Abstract
Aims: Model how moving from current disease-modifying drug (DMD) prescribing patterns for relapsing-remitting multiple sclerosis (RRMS) observed in the United Kingdom (UK) to prescribing patterns based on patient preferences would impact health outcomes over time.
Materials and methods: A cohort-based Markov model was used to measure the effect of DMDs on long-term health outcomes for individuals with RRMS. Data from a discrete choice experiment were used to estimate the market shares of DMDs based on patient preferences (i.e. preference shares). These preference shares and real-world UK market shares were used to calculate the effect of prescribing behavior on relapses, disability progression, and quality-adjusted life-years (QALYs). The incremental benefit of patient-centered prescribing over current practices for the UK RRMS population was then estimated; scenario and sensitivity analyses were also conducted.
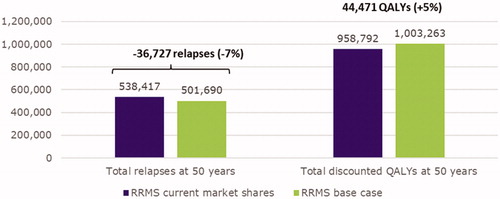
Results: Compared to current prescribing practices, when UK patients with RRMS were treated following patient preferences, health outcomes were improved. This population was expected to experience 501,690 relapses and gain 1,003,263 discounted QALYs over 50 years under patient-centered prescribing practices compared to 538,417 relapses and 958,792 discounted QALYs under current practices (−6.8% and +4.6%, respectively). Additionally, less disability progression was observed when prescribed treatment was based on patient preferences. In a scenario analysis where only oral treatments were considered, the results were similar, although the magnitude of benefit was smaller. Number of relapses was most sensitive to how the annualized relapse rate was modeled; disability progression was most sensitive to mortality rate assumptions.
Limitations: Treatment efficacy estimates applied to various models in this study were based on data derived from clinical trials, rather than real-world data; the impact of patient-centered prescribing on treatment adherence and/or switching was not modeled.
Conclusions: The population of UK RRMS patients may experience overall health gains if patient preferences are better incorporated into prescribing practices.
Introduction
Multiple sclerosis (MS) is a chronic and progressive disease that typically presents as relapsing-remitting MS (RRMS), which is characterized by periodic acute exacerbations of disease activity (relapses) followed by periods of remissionCitation1–5. There are several disease-modifying drugs (DMDs) available that help control the condition and delay disability progressionCitation5–8. Given the chronic and progressive nature of MS, patients will typically remain on a DMD indefinitelyCitation9; however, it is quite common to switch to another DMD due to a reduction in effectiveness and/or the occurrence of adverse events (AEs) on the current DMDCitation9,Citation10. Consequently, clinicians and patients are faced with selecting an appropriate DMD multiple times during the patient’s lifespan. In addition, research has found that adherence varies widely across studies, with estimates ranging from 47% to 93%Citation8,Citation11–14. Adherence is critically important given that it is associated with reduced relapse ratesCitation14–16, improved health-related quality of lifeCitation17, better physical outcomesCitation18, and lower healthcare utilization and costsCitation15,Citation19,Citation20.
Given the expanding treatment optionsCitation21, a large body of research has evolved around understanding patients’ preferences for various attributes of DMDsCitation22–27. These studies suggest that treatment frequency, mode of administration, and likelihood of side effects from a DMD are of importance to patientsCitation25–27. Studies show that patients strongly prefer oral administrationCitation22,Citation23,Citation27, particularly when oral treatment dosing is less frequent (e.g. less than three times daily) and the treatment has infrequent side effectsCitation26.
Information on patient preference is also important in facilitating shared decision-making between clinician and patient. Specifically, there have been calls for a shift to greater patient engagement and shared decision-making in the treatment of MSCitation28,Citation29. A recent systematic literature review found that shared decision-making significantly increases DMD adherenceCitation30. Patients who feel they do not have a voice in the clinical decision-making process, or lack in-depth understanding of the treatment options, are less likely to be adherent and more likely to discontinue treatmentCitation30,Citation31. Unfortunately, the treatment goals of patients and clinicians are often not alignedCitation32. Hence, taking into consideration the preferences and goals of patients is essential when making decisions regarding which DMD to use when initiating treatment as well as which DMD to switch to when the need arises.
The purpose of this study was to examine how moving from current DMD prescribing patterns observed in the United Kingdom (UK) to prescribing patterns based on patient preferences would impact long-term health outcomes, including disability progression, relapse rate, and quality-adjusted life-years (QALYs). Although research on patient preference and shared decision-making is invaluable for moving toward a more patient-centered approach to care, no study of which we are aware has assessed how taking a more patient-centered approach to treatment prescribing would affect long-term patient health outcomes in patients with RRMS. In clinical practice, treatment decisions would typically not be made solely by the patient; however, it is informative to see how health outcomes would differ if the decision was entirely patient-centered. This focus on patient-centered treatment decision-making is in line with the spirit of recent initiatives from United States (US) healthcare organizations/agencies such as the Patient-Centered Outcomes Research Institute (PCORI)Citation33, the Food and Drug Administration (FDA)Citation34, and the Centers for Medicare and Medicaid Services Quality Payment ProgramCitation35.
