Abstract
Objectives: To describe direct medical costs associated with each line of treatment among metastatic colorectal cancer (mCRC) patients in China.
Methods: Electronic medical records between 2011 and 2016 were extracted from 12 tertiary hospitals in China for adult patients who initiated third-line treatment at least nine months before the end of data collection. Direct medical costs included costs of wards, diagnostic tests, surgical procedures, special materials, drugs and others. Costs were assessed by line of treatment, and drug costs were further breakdown for patients receiving chemotherapy alone and those receiving chemo- and biologics-combined therapy.
Results: Of the 404 mCRC patients, the mean age was 55 years old and 62% were male. Oxaliplatin- and irinotecan-based regimens dominated first- and second-line treatment, respectively (44 and 37%). From first- to second- to third-line, the proportion of patients receiving targeted biologics increased from 18% at first-line and 12% at second-line to 34% at third-line; median number of treatment cycles reduced from 6 to 4 and to 2. The corresponding mean direct medical costs per person per cycle increased from $2,514 to $2,678 to $5,121. Mean drug costs per cycle increased from $2,314 to $2,673 to $4,316 among patients receiving chemotherapy alone and from $3,245 to $2,717 to $6,533 among patients receiving chemo- and biologics-combined therapy.
Conclusions: Before 2017, mCRC patients in China did not receive the maximum benefits of precision medicine breakthroughs. Reduced treatment cycles and increased costs per cycle from first- to third-line suggested poor healthcare resource utilization. With earlier initiation and more treatment cycles, targeted biologics may better demonstrate their effectiveness among Chinese patients. Our findings reflected the urgent need to increase drug accessibility in China before 2017 and underscore that including innovative biologics into Chinese health insurance plans can reduce patients’ economic burden and improve the management of mCRC.
Introduction
Colorectal cancer (CRC) ranks as the second most common cancer in China, with an estimated incidence rate of 36.6 per 100,000 population in 2018Citation1. Previous studies showed that the average five-year overall survival rate of patients diagnosed with CRC was 57%, and the rate fell below 20% for patients at stage IVCitation2,Citation3. With such a low survival rate, CRC is the fifth leading cause of cancer-related deaths in China, resulting in over 245,000 deaths in 2018Citation1. Screening for CRC is a cost-effective prevention and control strategy; however, the screening rates among the Chinese are sub-optimalCitation4,Citation5. Consequently, CRC is often diagnosed at a later stageCitation6 due to the absence of symptoms or the reluctance of populations to be involved in screening programsCitation5. Metastatic CRC (mCRC) patients are often harder to treat and have a poorer prognosisCitation7. The five-year survival rate among patients diagnosed with mCRC was about 14%, which was considerably lower than patients diagnosed at an earlier stageCitation7. Although surgical resection is one of the most effective treatments for CRC, almost four in every five mCRC are unresectableCitation8. Without any treatment, the five-year survival rate of patients with unresectable mCRC is only 0.9%Citation8.
For patients with unresectable mCRC, chemotherapy is usually used to prolong their survival and improve the quality of lifeCitation9. The combination of chemotherapy and targeted therapy can even further improve progression-free survival (PFS) and overall survival (OS) in patients with mCRCCitation9. Therefore, the China CRC treatment guidelines recommend FOLFOX, FOLFIRI, CapeOx, cetuximab or bevacizumab combined therapy as first- or second-line treatment of mCRCCitation10. For third-line treatment, 2017 China guidelines recommend regorafenib and irinotecan combined with cetuximab; however, these treatment options were not available yet during our study period.
Despite their clinical benefits, chemotherapeutic and biologic agent combinations have not been widely used as first- and second-line treatments in China partially due to their high costs. A cross-sectional study in China between 2012 and 2014 reported that even with a 46.5% reimbursement ratio, Chinese mCRC patients still had to spend 59.9% of their previous year’s household income, resulting in 75.0% of the families perceiving these treatments as an unmanageable burdenCitation11. The situation is even worse for patients at later stages of the disease, with patients at stage IV experiencing the highest financial pressureCitation11,Citation12.
