Abstract
Aim: To assess the cost-effectiveness of nivolumab monotherapy for recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) in the US.
Methods: We constructed a cohort-based partitioned survival model for three health states (progression-free, progressed disease, and death). Using overall survival and progression-free survival data from the nivolumab and investigator’s choice (IC) arms of the CheckMate 141 study, the proportion of patients in each health state was estimated by parametric modeling over a 25-year period. Cost, utility, adverse event, and disease management data inputs were obtained from relevant literature and applied to patients in each health state. A scenario analysis was conducted assuming increased uptake of subsequent immunotherapies. A one-way deterministic sensitivity analysis assessed the impact of variation in multiple parameters. A probabilistic sensitivity analysis in which probabilistic distributions were applied to each input during 1,000 model iterations was also conducted.
Results: Total costs incurred were higher with nivolumab ($101,552) than with IC ($38,067). Nivolumab was associated with a higher number of life-years (LY; 1.21) and quality-adjusted life-years (QALYs; 0.89), compared with IC (0.68 and 0.42, respectively). The incremental cost-effectiveness ratio for nivolumab compared with IC was $134,438 per QALY, and this remained qualitatively similar when increased uptake of subsequent immunotherapies was assumed ($129,603 per QALY). Sensitivity analyses supported these findings.
Conclusions: These results suggest that, at a willingness-to-pay threshold of $150,000 per QALY, nivolumab is a cost-effective option for therapy of SCCHN in the US.
Introduction
Head and neck cancers (inclusive of neoplasms of the oral cavity, pharynx, larynx, sinuses, and salivary gland) are major causes of cancer-related mortality in the US, with approximately 65,410 new cases and 14,620 deaths annuallyCitation1; an estimated 90% of all cases are squamous cell carcinoma of the head and neck (SCCHN)Citation2. The economic burden of recurrent/metastatic (R/M) SCCHN in the US is considerable, with estimated 6-month attributable costs of $20,000–60,000 per patientCitation3. Approximately three-quarters of patients diagnosed with SCCHN present with locoregionally advanced diseaseCitation4; despite curative treatment (generally with chemoradiotherapy and/or surgery), a substantial proportion of these patients will experience recurrence, progression or metastases.
In patients with R/M SCCHN, the addition of cetuximab to platinum-based chemotherapy in combination with fluorouracil (5-FU) as first-line therapy improves survivalCitation5, and until recently this regimen remained the standard of care first-line therapy in this settingCitation6,Citation7. More recently, programmed death receptor-1 (PD-1) antibodies such as pembrolizumab and nivolumab have been investigated in this clinical setting. These immune checkpoint inhibitors inhibit the programmed cell death 1 ligand (PD-L1)-mediated evasion of T-cell cytotoxicity exhibited by many tumor typesCitation8. The US Food and Drug Administration (FDA) recently approved pembrolizumab for first-line therapy of R/M SCCHN, either as monotherapy (in patients whose tumors express PD-L1), or in combination with platinum-based chemotherapy and 5-FUCitation9, based on observations of efficacy and safety from the KEYNOTE-048 trialCitation10.
In the second-line setting, pembrolizumab and nivolumab have demonstrated clinical efficacy and safety as therapy for SCCHNCitation11,Citation12. The randomized, open-label, phase 3 CheckMate 141 trial compared nivolumab with investigator’s choice (IC; methotrexate, docetaxel, or cetuximab) in patients with R/M SCCHN who had progressed after platinum-based chemotherapy, and reported that patients receiving nivolumab had a significantly longer overall survival (OS) (7.5 months) compared with those receiving IC (5.1 months), and 1-year survival was higher in the nivolumab group (36% vs 17%)Citation12. Based on these findings, in November 2016 the FDA approved nivolumab for the treatment of R/M SCCHN with disease progression on or after platinum-based therapyCitation13.
