3. While approximately 25% of patients present with metastases at initial diagnosis, the clinical outcome for patients with metastatic CRC (mCRC) has improved over the last decadeCitation4. Standard first-line treatment for patients with mCRC in Germany predominantly consists of an irinotecan- or oxaliplatin-based chemotherapy doublet (FOLFIRI or FOLFOX, respectively) combined with a biological targeted agentCitation5. Choice of targeted agent is based on RAS mutation status and includes cetuximab, an IgG1 monoclonal antibody (mAb) against the epidermal growth factor receptor (EGFR); panitumumab, an IgG2 mAb against the same receptor; or bevacizumab, which binds to vascular endothelial growth factor (VEGF). Cetuximab, bevacizumab, and panitumumab improve outcomes in patients with RAS wild-type (wt) mCRC when added to chemotherapy regimensCitation4.
Previous studies have demonstrated that patients with left-sided (LS) CRC tumors have a better prognosis than those with right-sided tumors, who tend to derive limited benefit from standard treatments as right-sided tumors tend to have a higher frequency of mutations associated with a decreased response to therapyCitation6. Moreover, primary tumor side has been established as a predictive factor in patients with RAS wt mCRC receiving EGFR antibody therapy in combination with chemotherapyCitation6–12. A retrospective analysis of 6 randomized trials (including the cetuximab studies CRYSTAL, FIRE-3, and CALGB 80405) comparing chemotherapy + EGFR antibody therapy with chemotherapy alone or chemotherapy + bevacizumab reported that the response to chemotherapy + EGFR inhibitors was greater than that with chemotherapy ± bevacizumabCitation6. This effect was more pronounced in patients with RAS wt, LS primary tumors. Thus, primary tumor location is an important prognostic and predictive factor in RAS wt mCRC. A previous study also highlighted the role of liver-limited disease (LLD) as a prognostic factor in RAS wt mCRC, which was independent of hepatic resection in patients treated with targeted therapyCitation13.
From the perspective of healthcare decision makers, it is important to analyze the relative effectiveness and cost-effectiveness of a treatment. To evaluate the cost-effectiveness to society of a health intervention in a standardized manner, mean total costs associated with the intervention for a population of interest are divided by mean total health gains. This incremental cost-effectiveness/cost-utility ratio (ICER) is often expressed as costs in terms of time and quality of life, or costs per quality-adjusted life-year (QALY). To determine whether a treatment is cost-effective, the ICER is compared with a willingness-to-pay (WTP) threshold. In the absence of formal German WTP thresholds, thresholds from other European countries, including the United KingdomCitation14,Citation15 and the NetherlandsCitation16, bear relevance for Germany; the incidence, prevalence, and mortality rates of mCRC are similar, and background mortality is alike. The randomized phase 3 FIRE-3 trial conducted by the Arbeitsgemeinschaft Internistische Onkologie (AIO) working group in Germany evaluated first-line FOLFIRI in combination with either cetuximab or bevacizumab (FOLFIRI + cetuximab or FOLFIRI + bevacizumab) for the treatment of patients with unresectable RAS wt mCRCCitation17. Overall survival (OS) favored the FOLFIRI + cetuximab arm, with median OS 7.5 months longer than that observed in the FOLFIRI + bevacizumab arm. Here, we report the results of a cost-effectiveness model based on patient-level trial data for first-line treatment of mCRC with FOLFIRI + cetuximab compared with FOLFIRI + bevacizumab in patients with RAS wt tumors from a German payer perspective as provided by the German Statutory Health Insurance (GSHI) healthcare systemCitation18.
Methods
Population
The modified intention-to-treat (mITT) patient population of FIRE-3 informed the model through a direct comparison of FOLFIRI + either mAb in adult (>18 years) patients with RAS (KRAS/NRAS, exons 2–4) wt mCRCCitation17.