Methods
Model overview
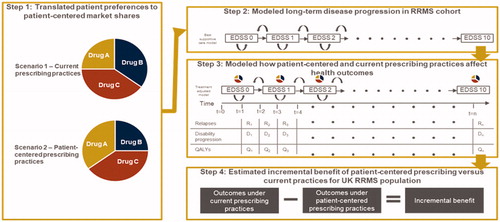
This study modeled the long-term health outcomes of the RRMS population in the UK using a cohort-based Markov model that evaluates the incremental clinical benefit of DMDs versus best supportive care (BSC) on long-term health outcomes. The analysis followed a multi-step approach. First, data from a recent discrete choice experiment (DCE) conducted in the UK and Germany was used to calculate the hypothetical market shares of DMDs if they were prescribed based on patient preferences (henceforth referred to as preference shares)Citation36. Second, a Markov model was used to calculate the effect of prescribing behavior (shares) on long-term health outcomes including relapses, disability progression, and QALYs. Third, the preference shares and current market shares (based on real-world data from patients who received a specified treatment in January 2019Citation37) were used in the model to identify the long-term health outcomes of patients under patient-centered and current prescribing practices. Lastly, the incremental benefit of patient-centered prescribing over current prescribing practices in the UK was estimated. The next four sub-sections describe each of these steps in greater detail and are accompanied by a visual overview of the study framework in . The latter two sub-sections describe a scenario analysis and various sensitivity analyses that were used to test the robustness of the base case findings.
Figure 1. Conceptual overview of study design. Notes. Study was executed in 4 steps: (1) a discrete choice experiment was performed to translate patient preferences into patient-centered market shared, (2) a Markov model was used to calculate the effect of prescribing behaviour (shares) on long-term health, (3) the preference shares and current market shares were used in the model to identify the long-term health outcomes of patients under patient-centered and current prescribing practices, (4) the incremental benefit of patient-centered prescribing over current prescribing practices in the UK was estimated. Abbreviations. DMD, disease-modifying therapy; EDSS, Expanded Disability Status Scale; QALY, quality-adjusted life-year.
Step 1. Translated patient preferences to patient-centered treatment market shares
This study used data extracted from a recent DCE study conducted in the UK and Germany to estimate DMD preference shares for all treatments approved at the time that the DCE was conducted: alemtuzumab, cladribine tablets, dimethyl fumarate, fingolimod, glatiramer acetate, interferon beta-1a, interferon beta-1b, natalizumab, peginterferon beta-1a, and teriflunomideCitation36. The treatment attributes that were used to evaluate patient preferences included administration frequency, location of administration, first administration monitoring, relapses, disability progression, immediate side effects, reversible side effects, irreversible side effects, monitoring for serious side effects, birth defects/pregnancy risk, and pre-pregnancy wait time requirements. This DCE was chosen because it is the most recent study we identified which measured the preferences of patients with RRMS in the UK. Further, the study had a larger sample size and was inclusive of more DMDs than previous DCEs in this therapeutic areaCitation22,Citation24.
The estimated probability of selecting each treatment was calculated using the individual level preference weights/coefficients for each respondent and the treatment with the highest predicted probability denoted the most preferredCitation38,Citation39. Preference share estimates were analyzed conditionally, dependent on whether respondents received treatment with DMDs; “no treatment” was not included as a treatment option. The number of respondents who preferred each treatment was aggregated to estimate the overall preference shares for each scenario. The preference weight estimation was implemented in Stata 15.1Citation40.
Step 2. Modeled long-term disease progression in RRMS cohort
This study used a cohort-based Markov model that compared the long-term effectiveness of DMDs to BSC in patients with RRMS. The model structure is similar to the one previously published by Hettle and colleagues, but Hettle et al. focused more narrowly on the high disease activity (HDA) RRMS populationCitation41. This model structure was chosen because it incorporates different state structures, assumptions on waning effects, and multiple sub-populations of the RRMS population. The model was created in Microsoft Excel 2016.
Model structure
The model comprised both a natural history module and a treatment-adjusted module. The natural history module was based on disability progression and relapse rate data from individuals receiving BSCCitation42–44. The treatment-adjusted module combined the natural history module with data on the comparative efficacy and safety of DMDs versus placebo, where placebo was used as proxy for BSCCitation45. The natural history model consisted of 11 states: 10 Expanded Disability Status Scale (EDSS) states and an all-cause death state, representing a simplified structure of disease progression that does not differentiate between patients with RRMS and secondary progressive MS. This model structure has previously been used in health technology appraisals of beta interferons and glatiramer acetate, as well as cladribine tablets and ocrelizumabCitation46–49. The treatment-adjusted module included the same states as the natural history module with an additional 10 EDSS states (i.e. where individuals in the cohort are on treatment).
In the treatment-adjusted module, all patients in the cohort started on treatment and switched to BSC when they discontinued DMDs due to either a treatment-specific AE or disability progression to EDSS ≥ 7.0 (i.e. the patient is essentially wheelchair bound and unable to walk beyond five meters even with aid). Patients were assumed to stay on BSC for the remainder of the study period such that the long-term effects of DMDs on health outcomes could be measured. Treatment switching was not modeled.