Given the high costs of these novel therapiesCitation14, rigorous cost-effectiveness analysis is crucial for payers to optimize healthcare spending. A recent cost-effectiveness analysis from a US healthcare system perspective assessed nivolumab for R/M SCCHN compared with other approved therapies (methotrexate, docetaxel, or cetuximab) and reported that nivolumab was cost-effective above a willingness-to-pay (WTP) threshold of $150,000Citation15; as only 15 months of published outcomes data were available at that time, the analysis used a time horizon of only 3 years. Guidance from the National Institute for Health and Care Excellence (NICE) recommends that cost-effectiveness evaluations should consider a “lifetime horizon” for patientsCitation16. Longer-term outcomes data have now been reported for CheckMate 141Citation17, and the 2-year survival rate of 17% in patients receiving nivolumab suggests that a 3-year time horizon may underestimate the value of nivolumab treatment over a longer period. Another analysis using a 30-year time horizon reported that nivolumab had an ICER of $294,400 compared with ICCitation18. Although that analysis used disease transition probabilities from CheckMate 141, corresponding utility values for progression-free (PF) and progressed disease (PD) were unavailable at that time and were instead derived from two different non-immunotherapy trials, with identical utilities applied to both the nivolumab and IC arms. Treatment-specific utility values from CheckMate 141 are now available for nivolumab and IC, and more accurately reflect the outcomes associated with each treatment. We assessed the incremental cost-utility of nivolumab for therapy of R/M SCCHN with disease progression on or after platinum-based therapy from a US healthcare system perspective, compared with the CheckMate 141 IC arm.
Methods
Design and structure
A cohort-based partitioned survival model was developed consisting of three mutually exclusive health states, representing the relevant primary stages of disease in R/M SCCHN: PF, PD, and death (). All patients were assumed to be PF at the start of the analysis. The proportion of patients in each health state was estimated by parametric modeling of OS and progression-free survival (PFS) data from the nivolumab and IC arms of CheckMate 141. Consistent with the design of CheckMate 141, the three individual agents comprising the IC arm were considered as an aggregate unit.
Figure 1. Schematic representation of partitioned survival model and disease health state transitions.
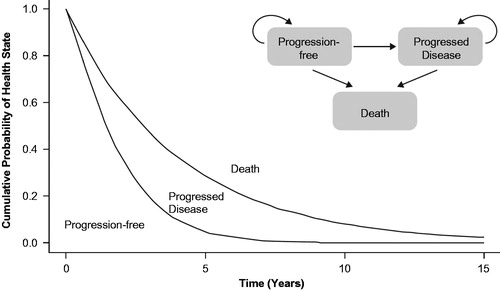
The process for fitting parametric survival curves to patient-level data was based on methodological guidance from the NICE Decision Support Unit, which advises that, if possible, the same parametric survival model should be selected when fitting independent parametric models to two arms for comparison when the proportional hazards assumption is not valid, as was the case for OS and PFS from CheckMate 141Citation16. Akaike and Bayesian Information Criterion goodness-of-fit statistics were used to identify the best-fitting survival models.
Model inputs
Incidence of adverse events (AEs) with nivolumab and IC were calculated using CheckMate 141 data reporting any grade 3, 4, or 5 AEs (i.e. AEs defined as severe, life-threatening/disabling, or causing death, respectively, according to “Common Terminology for Adverse Events v5.0”)Citation19 with an incidence of at least 5% in the nivolumab and IC armsCitation20 (Supplementary Table 1). Treatment-specific utilities for PF and PD health states were generated by applying a US population preference-weighting algorithmCitation21 to 3-level EuroQol 5-dimension health questionnaire (EQ-5D-3L)Citation22 data collected in CheckMate 141 (Bristol-Myers Squibb, data on file; OR NIVO 094, 2017) (Supplementary Table 2). As AEs were expected to occur within the first treatment cycle, disutility of AEs was applied to the PF health state, based on values obtained by systematic literature review (Supplementary Table 3).