Model structure
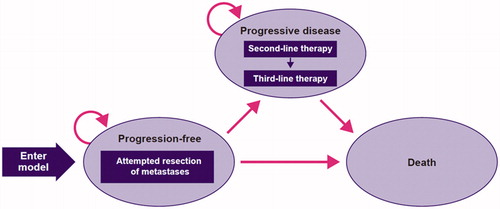
A standard oncology 3-state partitioned survival cost-utility model () was developed to analyze the costs and health benefits of FOLFIRI + cetuximab vs FOLFIRI + bevacizumab based on FIRE-3 study data and the literature. The model was designed to encapsulate the transition of patients with mCRC through 3 distinct health states: progression-free, progressive disease, and death. Patients entered the model in a progression-free, state and were at risk of progressive disease or death, and patients who transitioned to progressive disease remained in this state until death. The time horizon applied in the model was 37 years subsequent to the mean age of the trial population at diagnosis, thus considered lifetime. The lifetime horizon was sufficiently long to capture all relevant costs and health benefits. The cycle length was set at 1 month, and a half-cycle correction was applied. The model was developed in Microsoft Excel (Office 365). Parametric survival analyses were performed in R (3.6.1) using the “flexsurv” package of Jackson et al. 2019Citation19.
Transition probabilities
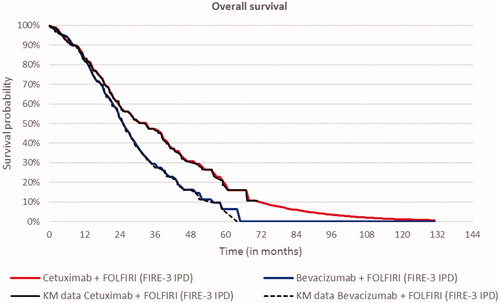
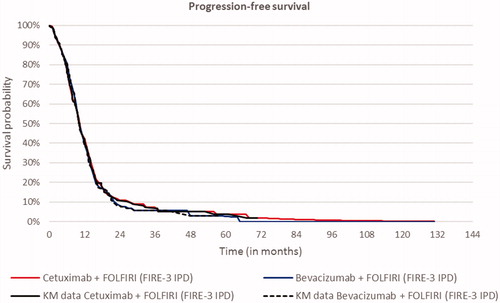
Transition probabilities to progressive disease and death were based on the progression-free survival (PFS) and OS Kaplan–Meier (KM) curves from the FIRE-3 trial. KM curves were directly used in the model. Transition probabilities were derived from the PFS and OS KM data. As a first step, the proportion of patients who progressed or died within each model cycle (one month) was determined based on the KM output, i.e. time, number at risk, number of events, survival, standard error, as well as lower and upper boundaries of the 95% confidence interval. Subsequently, based on survival probabilities of PFS and OS, the monthly hazard of progressing or dying was calculated for each cycle. PFS and OS hazards informed (a) the percentage of patients remaining progression free, (b) which percentage of patients progressed and (c) which percentage of patients died, following a similar approach to a partitioned survival model using parametric curves. KM curves were sufficiently mature for this purpose. The best statistical and visual goodness of fit was provided by the Weibull curve for OS of FOLFIRI-cet and the log-logistic curve for PFS ( and )
Costs, resource use, and utility inputs
A targeted literature search for information on healthcare resource utilization, unit costs, and utilities was performed. The model included direct and indirect medical costs, including drug costs across treatment lines, concomitant medication, clinical consultations and hospitalizations, laboratory tests and imaging, surgical interventions and other medical procedures, adverse event management, and best supportive care (BSC) (Tables S1 and S2). End-of-life costs were included for the GSHI perspective and constituted indirect medical costs. Direct and indirect nonmedical costs were not included in the model. Costs of second- and third-line therapies were calculated and included for all patients who experienced progressive disease and received further therapy in the trial. Costs of R0 or R1 liver metastasectomy were applied, irrespective of the outcome of the surgeryCitation20. Patients were assumed to receive treatment in the outpatient setting, in accordance with real-world practice. Adverse events of grade ≥3 occurring in ≥5% of patients in 1 comparator were included. Where necessary, costs were inflated to 2018 euro values. A targeted literature review was conducted to identify recent health-related quality-of-life publications to obtain utility values. Recently reported disutility values were identified for most adverse events. In several cases, multiple values for a single parameter were found in the literature. These, as well as assumptions on disutility values for adverse events that were not explicitly reported in literature, were presented to the FIRE-3 trial principal investigators for face validity and clinical plausibility in an interview setting. Weighted average baseline utility values for health states in first-, second-, and third-line settings were applied to the modelCitation21–23.