The key inputs in the model included disability progression, annualized relapse rate (ARR), health utilities, mortality, treatment discontinuation, and treatment waning effect. Each cycle, individuals could experience disease progression (move to a higher EDSS state), disease improvement (move to a lower EDSS state), remain stable (stay in the same state), or die. Disability progression of individuals with RRMS receiving BSC was modeled using Markov state transition probability matrices from the British Columbia Multiple Sclerosis (BCMS) registry published by Palace and colleaguesCitation42. In the treatment-adjusted module, these transition probabilities were adjusted using hazard ratios (HRs) for 6-month confirmed disability progression extracted from an indirect treatment comparisonCitation45. In addition to changes in disability, individuals could experience relapses in each cycle. The ARR for patients on BSC was extracted from the placebo arm of the CLARITY trial (mean ARR = 0.34; standard error SE = 0.20)Citation44,Citation50, and modeled to decline 22.9% every five years (independent from EDSS state)Citation43. Similar to disability progression, the reduction in ARR for DMDs were modeled using HRs from an indirect treatment comparisonCitation45.
To estimate QALYs, we extracted utilities by EDSS state, caregiver disutility, disutility per relapse, and disutility due to AEs from the literature. Health utilities by EDSS states were based on the EQ-5D data collected in the CLARITY trial and a study by Hawton and GreenCitation44,Citation50,Citation51. The model incorporated a disutility of 0.071 for each relapse as reported by Orme and colleaguesCitation52. Caregiver disutilities by EDSS states were extracted from Alcaster and colleaguesCitation53. The disutility parameters due to AEs were extracted from multiple sources in the literatureCitation54–56. DMDs impacted QALYs through their effects on relapse, disability progression, and the incidence of drug-related AEs.
All-cause mortality was derived by multiplying a standardized mortality rate with age- and sex-specific ratesCitation57; the mortality rate was then inflated to account for excess mortality risk associated with MS (HR = 1.68; SE = 0.171)Citation58 and converted to an annual mortality probability. DMDs did not affect mortality in the base case analysis. Treatment discontinuation rates were extracted from pooled discontinuation data from clinical trialsCitation44,Citation59–72. Individuals were assumed to retain the cumulative benefits of treatment until they discontinued treatment or when they progressed to an EDSS state of ≥ 7.0, after which they switched to BSC for the remainder of the study period. While on treatment, the effect of treatment reduced over time; specifically, treatments maintained 100% of their effect in years 0 to 2, 75% in years 2 to 5, and 50% in years 5 and on. This approach to waning has been previously used in the published literatureCitation41, as well as several models submitted to the National Institute for Health and Care Excellence (NICE)Citation54,Citation73,Citation74. Although alemtuzumab and cladribine are short-term courses, for any patient not continuing with the second course after the first course, they still received treatment effects of alemtuzumab and cladribine after the second course (so when they are no longer taking medication). This treatment efficacy undergoes the same waning schedule as other treatments. Additional detail on each key input parameter can be found in and the Supplemental Tables referenced therein.
Table 1. Key inputs of the economic model.
The primary outcomes of interest were QALYs, relapses, and disability progression. The QALYs for individuals were a function of the time spent in each EDSS state (which also incorporated caregiver disutility), the number of relapses experienced, and the disutility experienced due to AEs. The per-patient number of acute relapses experienced and discounted QALYs were estimated yearly and summed over a 50-year time horizon; the average EDSS state was calculated yearly. A discount rate of 3.5% for QALYs was assumed, as recommended by NICE guidelinesCitation75.
Study population
The study population of interest was the RRMS population in the UK. The mean age, proportion female, and EDSS state distribution of individuals in the cohort in year 1 was based on patients from the CLARITY trial who received either cladribine dosage 3.5 mg/kg or placebo (n = 870)Citation44,Citation50. Mean age was 38.7 years and almost two-thirds (65.9%) of the cohort was female. The intention-to-treat population in CLARITY has a similar baseline profile to that of patients enrolled to the UK multiple sclerosis Risk Sharing SchemeCitation42,Citation76 and was therefore considered by NICE to be generalizable to clinical practice in the UKCitation49.
Step 3. Modeled how patient-centered and current prescribing practices affect health outcomes
The DMD preference shares estimated in step 1 were incorporated into the model in step 2. Current market shares obtained from the Specialist Share Data from Wilmington HealthcareCitation37, which were based on patients who received a specified treatment in January 2019, were also incorporated into the model. Outcomes were modeled as the weighted sum of each outcome of all treatments of interest, both annually and over the entire 50-year time horizon of the model. The weights were determined by the DMD market shares based on prescribing patterns or preference shares. Total discounted QALYs per patient over 50 years, average number of relapses per patient over 50 years, and mean EDSS per year were calculated.