Cost input parameters applied to the model included those related to drug acquisition and administration, monitoring, disease management, treatment of AEs, and subsequent treatments (Supplementary Table 4–9). When a patient was assumed to have died, an end-of-life care cost of $10,528.07 was applied (based on reported costs for renal cell carcinoma, as no published SCCHN-specific costs were identified)Citation23. Cost data for IC used in this analysis represented a mean of the individual agents used in the IC arm of CheckMate 141.
The base case analysis assumed that all treatments were administered until disease progression, in line with their respective FDA-approved prescribing information. The base case analysis also assumed that 0.6% of patients receiving IC would subsequently receive immunotherapy (based on observations from CheckMate 141). An annual discount rate of 3% was applied to all costs and outcomes.
Outcomes
Health state (PF, PD or death) occupancy was evaluated at 4-week intervals over the hypothetical 25-year duration of the model. Total healthcare costs and health outcomes were calculated by combining the cost, medical resource use, and utilities (EQ-5D-3L) assigned to each health state (PF and PD). Health outcomes included life-years (LYs) and quality-adjusted life-years (QALYs). Total costs represented the sum of costs for disease management, treatment acquisition, treatment monitoring, treatment of AEs, and subsequent treatments, and are reported in 2017 US$per patient.
Scenario and sensitivity analyses
To better reflect evolving clinical practice and increasing use of immunotherapies, a “scenario analysis” was conducted assuming that 30% of patients receiving IC would subsequently receive immunotherapy. As it is possible that the disutilities associated with AEs may partly drive the lower utilities observed with IC treatment compared with nivolumab, a second scenario analysis removed the disutilities associated with AEs from the model, to account for the possibility of AE disutilities being double-counted during IC therapy. One-way deterministic sensitivity analyses (DSA) were conducted assessing variation in the multiple parameters. Individual parameters used in the base case scenario were replaced with estimated low (minimum) and high (maximum) values for sensitivity analyses; the range used was based on ± standard error for utility values, and ±20% for all other inputs. To evaluate the impact of uncertainty on the estimated cost-effectiveness, a probabilistic sensitivity analysis (PSA) was conducted using probabilistic distribution of input values during 1,000 model iterations. PSA input values were estimated from multivariate normal distribution (for OS and PFS), gamma distribution (for disease management costs, acquisition costs, administration costs, monitoring costs, AE costs, other costs, and disutility of AEs), and beta distribution (for utility weights).
Results
After preliminary evaluation of OS and PFS data from CheckMate 141, neither met the assumption of proportional hazards. The most appropriate models were log normal (for OS) and generalized gamma (for PFS); these were therefore selected for use in the base case analysis. Parametric extrapolation of OS in patients receiving nivolumab was externally validated against 5-year survival data from the squamous non-small cell lung cancer cohort from the phase 1 b, open-label CheckMate 003 study and found to be reliable (Supplementary Table 10).
Base case analysis
Total costs incurred with nivolumab ($101,552) and IC ($38,067) were largely driven by treatment acquisition costs ($75,981 and $14,599 respectively) and disease management costs ($20,816 and $16,316, respectively) (). Nivolumab was associated with a higher number of QALYs (0.89) and LY (1.21), compared with IC (0.42 and 0.68, respectively). Incremental cost-effectiveness ratios (ICERs) indicated that the cost per additional QALY with nivolumab was $134,438 compared with IC in the base case scenario (). The ICER for life-years gained with nivolumab was $118,455 per life-year. When the probability of patients receiving immunotherapies (nivolumab or pembrolizumab) in subsequent lines of therapy was increased in the IC arm (to reflect improved access to these treatments after their approval), total costs for IC increased. Consequently, the ICER for nivolumab versus IC decreased slightly, but all ICERs remained qualitatively similar to the base case (i.e. the ICER for nivolumab was $129,603 per QALY and $114,194 per life-year, compared with IC). An additional scenario analysis in which AE-related disutilities were removed from the model yielded a cost per QALY of $141,806 consistent with the base case findings.
Table 1. Absolute value estimates of health outcomes and costs associated with each treatment in the model.
Table 2. Incremental gains with nivolumab vs investigator’s choice.