Analyses
Costs and QALYs were both discounted at 3%, as recommended by the German Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). To contextualize the analysis, WTP thresholds from relevant European health technology assessment (HTA) agencies from England (£50,000 when appraising so-called end-of-life interventions, converted to euros) and the Netherlands (€80,000) were appliedCitation14–16,Citation24. In addition to the base-case analysis, several subgroup, scenario, and sensitivity analyses were performed. Subgroup of patients with RAS wt LS primary tumor location and those with metastases confined to the liver were identified from individual patient data (IPD) from the trial, and outcomes were modeled. In scenario 1, parametric survival curves were fitted over the entire mITT population survival data as opposed to using mITT KM data to model survival followed by parametric survival curves. Scenario 2 addressed the impact of potential double counting of costs borne during the terminal phase. As BSC is typically provided in the palliative setting preceding death, it potentially overlaps with end-of-life costs, resulting in double counting. Therefore, in this second scenario, end-of-life costs were excluded. Scenario 3 stratified PFS and OS over patients with and without resection. One-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA) projected the impact of parameter uncertainty on the model results.
Validation
Our model underwent an extensive quality control, performed by an independent third-party consultant who was not involved in its development, according to a pre-specified 57-item checklist used as part of a standard operating procedure for economic model development by the sponsor of this research. Validation included economic analysis design, clinical evidence used, resource use handling, baseline case results, and sensitivity analysis results. Additionally, the quality of programming was assessed against a 56-item checklist, covering functionality, model clarity, model accuracy, model platform, model engine/Markov traces, sensitivity analysis, and survival analysis. Any identified errors were resolved prior to reporting results.
Results
The cost-effectiveness analyses, using KM data combined with the best-fitting parametric survival curves, estimated 0.68 additional LYs and 0.53 additional QALYs for FOLFIRI + cetuximab vs FOLFIRI + bevacizumab at an additional cost of €24,841. The corresponding discounted ICERs were €36,360 and €47,250 per LY and QALY, respectively (). Patients with RAS wt, LS primary mCRC or those with RAS wt, LLD mCRC experienced survival gains without a corresponding increase in cost, thereby leading to lower ICERS (). For RAS wt, LS primary mCRC, FOLFIRI + cetuximab had an ICER of €39,999 and for LLD, the ICER was €27,075, both discounted, per QALY. Scenario analyses are presented in the Supplementary material (Table S3, S4 and S5).
Table 1. Incremental cost-effectiveness ratios.
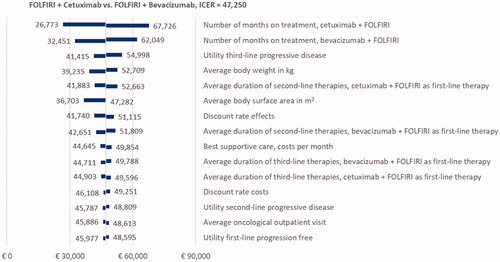
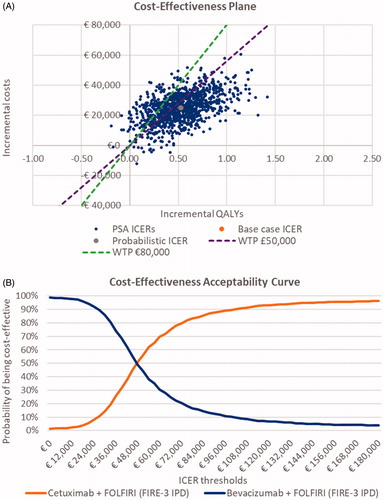
OWSA of the RAS wt population indicated that the model was sensitive to first-line treatment duration, utility of third-line progressive disease, body weight, body surface area, duration of second-line treatment, and discounting rate effects (). The explanation for the model being sensitive to variations in average patient weight and associated body surface area is due to the fact that medication is dosed according to these characteristics. PSA showed that FOLFIRI + cetuximab had a 63.5% and 84.2% probability of being cost-effective vs FOLFIRI + bevacizumab in the RAS wt patient population at WTP thresholds of £50,000 and €80,000, respectively (). In both subgroup analyses, the PSA showed that the probability of FOLFIRI + cetuximab being cost-effective was higher, at 80.8% and 90.3% for patients with an LS primary tumor location and LLD, respectively, against a threshold of £50,000/QALY (Figure S1).