Step 4. Estimated the incremental benefit of patient-centered prescribing versus current practices on health outcomes for the UK RRMS population
Finally, this study calculated the difference between patient-centered prescribing practices compared to current prescribing practices for the number of relapses, disability progression, and discounted QALYs gained to obtain the incremental benefit of patient-centered prescribing practices for the total UK RRMS population. The incremental benefit was calculated at the patient-level and then translated into population-level results by multiplying this incremental benefit by the size of the total RRMS population in the UK (126,669 total MS patients in the UK × 85% of MS patients who have RRMS = 107,669 patients)Citation77,Citation78.
Scenario analysis
A scenario analysis was conducted on the RRMS population that included oral DMDs only: cladribine tablets, dimethyl fumarate, fingolimod, and teriflunomide. Patient preference shares for each oral therapy were estimated based on the results of the DCE as a fraction of the oral therapy market. For example, if altogether oral therapies were preferred by 50% of patients and oral therapy A was preferred by 10% of patients considering all DMDs, then among orals, therapy A would have a 20% preference share.
Sensitivity analyses
A series of one-way and multi-way sensitivity analyses were performed on the preference shares analysis for patients with RRMS. The sensitivity analyses tested whether results were robust to changes in specific parameters that were chosen as they were important inputs in the model and were based on assumptions/methodologies that may have had a significant effect on the results. In the one-way analyses the following parameter inputs were varied: (1) ARR, (2) mortality rate, and (3) treatment discontinuation. In the base analysis, ARR is modeled as a function of disease duration, independent of EDSS states. This is to avoid double counting of the treatment effect of DMDs on disability progression, meaning the effect of treatment on disability progression may also be associated with ARR. As the number of relapses increases with higher states, we modeled the ARR as a function of EDSS states, independent of disease duration, in the sensitivity analyses. Similarly, mortality was modeled independent of EDSS states in the base analysis, but dependent on EDSS states in the sensitivity analyses. For treatment discontinuation, we varied the data sources this key input was estimated on between the base and sensitivity analyses. In the base analyses, we estimated treatment discontinuation on pooled clinical trial data, a method used in a prior NICE submissionCitation47. In the sensitivity analyses, we estimated treatment discontinuation using a network meta-analysis of all-cause discontinuation dataCitation45. Combinations of the parameter inputs that were varied within the one-way sensitivity analyses were varied within the two multi-way sensitivity analyses. In the first multi-way sensitivity analysis, we only varied ARR and mortality rate. In the second multi-way sensitivity analysis, we varied all three parameters (ARR, mortality rate, and treatment discontinuation).
Results
Preference share estimates
Patients with RRMS in the UK preferred infusions (alemtuzumab and natalizumab; 43.0% combined) and oral treatments (cladribine tablets, dimethyl fumarate, fingolimod, and teriflunomide; 38.8% combined) over injections. In addition, these patients preferred DMDs with less frequent administrations, such as those administered once per month or less (natalizumab, cladribine tablets, alemtuzumab; 58.0% combined). When only oral treatments were considered in the scenario analysis, cladribine tablets were the most preferred treatment with a preference share of 38.8%. Detailed preference shares can be found in .
Table 2. Preference share estimation results.
These results indicated large discrepancies between current market shares (as reported based on the January 2019 Specialist Share Data from Wilmington HealthcareCitation37) and patient preference shares (based on the DCE) among patients with RRMS in the UK. For example, while natalizumab had the largest preference share (28.3%) among RRMS patients, this treatment occupied just 12.6% of the RRMS market in January 2019. Cladribine tablets, having been available only more recently, occupied 1.3% of the market in January 2019, despite a preference share of 15.0% among RRMS patients. Dimethyl fumarate had the largest market share of 28.5% in January 2019, yet only had a preference share of 4.9%.
Impact on health outcomes
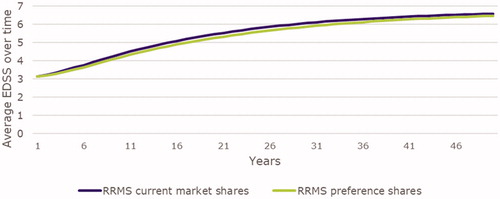
Long-term health outcomes were improved when UK RRMS patients were prescribed DMDs according to patient preferences versus current prescribing practices. For example, the UK RRMS population was expected to experience 501,690 total relapses over 50 years under patient-centered prescribing practices compared to 538,417 relapses under current prescribing practices, representing a reduction of 36,727 relapses (−6.8%; ). Similarly, the UK RRMS population gained 1,003,263 discounted QALYs over 50 years under patient-centered prescribing practices versus 958,792 discounted QALYs under current prescribing practices; an incremental gain of 44,471 discounted QALYs (+4.6%; ). Finally, in each year, less disability progression was observed among the UK RRMS population when prescribed treatment based on patient preferences (−0.16 EDSS per year; ).
Scenario analysis
In the scenario analysis where only oral treatments were considered, the results were qualitatively similar as those of the base analysis in which all RRMS treatments are considered, although the magnitude of benefit to patients was smaller. Compared to current prescribing practices, the UK RRMS population experienced a reduction of 20,910 relapses (−3.9%) under patient-centered prescribing practices, gained 5,717 discounted QALYs (+0.6%), and experienced less disability progression over 50 years.