Deterministic sensitivity analyses
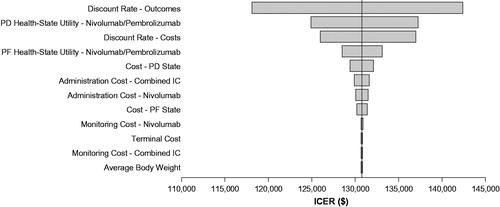
A tornado plot representing DSA for nivolumab compared with IC is presented as . The ICER for nivolumab versus IC did not change substantially with variation in individual parameters. ICERs were generally influenced most strongly by variation in discount rate on outcomes and costs, and health state utility values for nivolumab.
Figure 2. Deterministic sensitivity analysis of ICER response to variability of input parameters for nivolumab vs investigator’s choice arm. Abbreviations. ICER, incremental cost-effectiveness ratio; OS, overall survival; PD, progressed disease; PF, progression-free. Range of input variability: ±standard error for utility values; ±20% for all other inputs values.
Probabilistic sensitivity analyses
The results of the PSA for 1,000 model iterations are presented in . These results supported the findings from the base case analysis, with the ICER for nivolumab ($137,927) considered cost-effective compared with IC at a WTP threshold of $150,000. A scatter plot of individual model iterations during the PSA is presented in Supplementary Figure 1. At a WTP threshold of $150,000, 62.2% of PSA model iterations were deemed cost-effective; a cost-effectiveness acceptability curve describing this is presented in Supplementary Figure 2.
Table 3. Probabilistic sensitivity analysis of health outcomes and costs associated with each treatment in 1,000 model iterations.
Discussion
The findings from the base case analysis in our model suggest that, at the $150,000 per QALY threshold generally considered acceptable in the USCitation24, nivolumab would be cost-effective compared with IC (consisting of methotrexate, docetaxel, or cetuximab). PSA results were similar to the base case analysis, and found that the majority (>60%) of model iterations estimated the ICER to be less than $150,000. One-way DSA demonstrated that variations in discount for costs and outcomes, and utility values for PD and PF, had the highest impact on resultant ICER estimates. When increased use of immunotherapies subsequent to IC therapy was assumed, in keeping with contemporaneous clinical observations, the incremental costs between nivolumab and IC decreased slightly. Consequently, the ICER for nivolumab was less than $130,000 per QALY versus IC in this scenario.
Existing ICER thresholds continue to foment debate in the face of rising costs associated with novel therapiesCitation25, and vary greatly across countries in both their magnitude and the way they are appliedCitation26. The WTP threshold of $150,000 generally used in the US follows World Health Organization recommendations that the upper limit for cost-effectiveness of an intervention should be considered to be approximately three times gross domestic product per capitaCitation24. However, ICERs for oncology treatments are more than double those of non-oncology treatmentsCitation27, and recent oncology-specific studies have suggested that the “true” threshold should be considered to be above $150,000: surveys from academic oncologistsCitation28 and observational analyses of patient behaviorsCitation29 suggest that a threshold as high as $250,000 may be acceptable, particularly in the metastatic setting. Given the spiraling costs of healthcare in the US, which are approximately double that of other high income countries per capita, optimization of healthcare resources is increasingly important.Citation30 Initiatives such as the ASCO frameworkCitation31 have attempted to provide objective guidance for assessing the cost-effectiveness of therapies but may not reflect affordability or additional “value” of treatments (such as novel mechanisms of action, providing treatment options when few are currently available, or therapies for rare or high-morbidity conditions)Citation32,Citation33.
To the authors’ knowledge, two other analyses have used CheckMate 141 data to estimate the cost-effectiveness of nivolumab as therapy of R/M SCCHN from a US healthcare system perspective. Our findings broadly concur with those of Ward et al, who reported that nivolumab was cost-effective at a WTP threshold of $150,000 compared with ICCitation15. However, our model extends over a more appropriate time horizon (25 years) and is, therefore, more likely to capture the “lifetime” perspective of patients receiving nivolumab in US clinical practice. A further economic analysis reported that nivolumab would not be cost-effective at currently accepted thresholdsCitation18. That study used a longer-term time horizon (30 years), but drew utility values from older, non-immunotherapy trials, and applied identical utility values for PF and PD to both nivolumab and IC arms. Both of these studies excluded the likelihood of subsequent therapy in patients with progressed disease after platinum-based therapy. Following the approval of immunotherapies, patients now have improved treatment options following disease progression.