Figure 5. Probabilistic sensitivity analyses. A, Cost-effectiveness plane, RAS wild-type (wt) patient population (base case). B, Cost-effectiveness acceptability curve, RAS wt patient population (base case). Abbreviations. ICER, incremental cost-effectiveness ratio; IPD, individual patient data; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year; WTP, willingness to pay.
Discussion
The objective of this study was to evaluate cost-effectiveness of FOLFIRI + cetuximab compared with FOLFIRI + bevacizumab as first-line treatment of patients with RAS wt mCRC in the German setting. While Germany does not apply a WTP threshold as part of its HTA framework, when considering established WTP thresholds from other European HTA bodies, FOLFIRI + cetuximab is cost-effective compared with FOLFIRI + bevacizumab, with an ICER of €47,250/QALY. Notably, an improved ICER was seen in patients with RAS wt, LS primary tumors and patients with LLD. Patients in these post hoc subgroup analyses benefited from a further increase in OS gains.
The model structure used in this analysis bears resemblance to a model presented by Graham et al.Citation25 in which survival outcomes for patients who underwent a resection were modeled separately based on a previously published studyCitation26. In our base-case analyses, we did not model survival separately for patients who underwent a resection because the effects of resection were captured within PFS and OS of the mITT population and the costs of resection were explicitly included. A scenario analysis stratifying PFS and OS over patients with and without resection provided results comparable to the base-case results (Table S3). This validates the use of a more conventional 3-state model structure vs a more complex model in which PFS and OS are modeled separately for patients without or with surgical resection of liver metastases. In addition, cost inputs differ between our model and that of Graham et al. due to the chosen healthcare perspectives. Furthermore, utilities of the health states differ, as our study used RAS wt utilities compared with KRAS wt utilities.
To our knowledge, our study is the first full report on a model based on patient-level data from the FIRE-3 trial. The clinical efficacy and cost-effectiveness of cetuximab for the first-line treatment of RAS wt mCRC in the United Kingdom were previously assessed by the Peninsula Technology Assessment Group and published by Huxley et al.Citation27. It should be noted that ICERs reported in Huxley et al. differ from our ICER calculations. Huxley et al. predicted a higher ICER resulting from different assumptions around OS. Huxley et al. considered survival after first-line progression to be independent of first-line treatment. According to assumptions presented by Huxley et al., similarity in PFS between FOLFIRI + cetuximab vs FOLFIRI + bevacizumab as observed in the FIRE-3 trial would translate into similar OS estimates. Similar OS results, meaning small incremental LYs and QALYs for FOLFIRI + cetuximab over FOLFIRI + bevacizumab, lead to high ICERs. However, in their paper, Huxley et al. describe another scenario, in which post-progression survival is modeled by extrapolating the OS and PFS curves from the trials. The latter approach is quite similar to our approach depicted in this manuscript. ICER estimates for the alternative scenario analysis were not provided in the Huxley et al. manuscript. Our results in terms of undiscounted life years align with the Huxley scenario, while residual differences between ICER estimates can be explained by modeling based on KM data (our study) versus parametric survival curvesCitation27. This narrows the gap between calculated ICERs substantially. In line with data reported here and based on the FIRE-3 study, first-line treatment with FOLFIRI + cetuximab in KRAS and extended RAS wt mCRC was reported to be cost-effective compared with FOLFIRI + bevacizumab in the United States, with an ICER of $73,731/LY for the RAS wt patient population scenario analysisCitation28. It should be noted that cetuximab has a different marketing authorization holder in the United States, and costs differ notably between jurisdictions.