In this study, we aimed to describe the disease burden of mCRC with regards to direct medication costs by line of treatment and consequently explore unmet treatment needs for Chinese mCRC patients using real-world data in China.
Methods
Data source
Data on mCRC patients between 1 January 2011 and 30 September 2016 were extracted from a multi-center oncology database, which collected information from electronic medical records (EMRs) of multiple tertiary hospitals in China. To obtain good geographic coverage and a sufficient number of patients, we selected a sample of 12 hospitals from three directly-administered municipalities (Beijing, Shanghai, and Chongqing) and six provinces (Jiangsu, Zhejiang, Guangdong, Sichuan, Fujian, and Shaanxi).
Study population
Patients who had a primary diagnosis of mCRC between 1 January 2011 and 1 January 2016, were aged 18 or older at primary diagnosis and had evidence of receiving all three lines of treatment were included. To ensure a full observation period of third-line treatment, all patients had a minimum of nine months of observation time since the initiation of third-line treatment. This is because a previous phase III clinical trial suggested that the maximum progression-free-survival time of mCRC patients receiving third-line treatment was nine monthsCitation13.
Patients with mCRC were defined as those who were classified as TNMCitation14 stage IV at primary diagnosis, and those whose tumor metastasized before the database lock (30 September 2016). Primary diagnosis dates (baseline) were defined as the first clinical or pathological diagnosis dates recorded in the selected hospital’s EMRs, whichever occurred earlier.
Study variables
Patient demographics and clinical characteristics at primary diagnosis were analyzed for the mCRC population. As the first appearance of metastases was not recorded in EMRs, dates of metastasis were defined by the dates for initiation of palliative chemotherapy. Treatment patterns of first-, second- and third-line palliative chemotherapy were analyzed, with regimens and cycles reported. Chemotherapeutic lines were determined by physicians and recorded in EMRs. Direct medical costs included costs of wards, diagnostic tests, surgical procedures, special materials, drugs and others. Costs were assessed by line of treatment, and drug costs were further breakdown for patients receiving chemotherapy alone and those receiving combined therapies including both chemotherapy and targeted biologics.
Statistics
For continuous variables, mean, standard deviation (SD), median, interquartile range (IQR), minimum (min) and maximum (max) values were presented as appropriate; for categorical variables, the numbers of missing values, frequency distributions and percentages were presented. Missing data were excluded in percentage calculations. All statistical analyses were performed using STATA version 14.0 (Stata Corporation, Texas, USA).
The direct medical costs were originally recorded in RMB in EMRs, and all were inflated to 2016 RMB during calculation. To make it more explicit and comparable with other studies, we have conserved it to US dollars by multiplying the exchange rate which was 0.145 in 2016.
Ethics approvals
De-identification of personal information was performed at the data entry stage in the multi-center oncology database. Ethics approvals were obtained for this study from selected hospitals.
Results
Baseline characteristics
In total, 404 patients with mCRC meeting the inclusion criteria were identified. The average age of mCRC patients at the primary diagnosis date was 55 ± 12 years old. Of the 404 mCRC patients, 62% were male, 85% presented in internal medicine departments, 44% had a KRAS mutation testing, and 98% were physically well at baseline (ECOG PS = 0–1). The majority of CRC patients had left-sided primary tumor sites (64%), with the average primary tumor size being 4.3 ± 2.3 cm (). Of patients who were diagnosed as stage IV at first visit (339), 56% had liver metastasis (191).
Table 1. Baseline characteristics of mCRC patients.