From a non-US approach, one analysis from the perspective of Canadian healthcare payers reported that the ICER for nivolumab compared with docetaxel was CAD $144,000 above the conventional WTP threshold of CAD $100,000 suggested by the authorsCitation34. However, by comparing nivolumab with docetaxel alone, these observations are unlikely to reflect US clinical practice. A further analysis from the perspective of the Swiss healthcare system assessed the cost-effectiveness of nivolumab compared with IC using a Markov model, and estimated that the ICER for nivolumab would be CHF 102,957, just above the authors’ proposed WTP threshold of CHF 100,000Citation35. It should be noted that both of these analyses used a 5-year time horizon and are therefore unlikely to represent the “lifetime” perspective for patients with R/M SCCHN. Indeed, in the latter publication, a scenario analysis extending the time horizon to 10 years found nivolumab to be cost-effective, with an estimated ICER of CHF 93,325Citation35. This illustrates the importance of considering the long-term benefit of immunotherapies in cost-effectiveness analyses; clinical trials have shown such treatments to be associated with delayed responses and “responder” subpopulations, both of which require sufficient time horizons to become apparentCitation36.
There are several limitations to this analysis. The probabilities of patients experiencing AEs were based on clinical trial data for only grade 3 or above AEs and AEs occurring in more than 5% of patients. Consequently, the presence of rare or low-grade AEs with nivolumab may be underestimated in the model. However, the contribution of AE treatment to total costs was minimal, and DSA did not identify AE treatment as having a strong influence on the resultant ICERs. Disutility of AEs was not reported in the CheckMate 141 trial; the disutilities used in the present model have therefore been taken from other published literature (as listed in Supplementary Table 3). Lastly, it should be noted that WTP thresholds are variable and no clear consensus on their implementation or interpretation is presently available; caution should be taken when considering the results from this analysis.
In conclusion, this analysis used survival models informed by data from the CheckMate 141 clinical trial to compare the cost-effectiveness of nivolumab versus IC for therapy of R/M SCCHN. Despite higher treatment costs compared with standard care (IC), nivolumab is associated with a considerable improvement in QALYs and LY gained, and is a cost-effective option for therapy of R/M SCCHN in the US.
Transparency
Declaration of funding
This study was sponsored by Bristol-Myers Squibb.
Declaration of financial/other relationships
Robert Haddad has received research funding and/or consulting fees from AstraZeneca, Bristol-Myers Squibb, Genentech, Glenmark, GSK, Immunomic, Kura, Loxo, Merck and Pfizer. Ezra E.W. Cohen has received research funding and/or consulting fees from Amgen, AstraZeneca, Bristol-Myers Squibb, EMD Serono, Incyte, Merck, and Pfizer. Meena Venkatachalam and Kate Young are employees of Parexel, which has received payment from Bristol-Myers Squibb for contracted analyses. Prianka Singh, James W. Shaw, Beata Korytowsky, and Pranav Abraham are employees and shareholders of Bristol-Myers Squibb. Kevin J. Harrington has received research funding and/or consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck-Serono, MSD, Oncolys, Pfizer, Replimune, Vyriad. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study design. MV and KY developed the model. All authors reviewed the model and data, and contributed critical revisions to the manuscript. All authors agree to be held accountable for the manuscript content, and approve the final version for publication.
Supplemental Material
Download MS Word (307.5 KB)Acknowledgements
Under direction of the authors, Martin Bell, PhD, of Evidence Scientific Solutions, Inc., provided writing assistance for this manuscript.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
- Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141.