Results for the RAS wt and RAS wt, LS patient populations have been published based on an earlier version of this modelCitation18. The results we present here reflect updated utility and resource use inputs in this model following a targeted literature review performed in November 2017. With inclusion of utilities specific for patients with RAS wt mCRCCitation29, QALY values have improved compared with those of the previous model. Additionally, BSC costs were updated to match Bekelman et al.Citation30, and end-of-life costs were incorporated into the base case to be in line with previous comparable cost-effectiveness modelsCitation25,Citation27,Citation31,Citation32. Since BSC costs cannot always be distinguished from end-of-life costs, the potential to overestimate costs cannot be ruled out, notably in the cetuximab arm, where survival was longer. The marginal increase in ICERs from €47,250 to €47,391, both per QALY, suggests no impact of double counting, contrary to our assumptions. End-of-life costs were removed from both arms, so although the absolute cost of both treatments was lower when end-of-life costs were excluded, the difference was numerically small.
Linked to the addition of the substantial monthly BSC costs, we report higher ICERs compared with those of the earlier version of the model, as BSC costs were applied to patients with progressive disease, and time lived with progressive disease was longer for FOLFIRI + cetuximab than FOLFIRI + bevacizumab.
Our scenario analyses showed higher ICERs for all subgroups of patients when the best-fitting parametric survival curves were applied. This can be explained by the OS KM data of the FOLFIRI + bevacizumab arm, which ends with no patients at risk of an event in most subgroups. If parametric survival curves were fitted, the point where these curves reach 0% survival occurred later than that in the KM data. Therefore, the modeled survival of the FOLFIRI + bevacizumab arm was longer when parametric survival curves were applied. When comparing these 2 approaches (KM data combined with parametric survival curves vs parametric survival curves) from an HTA perspective, there was no single preferred method. The National Institute for Health and Care Excellence (NICE) has taken different positions in technology appraisals in oncology with regard to directly applying KM data to model OS and PFSCitation33–35. In TA259 in 2012, the evidence review group argued that patient-level KM data, although representing observed data, may be less applicable to patients outside the trial than well-fitting parametric distributions. However, more recently, the NICE committee stated they were aware of 2 divergent views: (1) modeled data could be more generalizable than data from a single trial and (2) they can also be considered preferable to “maximize” use of trial data, particularly when the data are matureCitation34. Thus, due to changing views over time, it remains important to consider the results of both approaches.
Conclusions
The cost-effectiveness of treatment is an important consideration when evaluating the value of a therapy, both for decision makers and clinicians. We conclude that FOLFIRI + cetuximab is a cost-effective treatment option for patients diagnosed with RAS wt mCRC in Germany. In addition, our model shows that the cost-effectiveness of FOLFIRI-cet in patients with RAS wt mCRC with LS primary tumors is associated with lower ICERs.
Transparency
Declaration of funding
This study was funded by Merck KGaA, Darmstadt, Germany.