Treatment patterns and sequences
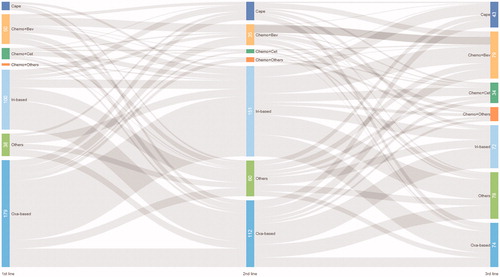
Fluorouracil, leucovorin, and oxaliplatin (FOLFOX) and other oxaliplatin-based regimens were most frequently administered in first-line treatment (179/404, 44%), followed by fluorouracil, leucovorin, and irinotecan (FOLFIRI) and other irinotecan-based regimens (25%) (). These two regimens accounted for 28% and 37% for second-line treatment, respectively (). At third-line, there were still 66% of mCRC patients receiving chemotherapy alone including 36% (146/404) receiving oxaliplatin-based and irinotecan-based chemotherapy. Only 34% received combined chemotherapy and targeted biological treatment as third-line treatment (). Among patients receiving targeted biologics, bevacizumab was the dominant choice of biotherapy for all three lines (50/73, 68%; 35/50, 70%; and 79/137, 58% in first-, second- and third-lines, respectively) ().
Figure 1. Treatment patterns of palliative chemotherapy for mCRC patients. (A) First-line treatment (n = 404). (B) Second-line treatment (n=404). (C) Third-line treatment (n=404).
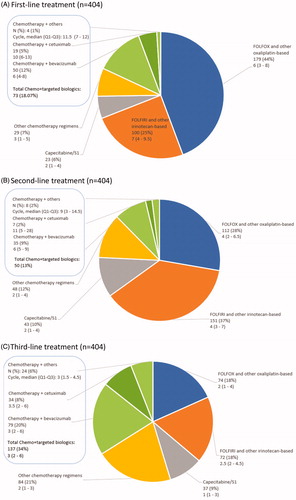
Table 2. Treatment pattern of palliative chemotherapy for mCRC patients.
Treatment duration decreased in later lines of treatment. Median number of cycles were six in first-line, four in second-line and two in third-line treatment (); in addition, at least half of the patients reported disease progression as the reason for switching (). The majority of patients switching between two common chemotherapy regimens: 25% (100/404) switched from FOLFOX and other oxaliplatin-based regimens at first-line to FOLFRI and other irinotecan-based regimens at second-line (), followed by 12% of the patients receiving the reverse sequence; 8.9% (36/404) switched from FOLFIRI and other irinotecan-based regimens at second-line to FOLFOX and other oxaliplatin-based regimens at third-line, followed by the reverse sequence (8.7%).
Table 3. Reasons for line switching.
Direct medical costs
The mean direct medical costs per patient per cycle increased from $2,514 (SD: 2,599) for first line to $2,679 (SD:5,383) for second-line and $5,121 (SD:7,785) for third-line. The mean cost per cycle for chemotherapy alone was $2,314 (SD: 2,603)) for first-line and $2,673 (SD: 5,728) for the second-line, respectively (). The cost increased to $4,316 (SD: 5,109) for the third-line treatment. Similarly, the mean direct medical cost per cycle for combined chemotherapy and targeted biologics was also significantly higher for the third-line compared with that for first- and second-line treatments ($6,534 [SD: 10,898] for the third-line vs. $2,717 [SD: 2,347] for the second-line and $3,245 [SD: 2,473] for first-line).
Table 4. Direct medical cost by types of regimens.
Discussion
Using real-world data in China, this study described the characteristics of mCRC patients, treatment sequences and direct medical costs of each line of treatment. We found that in line with the 2014 Asian consensus on mCRCCitation15 and 2017 Chinese Protocol of Diagnosis and Treatment of Colorectal CancerCitation10, oxaliplatin-based regimens, and irinotecan-based regimens was the dominated treatments at first-line and second-line, respectively. In contrast, there was no dominated chemotherapy regimen at third-line. The proportion of patients receiving chemotherapy and targeted biological combined therapy was low for all three lines (18, 13 and 34%, respectively). Among those receiving combined therapy, bevacizumab and cetuximab were the two main biologics accounting for over 80% at each line. Of patients receiving combined therapy at first- and second- lines, about half switched to chemotherapy alone for subsequent lines. At third-line treatment, some patients only received combined therapy for no more than three cycles and then switched likely due to disease progression. Direct medical costs per cycle were higher at third-line regardless of regimen type.