Sensitivity analyses
The total number of relapses over 50 years among the UK RRMS population was most sensitive to how ARR was modeled (see Supplemental Figure 1). Specifically, when ARR was modeled as a function of EDSS state, independent of disease duration in the sensitivity analyses, the total number of relapses was higher compared to the preference share base analysis (1,620,844 versus 501,690) where ARR was modeled as a function of disease duration, independent of EDSS state. The increase in relapses is likely due to the fact that ARR has a higher correlation with EDSS state rather than disease duration. We abstained from modeling ARR as a function of EDSS state, independent of disease progression, in the base analysis to avoid “double counting” of the treatment effect on disability progression (i.e. the effect of treatment on disability progression may also be associated with ARR)Citation52,Citation79. The total discounted QALYs calculated over 50 years was largely insensitive (see Supplemental Figure 2) to assumptions around key parameters, such as ARR, mortality rate, and treatment discontinuation. In the preference share base analysis, the RRMS population benefited from 1,003,263 discounted QALYs over 50 years; in the preference share sensitivity analyses, our estimates ranged between 986,918 and 999,212 total discounted QALYs.
The average disability progression over time was most sensitive to assumptions around how mortality rate was modeled (see Supplemental Figure 3). When mortality rate was modeled such that it differed by EDSS state, UK RRMS patients experienced a reduction in mean EDSS in the later years of the study period. This reduction in mean EDSS is not observed in the preference share base analysis and is likely due to the fact that individuals in higher EDSS states have higher mortality rates.
Discussion
Similar to prior literature, our model showed that patients with RRMS in the UK prefer oral DMDs and infusions relative to injectionsCitation22,Citation23,Citation27, as well as DMDs with less frequent administrationCitation26. However, our results also showed that current DMD market shares within the UK RRMS population do not reflect these patient preferences. To our knowledge, no prior studies have examined the long-term clinical impact of more explicitly following patient preferences on clinical health outcomes. This study contributes to the literature by estimating the benefits of patient-centered prescribing for long-term health outcomes, with patients experiencing less disease progression and fewer relapses, as well as gaining more QALYs.
According to this model, patient outcomes would be largely improved if prescribing practices were more aligned with patient preferences. On average, RRMS patients would experience reductions in relapses and disability progression, and improvements in quality of life compared with current prescribing practices. When examined over the entire RRMS population in the UK, this would lead to a total of almost 37,000 avoided relapses and over 44,000 discounted QALYs gained by RRMS patients over 50 years. In an alternative scenario analysis where only oral treatments were considered, as well as in sensitivity analyses based on total discounted QALYs, reported results were similar to those of the preference share base analysis. Varying input parameters on the preference share base analyses showed that the total number of relapses in the UK RRMS population was most sensitive to modeling ARR as a function of EDSS state, independent of disease duration, whereas average disability progression over time was most sensitive to mortality rate assumptions. This substantial increase in the number of relapses may reflect the correlation between ARR and long-term disability progression, a relationship that has been much debated in the literatureCitation80.
Although DMDs for RRMS have the potential to improve health outcomes for patients, a number of barriers to treatment exist as only 21% of individuals with MS in the UK currently receive DMDsCitation81. The NICE Quality Standards for MS recommend that patients receive care from a multidisciplinary team with MS expertise and undergo a comprehensive review of their treatment and care annuallyCitation82, yet it is estimated that 36% of patients with MS had not seen a neurologist in the past 12 months, with one in ten patients reporting that they had not seen a neurologist despite having a medical need to do soCitation82. Moreover, DMDs are typically only prescribed by neurologists, but the most common point of contact for patients with MS tends to be a MS specialist nurseCitation83. Patients may therefore not be receiving sufficient information regarding DMDs, which could explain the relatively low DMD utilization rate in the UK, as well as the discrepancy in market shares and patient preferences. Improving access to prescribers would improve the prognosis of RRMS patients in the UK.
Incorporating patient preferences into treatment decisions requires that prescribers engage in shared decision-making with patients. Previous research has indicated that most people with MS prefer to be more involved in their treatment decision, with 91% of patients preferring to make shared or autonomous decisionsCitation84. Prescribers often assume that they understand patients’ priorities, but previous research has shown that they are not always adept at judging the quality and quantity of information patients want when making medical decisionsCitation85–87, which may complicate shared decision-making. Even within the prescriber community, preferences may differ. For instance, an interview of DMD-prescribing neurologists in the UK revealed that prescribers in England generally viewed NICE guidelines as mandatory criteria they were obligated to follow, whereas neurologists in Scotland and Wales were more varied – some followed NICE guidelines strictly, while others exercised more flexibility in prescribing decisions, prioritizing patient welfareCitation88. Furthermore, neurologists tend to prescribe DMDs with which they are familiar and are influenced by prescribing cultures in their peer networkCitation88. Additional effort should be focused on improving shared decision-making in the UK through tools such as decision aids, multi-criteria decision analysisCitation89, and on physician education around newer, more efficacious treatments, so that prescribing practices sufficiently address patients’ needs.