Declaration of financial/other relationships
SS discloses an advisory role and has received honoraria for talks from Amgen, Bayer, Eli Lilly, Merck KGaA, Roche, Sanofi, and Takeda. IvO served as a consultant for Amgen, AstraZeneca, Janssen Pharmaceutical, Merck, Pfizer, and Sanofi, and received funding from Amgen, AstraZeneca, Janssen Pharmaceutical, Merck, Pfizer, and Sanofi. CP and PR are employees of Merck KGaA, Darmstadt, Germany. BH served as a consultant for Amgen, AstraZeneca, Celgene, Johnson & Johnson, Merck, Pfizer, and Sanofi; received funding from Amgen, AstraZeneca, Celgene, Johnson & Johnson, Merck, Pfizer, and Sanofi. VH discloses honoraria from Merck, Roche, Celgene, AMGEN, Sanofi, Lilly, SIRTEX, Boehringer-Ingelheim, Taiho, Servier; consulting or advisory role with Merck, Roche, AMGEN, Sanofi, SIRTEX, Servier, Celgene, Boehringer-Ingelheim, Halozyme, MSD, BMS; speaker’s bureau for MERCK, Roche, AMGEN, SIRTEX, Servier, Shire, MSD, BMS; research funding from MERCK, Roche, AMGEN, SIRTEX, Servier, Celgene, Boehringer-Ingelheim, Shire; expert testimony for Servier; and travel, accommodations, expenses from MERCK, Roche, AMGEN, SIRTEX, Servier, Shire, MSD, BMS. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
CP, SS IvO, and BH contributed to study concepts and study design. CP, SS, and VH contributed to data acquisition. CP, IvO, and BH contributed to quality control of data and algorithms. CP, SS, IvO, BH, PR, and VH contributed to data analysis and interpretation. IvO and BH contributed to statistical analysis. All authors contributed to manuscript preparation, manuscript editing, and manuscript review. In addition, all authors agree to be accountable for all aspects of the work.
FIRE-3_CE_SUPPL_9Aug2019.docx
Download MS Word (249.1 KB)Acknowledgements
Model quality control was performed by Covance Market Access Services Inc, London, United Kingdom. Medical writing support was provided by ClinicalThinking, Inc, Hamilton, NJ, USA, and funded by Merck KGaA, Darmstadt, Germany. The funder of the study had a role in study design, data collection, data analysis, and data interpretation. The authors had access to all of the data in the study and had final responsibility for the decision to submit for publication.
References
- World Health Organization: International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012. [cited 2020 Jan 06]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- Bray F, Ren J-S, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145.
- Rafiemanesh H, Mohammadian-Hafshejani A, Ghoncheh M, et al. Incidence and mortality of colorectal cancer and relationships with the human development index across the world. Asian Pac J Cancer Prev. 2016;17(5):2465–2473.
- Van Cutsem E, Cervantes A, Nordlinger B, ESMO Guidelines Working Group, et al. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–iii9.
- German Guideline Program in Oncology (GGPO) 2014. Evidenced-based guideline for colorectal cancer. Version 2.1. Berlin, Germany: Deutsche Krebsgesellschaft; 2019. [cited 2019 July 31]. Available from: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Kolorektales_Karzinom/Version_2/GGPO_Guideline_Colorectal_Cancer_2.1.pdf
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol. 2017;28(8):1713–1729.
- Moretto R, Cremolini C, Rossini D, et al. Location of primary tumor and benefit from anti-epidermal growth factor receptor monoclonal antibodies in patients with RAS and BRAF wild-type metastatic colorectal cancer. Oncologist. 2016;21(8):988–994.
- Chen KH, Shao YY, Chen HM, et al. Primary tumor site is a useful predictor of cetuximab efficacy in the third-line or salvage treatment of KRAS wild-type (exon 2 non-mutant) metastatic colorectal cancer: A nationwide cohort study. BMC Cancer. 2016;16(1):327.
- Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1°) tumor location on overall. survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(15_suppl):3504–3504.
- Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194–201.
- Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: Differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57–64.
- Schrag D, Weng S, Brooks G, et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol. 2016;34(15_suppl):3505–3505.
- Holch JW, Ricard I, Stintzing S, et al. Relevance of liver-limited disease in metastatic colorectal cancer: subgroup findings of the FIRE-3/AIO KRK0306 trial. Int J Cancer. 2018;142(5):1047–1055.