Our study has highlighted two important findings in relation to mCRC patients in China between 2011 and 2016. The relatively low proportion of patients using targeted biologics in China between 2011 and 2016 and the high costs of targeted biologics may limit patients’ accessibility to combined therapies. This reflected the gap between clinical practices and national guidelines on the use of biologics in China. Multiple clinical trials have confirmed that, compared with chemotherapy alone, chemotherapy combined with targeted therapy, such as bevacizumabCitation16 and cetuximab, significantly improved PFS and OS in patients with mCRCCitation17. As a result, chemotherapy- and targeted biologics-combined therapies have been recommended as first-line treatment to mCRC patients in two recent guidelinesCitation10,Citation18. Observational studies have also provided real-world evidence that earlier use of targeted biologics is beneficial and tolerableCitation19. In the US and Europe, observational studies show that 45–77% of mCRC patients received a targeted biologic as part of their first-line treatment, and more than 70% of patients received biologics at any line of treatmentCitation20–22. In contrast, we found that only a small proportion of mCRC patients in China ever received chemotherapy and biologics combined regimens and an even smaller proportion of mCRC patients received combined regimens at first-line. In addition, among those receiving combined therapy at third-line, the vast majority stopped after only three cycles. These findings raise a major concern about the delayed initiation of combined therapy among Chinese mCRC patients, which may consequently lead to suboptimal treatment outcomes. Given the high cost of combined therapy, delayed initiation may not only lead to low effectiveness but also low cost-effectiveness. Secondly, our results showed that, despite receiving targeted biologics as first-line treatment, when the disease progressed, patients might have to switch back to chemotherapy alone due to limited options of biologics. Since 2017, there have been some promising policy changes for mCRC treatment, especially concerning third-line treatments. The Centre for Drug Evaluation, China Food and Drug Administration (CDE, CFDA) has established Priority Review and Conditional Approval to expedite market launches of clinically urgent and effective drugs. Bevacizumab (Avastin, South San Francisco, Genentech, USA) has been included in the national reimbursement list. Regorafenib (Stivarga, Berlin, Bayer, Germany) and Fruquintinib (Elunate, HongKong, Chi-Med, China) were approved in China’s market. Along with these new drugs going on the market, in January 2018, the Pan-Asian ESMO Consensus Guidelines for the Management of mCRC Patients recommended cytotoxics and targeted biologics in first- and subsequent-line treatments unless contraindicatedCitation23. Real-world evidence from this study implies unmet needs in treatment and highlights the need to optimize treatment sequences following recently updated clinical guidelines, particularly for the earlier use of targeted biologics and for increasing third-line treatment options.
There are a few limitations to this study. First of all, the study retrospectively selected mCRC patients from 12 hospitals and only 404 patients were eligible and included in our analyses. However, representativeness analyses found that the demographics of mCRC patients excluded due to missing key outcome information were not significantly different from patients included in this study. Similarities between included and excluded patients suggested low selection bias and consequently a marginal impact on the study results. Secondly, patients included in the current study were those who had received all three lines of therapy, which may lead to an overestimation of the costs of first- and second-line. This is because patients might be died during the first two lines of therapy or decided to stop treatment altogether because of toxicities or other reasons, for instance, financial reason. However, the current study aims to explore direct medical costs of patients who received three lines of therapy, and the main reason for switching line of therapy is disease progression (), it is unlikely that the overestimation of costs of first- and second-line therapies would have a substantial impact on the overall conclusion. Thirdly the study did not consider the indirect costs due to the data availability issue. Indirect costs, such as the productivity losses due to illness could provide more insights into the economic burden of patients with mCRC. Without data on patients’ household income and their insurance types, we could not be able to calculate the costs borne by patients and their families. To better understand the economic burden of mCRC treatment, different cost models should be integrated into future studies.
Conclusions
In conclusion, during our study period, patients in China did not receive the maximum benefits of innovative targeted biologics most likely due to limited biological options on the Chinese market, high costs of biologics, unpromising effectiveness and a lack of established third-line treatment guidelines. With earlier initiation and more treatment cycles, targeted biologics may better demonstrate their effectiveness among Chinese patients. Our findings reflected the urgent need to increase drug accessibility in China before 2017 and underscore that including innovative biologics into Chinese health insurance plans can reduce mCRC patients’ economic burden and improve the management of mCRC.