- Kim Le T, Winfree KB, Yang H, et al. Treatment patterns and economic burden of metastatic and recurrent locally-advanced head and neck cancer patients. J Med Econ. 2012;15(4):786–795.
- SEER. Cancer stat facts: oral cavity and pharynx cancer. 2017. Available from: https://seer.cancer.gov/statfacts/html/oralcav.html.
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127.
- Magnes T, Egle A, Greil R, et al. Update on squamous cell carcinoma of the head and neck: ASCO annual meeting 2017. Memo. 2017;10(4):220–223.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: head and neck cancers. Version 1.2019 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- Ling DC, Bakkenist CJ, Ferris RL, et al. Role of immunotherapy in head and neck cancer. Semin Radiat Oncol. 2018;28(1):12–16.
- US Food and Drugs Administration. FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma 2019. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma.
- Rischin D, Harrington KJ, Greil R, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. 2019;37(15_suppl):6000–6000.
- Cohen EEW, Soulieres D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167.
- Ferris RL, Blumenschein G, Jr., Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867.
- US Food and Drugs Administration. Nivolumab for SCCHN 2016. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-scchn.
- da Veiga CRP, da Veiga CP, Drummond-Lage AP. Concern over cost of and access to cancer treatments: a meta-narrative review of nivolumab and pembrolizumab studies. Crit Rev Oncol Hematol. 2018;129:133–145.
- Ward MC, Shah C, Adelstein DJ, et al. Cost-effectiveness of nivolumab for recurrent or metastatic head and neck cancer. Oral Oncol. 2017;74:49–55.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data. NICE Decision Support Unit Technical Support Documents. London 2013. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf
- Ferris RL, Blumenschein G, Jr., Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51.
- Tringale KR, Carroll KT, Zakeri K, et al. Cost-effectiveness analysis of nivolumab for treatment of platinum-resistant recurrent or metastatic squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 2018;110(5):479–485.
- National Cancer Institute Cancer Therapy Evaluation Program (CTEP). Common Terminology Criteria for Adverse Events (CTCAE) v5.0 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
- Venkatachalam M, Bobiak S, Shaw JW, et al. Estimated costs of treatment-related adverse events (TRAEs) for recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) in the checkmate 141 trial. Ann Oncol. 2017;28(suppl_5)1056P
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220.
- EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
- Perrin A, Sherman S, Pal S, et al. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ. 2015;18(3):200–209.
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124.
- Carrera P, IJzerman M. Are current ICER thresholds outdated? Valuing medicines in the era of personalized healthcare. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):435–437.
- Schwarzer R, Rochau U, Saverno K, et al. Systematic overview of cost-effectiveness thresholds in ten countries across four continents. J Comp Eff Res. 2015;4(5):485–504.
- Bae YH, Mullins CD. Do value thresholds for oncology drugs differ from nononcology drugs? J Manag Care Spec Pharm. 2014;20(11):1086–1092.
- Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11(2):90–95.
- Seabury SA, Goldman DP, Maclean JR, et al. Patients value metastatic cancer therapy more highly than is typically shown through traditional estimates. Health Aff (Millwood). 2012;31(4):691–699.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039.
- Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American society of clinical oncology value framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34(24):2925–2934.
- Dubois RW. Cost-effectiveness thresholds in the USA: are they coming? Are they already here? J Comp Eff Res. 2016;5(1):9–11.
- Neumann PJ, Cohen JT. Measuring the value of prescription drugs. N Engl J Med. 2015;373(27):2595–2597.
- Zargar M, McFarlane T, Chan KKW, et al. Cost-effectiveness of nivolumab in recurrent metastatic head and neck squamous cell carcinoma. Oncologist. 2018;23(2):225–233.
- Hirschmann A, Lupatsch JE, Schwenkglenks M, et al. Cost-effectiveness of nivolumab in the treatment of head and neck cancer. Oral Oncol. 2018;87:104–110.
- Chen TT. Statistical issues and challenges in immuno-oncology. J Immunother Cancer. 2013;1:18.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. 2014; Available from: https://hcupnet.ahrq.gov/#setup.