- NICE. Value based assessment of health technologies. London, UK: NICE; 2018. [cited 2019 Jan 31]. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/VBA-TA-Methods-Guide-for-Consultation.pdf
- NHS England Cancer Drugs Fund Team. Appraisal and funding of cancer drugs July 2016. Leeds, UK: NHS England; 2018. [cited 2019 Jan 31]. Available from: https://www.england.nhs.uk/wp-content/uploads/2013/04/cdf-sop.pdf
- Zorginstituut Nederland. Kosteneffectiviteit in de praktijk. Diemen, the Netherlands: Zorginstituut Nederland; 2015. [cited 2018 Jan 4]. Available from: https://www.zorginstituutnederland.nl/publicaties/rapport/2015/06/26/kosteneffectiviteit-in-de-praktijk
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075.
- Stintzing S, van Oostrum I, Pescott C, et al. Cost-effectiveness of FOLFIRI + cetuximab vs FOLFIRI + bevacizumab in the first-line (1L) treatment of RAS wild-type (wt) metastatic colorectal cancer (mCRC) in Germany: data from the FIRE-3 (AIO KRK-0306) study. J Clin Oncol. 2018;36(4_suppl):800–800.
- Jackson C, Metcalfe P, Amdahl J. Package 'flexsurv”, Flexible Parametric Survival and Multi-State Models [accessed 2019 Dec 6]. R package. Available from: https://cran.r-project.org/web/packages/flexsurv/flexsurv.pdf.
- InEK—Institut für das Entgeltsystem im Krankenhaus. G-DRG system 2018. Siegburg, Germany: InEK; 2017–2018. [cited 2019 Jan 31]. Available from: https://www.g-drg.de/G-DRG-System_2018
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–4713.
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28(31):4697–4705.
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–1664.
- XE. Live exchange rates. Newmarket, Canada: XE.com Inc; 2018. [cited 2018 Mar 13]. Available from: https://www.xe.com/
- Graham CN, Hechmati G, Hjelmgren J, et al. Cost-effectiveness analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2014;50(16):2791–2801.
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg. 2004;240(4):644–657.
- Huxley N, Crathorne L, Varley-Campbell J, et al. The clinical effectiveness and cost-effectiveness of cetuximab (review of technology appraisal no. 176) and panitumumab (partial review of technology appraisal no. 240) for previously untreated metastatic colorectal cancer: a systematic review and economic evaluation. Health Technol Assess. 2017;21(38):1–294.
- Shankaran V, Ortendahl JD, Purdum AG, et al. Cost-effectiveness of cetuximab as first-line treatment for metastatic colorectal cancer in the United States. Am J Clin Oncol. 2018;41(1):65–72.
- Koukakis R, Gatta F, Hechmati G, et al. Skin toxicity and quality of life during treatment with panitumumab for RAS wild-type metastatic colorectal carcinoma: Results from three randomised clinical trials. Qual Life Res. 2016;25(10):2645–2656.
- Bekelman JE, for the International Consortium for End-of-Life Research (ICELR), Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315(3):272–283.
- Graham CN, Maglinte GA, Schwartzberg LS, et al. Economic analysis of panitumumab compared with cetuximab in patients with wild-type KRAS metastatic colorectal cancer that progressed after standard chemotherapy. Clin Ther. 2016;38(6):1376–1391.
- Hoyle M, Peters J, Crathorne L, et al. Cost-effectiveness of cetuximab, cetuximab plus irinotecan, and panitumumab for third and further lines of treatment for KRAS wild-type patients with metastatic colorectal cancer. Value Health. 2013;16(2):288–296.
- NICE. Abiraterone for castration-resistant metastatic prostate cancer previously treated with a docetaxel-containing regimen (TA259). London, UK: NICE; 2018. [cited 2019 Jan 31]. Available from: https://www.nice.org.uk/guidance/ta259
- NICE. Nintedanib for previously treated locally advanced, metastatic, or locally recurrent non-small-cell lung cancer (TA347). London, UK: NICE; 2015. [cited 2019 Jan 31]. Available from: https://www.nice.org.uk/guidance/ta347
- NICE. Ramucirumab for treating advanced gastric cancer or gastro–oesophageal junction adenocarcinoma previously treated with chemotherapy (TA378). London, UK; NICE; 2016. [cited 2019 Jan 31]. Available from: https://www.nice.org.uk/guidance/ta378