Transparency
Declaration of funding
This study was funded by Lilly Suzhou Pharmaceutical Company Limited.
Declaration of financial/other relationships
No author relationships to be declared. JME peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Author contributions
Ruihua Xu designed the study, Cike Peng conducted the analysis, the remaining authors edited the manuscript. All authors discussed the results and contributed to the final manuscript.
Acknowledgements
We thank Medbanks for data provision.
References
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer [cited 2019 Jan 20]. Available from: https://gco.iarc.fr/today.
- Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6(5):e555–e567.
- Yuan Y, Li MD, Hu HG, et al. Prognostic and survival analysis of 837 Chinese colorectal cancer patients. WJG. 2013;19(17):2650–2659.
- Koo JH, Leong RWL, Ching J, et al. Knowledge of, attitudes toward, and barriers to participation of colorectal cancer screening tests in the Asia-Pacific region: a multicenter study. Gastrointest Endosc. 2012;76(1):126–135.
- Leung DY, Chow KM, Lo SW, et al. Contributing factors to colorectal cancer screening among Chinese people: a review of quantitative studies. IJERPH. 2016;13(5):506.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics. CA Cancer J Clin. 2017;67(3):104–117.
- American Cancer Society. Survival rates for colorectal cancer by stage. American Cancer Society [cited 2018 Nov 2]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html.
- René A, Emir H, Luis César B. Evolution of neoadjuvant therapy for extended hepatic metastases–have we reached our (non-resectable) limit? J Surg Oncol. 2015;102(8):922–931.
- Zhang Y, Chen Z, Li J. The current status of treatment for colorectal cancer in China: a systematic review. Medicine. 2017;96(40):e8242.
- Hospital Authority of National Health and Family Planning Commission of the People′s Republic of China, Chinese Society of Oncology. Chinese protocol of diagnosis and treatment of colorectal cancer (2017 edition). Zhonghua Wai Ke Za Zhi. 2018;56(4):241–258.
- Huang HY, Shi JF, Guo LW, et al. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: a hospital-based, multicenter, cross-sectional survey. Chin J Cancer 2017;36(8):41.
- Liu CC, Huang HY, Shi JF. Economic burden of colorectal cancer in China from 1996 to 2015: a systematic review. Zhongguo Zhong Liu. 2017;26:859–867.
- Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA 2018;319(24):2486.
- Ueno H, Mochizuki H, Akagi Y, et al. Optimal colorectal cancer staging criteria in TNM classification. J Clin Oncol. 2012;30(13):1519–1526.
- Adaptation of international guidelines for metastatic colorectal cancer: an Asian consensus. Clin Colorectal Cancer 2014;13(3):145–155.
- Ranhua C, Shuai Z, Dedong M, et al. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med Oncol. 2015;32(1):325.
- Volker H, Ludwig Fischer VW, Thomas D, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075.
- Department of Medical Administration of the Ministry of Health of the People’s Republic of China. Chinese protocol of diagnosis and treatment colorectal cancer (2010 edition). Zhongguo Yi Xue Qian Yan Za Zhi. 2011;3(6):130–146.
- Zhu LM, Zhao YZ, Ju HX, et al. Efficacy and safety of bevacizumab in Chinese patients with metastatic colorectal cancer. Asian Pac J Cancer Prev. 2014;15(16):6559–6564.
- Abrams TA, Meyer G, Schrag D, et al. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106(2):djt371–djt371.
- Hess GP, Peter Feng W, David Q, et al. Systemic therapy for metastatic colorectal cancer: patterns of chemotherapy and biologic therapy use in US medical oncology practice. JOP. 2010;6(6):301–307.
- Carlomagno C, Stefano AD, Rosanova M, et al. Multiple treatment lines and prognosis in metastatic colorectal cancer patients. Cancer Metastasis Rev. 2019;38(1–2):307–313.
- Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer; a JSMO–ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70.