There are several limitations to our study that should be considered. First, treatment efficacy estimates were based on clinical trials, rather than real-world data. Previous studies have shown that patient adherence to DMDs ranges from 47% to 93%Citation8,Citation11–14. To the extent that treatment adherence is lower and discontinuation more frequent in the real world compared to clinical trials, our model may overestimate effectiveness. Furthermore, our model may also overestimate effectiveness if medical and supportive care methods have improved in the time period since the clinical trials were conducted (assuming supportive care in clinical trials is equivalent to that in the real-world). Second, the benefits of patient-centered prescribing may be underestimated in our model as we did not take into account how patient-centered prescribing would affect outcomes when treatment adherence improves or patients switch therapies. For example, studies in other diseases areas have shown that patient-centered care may improve medication adherenceCitation90–92; this would be an additional benefit not captured in the model to the extent that it is translatable to MS. Third, certain analyses were not feasible given the limited evidence. For example, this study was not able to obtain the patient preference market share for ocrelizumab because the treatment attributes assessed in the DCE did not match the profile of ocrelizumab; this treatment was therefore excluded from our analysis. Additionally, disability progression for peginterferon beta-1a was not included in the indirect treatment comparisonCitation45 used to measure treatment efficacy within our Markov model (analysis was not feasible considering limited evidence); we, therefore, used a conservative estimate that efficacy was the same as BSC. As the market share and preference shares for peginterferon beta-1a are both <5%, our results would be qualitatively similar if we pooled peginterferon beta-1a effectiveness with other interferons. Fourth, our model was limited to patients with RRMS who were being treated with therapies approved for the treatment of RRMS. Patients with primary progressive MS and those treated with off-label and/or investigational agents were not considered in the model. Fifth, aggregating to the total number of individuals with RRMS in UK could be a potential overestimation as we do not incorporate those patients not on treatment at model start (100% of individuals in the cohort started on treatment). Finally, the model did not segment patients into subgroups based on disease activity and/or prognosis, which can influence access to the range of DMD options.
Conclusions
The population of patients with RRMS in the UK may experience overall health gains if patient preferences are incorporated into current prescribing practices. Based on our model projections, the UK RRMS population would avoid almost 37,000 relapses and gain discounted QALYs by more than 44,000 over a 50-year period if patient preferences were fully accounted for in prescribing decisions. Future research is needed to investigate the impact of real-world patient-centered prescribing on clinical, humanistic, economic, and societal outcomes.
Transparency
Declaration of funding
Financial support was provided by EMD Serono, Inc. (a business of Merck KGaA, Darmstadt, Germany) to Precision Health Economics to conduct this study.
Declaration of financial/other relationships
EvE, MB, RK, JM and JS are employees of Precision Health Economics, who received consultancy fees to conduct this study. SLW and AD are employees of EMD Serono, Inc. (a business of Merck KGaA, Darmstadt, Germany). LM was employed by EMD Serono, Inc. (a business of Merck KGaA, Darmstadt, Germany) at the time the study was conducted.
A peer reviewer on this manuscript has disclosed that they present talks involving all MS disease modifying treatments. A peer reviewer on this manuscript has disclosed that they have worked as a consultant or received travel grants from Biogen, TEVA, Genzyme, Merck, Roche and Novartis. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors were involved in the conception and design of the study, collection of the data, analysis and interpretation of the data, drafting of the article, and the provision of final approval of the article for submission/publication. All authors agree to be accountable for all aspects of this work.
Supplemental Material
Download MS Word (171.3 KB)Acknowledgements
The authors would like to thank Jason Allaire, PhD of Generativity Solutions Group for his assistance with editing this manuscript, funded by EMD Serono, Inc. (a business of Merck KGaA, Darmstadt, Germany).
References
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517.
- Luessi F, Siffrin V, Zipp F. Neurodegeneration in multiple sclerosis: novel treatment strategies. Exp Rev Neurother. 2012;12(9):1061–1077.
- Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952.
- Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. Eur Neurol. 2014;72(3–4):132–141.
- Jongen PJ. Health-related quality of life in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2017;31(7):585–602.
- Farber RS, Sand IK. Optimizing the initial choice and timing of therapy in relapsing-remitting multiple sclerosis. Ther Adv Neurol Disord. 2015;8(5):212–232.
- Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9:S5–S48.
- Johnson KM, Zhou H, Lin F, et al. Real-world adherence and persistence to oral disease-modifying therapies in multiple sclerosis patients over 1 year. JMCP. 2017;23(8):844–852.
- Grand'Maison F, Yeung M, Morrow SA, et al. Sequencing of disease-modifying therapies for relapsing-remitting multiple sclerosis: a theoretical approach to optimizing treatment. Curr Med Res Opin. 2018;34(8):1419–1430.
- Merkel B, Butzkueven H, Traboulsee AL, et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev. 2017;16(6):658–665.
- Duquette P, Yeung M, Mouallif S, et al. A retrospective claims analysis: compliance and discontinuation rates among Canadian patients with multiple sclerosis treated with disease-modifying therapies. PloS One. 2019;14(1):e0210417.
- Setayeshgar S, Kingwell E, Zhu F, et al. Persistence and adherence to the new oral disease-modifying therapies for multiple sclerosis: a population-based study. Mult Scler Relat Disord. 2019;27:364–369.
- Evans C, Marrie RA, Zhu F, et al. Adherence and persistence to drug therapies for multiple sclerosis: a population-based study. Mult Scler Relat Disord. 2016;8:78–85.
- Cohen BA, Coyle PK, Leist T, et al. Therapy optimization in multiple sclerosis: a cohort study of therapy adherence and risk of relapse. Mult Scler Relat Disord. 2015;4(1):75–82.
- Lizan L, Comellas M, Paz S, et al. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Prefer Adherence. 2014;8:1653–1664.
- Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30(2):89–100.
- Devonshire V, Lapierre Y, Macdonell R, for the GAP Study Group, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(1):69–77.
- Hao J, Pitcavage J, Jones JB, et al. Measuring adherence and outcomes in the treatment of patients with multiple sclerosis. J Am Osteopath Assoc. 2017;117(12):737–747.
- Burks J, Marshall T, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. CEOR. 2017;9:251–260.
- Yermakov S, Davis M, Calnan M, et al. Impact of increasing adherence to disease-modifying therapies on healthcare resource utilization and direct medical and indirect work loss costs for patients with multiple sclerosis. J Med Eco. 2015;18(9):711–720.
- Linker RA, Kieseier BC, Gold R. Identification and development of new therapeutics for multiple sclerosis. Trend Pharmacol Sci. 2008;29(11):558–565.
- Bottomley C, Lloyd A, Bennett G, et al. A discrete choice experiment to determine UK patient preference for attributes of disease modifying treatments in Multiple Sclerosis. J Med Econ. 2017;20(8):863–870.
- Carlin CS, Higuera L, Anderson S. Improving patient-centered care by assessing patient preferences for multiple sclerosis disease-modifying agents: a stated-choice experiment. PERMJ. 2017;21:16–102.
- Poulos C, Kinter E, van Beek J, et al. Preferences of patients with multiple sclerosis for attributes of injectable multiple sclerosis treatments in the United Kingdom and France. Int J Technol Assess Health Care. 2018;34(4):425–433.
- Garcia-Dominguez JM, Muñoz D, Comellas M, et al. Patient preferences for treatment of multiple sclerosis with disease-modifying therapies: a discrete choice experiment. PPA. 2016;10:1945–1956.
- Utz KS, Hoog J, Wentrup A, et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: a choice-based conjoint analysis. Ther Adv Neurol Disord. 2014;7(6):263–275.
- Wilson LS, Loucks A, Gipson G, et al. Patient preferences for attributes of multiple sclerosis disease-modifying therapies: development and results of a ratings-based conjoint analysis. Int J MS Care. 2015;17(2):74–82.
- Rieckmann P, Centonze D, Elovaara I, et al. Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: a combined perspective from the MS in the 21st Century Steering Group. Mult Scler Relat Disord. 2018;19:153–160.
- Yeandle D, Rieckmann P, Giovannoni G, et al. Patient power revolution in multiple sclerosis: navigating the new frontier. Neurol Ther. 2018;7(2):179–187.
- Ben-Zacharia A, Adamson M, Boyd A, et al. Impact of shared decision making on disease-modifying drug adherence in multiple sclerosis. Int J MS Care. 2018;20(6):287–297.
- Lugaresi A, Ziemssen T, Oreja-Guevara C, et al. Improving patient-physician dialog: commentary on the results of the MS Choices survey. Patient Prefer Adherence. 2012;6:143–152.
- Col NF, Solomon AJ, Springmann V, et al. Whose preferences matter? A patient-centered approach for eliciting treatment goals. Med Decis Making. 2018;38(1):44–55.
- Patient-Centered Outcomes Research Institute. Fiscal Year 2016 Annual Report. 2016.
- US Food and Drug Administration. Plan for Issuance of Patient‐Focused Drug Development Guidance. 2017.
- Centers for Medicare & Medicaid Services (CMS). CMS Quality Measure Development Plan: Supporting the Transition to The Quality Payment Program 2017 Annual Report. 2017.
- Jonker MF, Donkers B, Gossens L, et al. Summarizing patients’ preferences for the competitive landscape of multiple sclerosis treatment options. Medical Decision Making. In Press.
- Wilmington Healthcare. Specialist Patient Share – Multiple Sclerosis. 2018.
- Rosen HS, Small KA. Applied welfare economics with discrete choice models. Econometrica. 1981;49:105–130.
- Lancsar E, Savage E. Deriving welfare measures from discrete choice experiments: inconsistency between current methods and random utility and welfare theory. Health Econ. 2004;13(9):901–907.
- Train KE. Discrete choice methods with simulation. Cambridge, UK: Cambridge university press; 2003.
- Hettle R, Harty G, Wong SL. Cost-effectiveness of cladribine tablets, alemtuzumab, and natalizumab in the treatment of relapsing-remitting multiple sclerosis with high disease activity in England. J Med Eco. 2018;21(7):676–686.
- Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open. 2014;4(1):e004073.
- Tremlett H, Zhao Y, Rieckmann P, et al. New perspectives in the natural history of multiple sclerosis. Neurology. 2010;74(24):2004–2015.
- Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426.
- Siddiqui MK, Khurana IS, Budhia S, et al. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2018;34(8):1361–1371.
- National Institute for Health and Care Excellence. Beta interferon and glatiramer acetate for the treatment of multiple sclerosis. Technology appraisal guidance [TA32]. 2002.
- National Institute for Health and Care Excellence. Daclizumab for treating relapsing–remitting multiple sclerosis [TA441]. Technology appraisal guidance. 2017.
- National Institute for Health and Care Excellence. Ocrelizumab for treating relapsing–remitting multiple sclerosis [TA533]. 2018.
- National Institute for Health and Care Excellence. Cladribine tablets for treating relapsing–remitting multiple sclerosis [TA493]. 2017.
- Giovannoni G, Soelberg Sorensen P, Cook S, et al. Efficacy of cladribine tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: a post hoc analysis of the CLARITY study. Mult Scler. 2019;25(6):819–827.
- Hawton A, Green C. Health utilities for multiple sclerosis. Value Health. 2016;19(4):460–468.
- Orme M, Kerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10(1):54–60.
- Acaster S, Perard R, Chauhan D, et al. A forgotten aspect of the NICE reference case: an observational study of the health related quality of life impact on caregivers of people with multiple sclerosis. BMC Health Serv Res. 2013;13(1):346.
- National Institute for Health and Care Excellence. Alemtuzumab for treating relapsing–remitting multiple sclerosis [TA312]. Technology appraisal guidance. 2014.
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230.
- Phillips JT, Selmaj K, Gold R, et al. Clinical significance of gastrointestinal and flushing events in patients with multiple sclerosis treated with delayed-release dimethyl fumarate. Int J MS Care. 2015;17(5):236–243.
- Merck. Cost-effectiveness analysis of Mavenclad (cladribine tablets) in relapsing remitting mutliple sclerosis technical report. 2018.
- Jick SS, Li L, Falcone GJ, et al. Mortality of patients with multiple sclerosis: a cohort study in UK primary care. J Neurol. 2014;261(8):1508–1517.
- Coles AJ, Compston DA, Selmaj KW, CAMMS223 Trial Investigators, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–1801.
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–1828.
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet2012;380(9856):1829–1839.
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–1097.
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–1107.
- Kappos L, Radue E-W, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
- Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556.
- Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–914.
- Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976–1983.
- Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–1504.
- Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015;373(15):1418–1428.
- Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet. 2002;359(9316):1453–1460.
- Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
- O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–1303.
- National Institute for Health and Care Excellence. Teriflunomide for treating relapsing–remitting multiple sclerosis [TA303]. Technology appraisal guidance. 2014.
- National Institute for Health and Care Excellence. Dimethyl fumarate for treating relapsing–remitting multiple sclerosis [TA320]. Technology appraisal guidance. 2014.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013.
- Palace J, Duddy M, Bregenzer T, et al. Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator. Lancet Neurol. 2015;14(5):497–505.
- Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–1024.
- Mackenzie IS, Morant SV, Bloomfield GA, et al. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatr. 2014;85(1):76.
- Ruutiainen J, Viita AM, Hahl J, et al. Burden of illness in multiple sclerosis (DEFENSE) study: the costs and quality-of-life of Finnish patients with multiple sclerosis. J Med Eco. 2016;19(1):21–33.
- Kalincik T. Multiple sclerosis relapses: epidemiology, outcomes and management. a systematic review. Neuroepidemiology. 2015;44(4):199–214.
- European Multiple Sclerosis Platform. Multiple sclerosis in Europe 2015 [2019]. [cited 2020 Jan 6]. Available from: http://www.emsp.org/wp-content/uploads/2015/08/MS-in-EU-access.pdf.
- National Institute for Health and Care Excellence. NICE Guidance: Multiple Sclerosis 2016 [2019]. [cited 2020 Jan 6]. Available from: https://www.nice.org.uk/guidance/QS108/chapter/List-of-quality-statements.
- Multiple Sclerosis Society. My MS My Needs 2016: Access to treatment and health care technical report. 2016.
- Heesen C, Kasper J, Kopke S, et al. Informed shared decision making in multiple sclerosis–inevitable or impossible? J Neurol Sci. 2007;259(1-2):109–117.
- Brody DS. The patient's role in clinical decision-making. Ann Intern Med. 1980;93(5):718–722.
- Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Preven Med. 1999;17(4):285–294.
- Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? JAMA. 1984;252(21):2990–2994.
- Cameron E, Rog D, McDonnell G, et al. Factors influencing multiple sclerosis disease-modifying treatment prescribing decisions in the United Kingdom: a qualitative interview study. Mult Scler Relat Disord. 2019;27:378–382.
- Vermersch P, Martinelli V, Pfleger C, et al. Benefit-risk assessment of cladribine using multi-criteria decision analysis (MCDA) for patients with relapsing-remitting multiple sclerosis. Clin Ther. 2019;41(2):249–260.e18.
- Roumie CL, Greevy R, Wallston KA, et al. Patient centered primary care is associated with patient hypertension medication adherence. J Behav Med. 2011;34(4):244–253.
- Kahn KL, Schneider EC, Malin JL, et al. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45(5):431–439.
- Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Prac. 2008;20(12):600–607.