Abstract
Objective: We determined the percentage of patients with severe asthma and exacerbations and evaluated the costs of the disease based on blood eosinophil counts.
Methods: A retrospective observational study based on the review of medical records in Spain was carried out. Patients ≥18 years of age requiring care during the years 2014–2015; diagnosed with asthma with at least 2 years of continuous records (at least one year prior to the index date defined as the first asthma medication prescription and at least one year after the index date) were included. Study groups: eosinophil counts <300 cells/μl and ≥300 cells/μl. Main variables: comorbidity, clinical parameters, exacerbations and annual asthma total costs.
Results: A total of 268 severe asthmatic patients in Spain were included, representing 6.3% of the asthma population, with 58.6% having eosinophil count ≥300 cells/μl and 41.4% eosinophil count <300 cells/μl. The mean age was 56.1 years (63.4% women). Patients with eosinophilic inflammation (≥300 cells/μl) had lower FEV1 values (54.3% vs. 60.7%; p < .001), poorer treatment adherence (65.6% vs. 77.3%; p < .001), and a greater mean number of exacerbations (3.3 vs. 1.9; p < .001). Exacerbations were correlated to FEV1 (β=‒.606), eosinophils (β = .255), immunoglobulin E (β = .152), and age (β = .128), p < .001. The mean total asthma annual cost (ANCOVA) was 6222 vs. 4152 euros, respectively (p = .016). Health costs were associated with age (β = .323), FEV1 (β = .239), eosinophils (β = .177) and exacerbations (β = .158), p < .01.
Limitations: Those inherent to retrospective studies; the possible inaccuracy of diagnostic coding referring to severe asthma and other comorbidities and the external validity of the results.
Conclusions: Health costs of patients with severe asthma were high. Total annual asthma costs and resource use were greater in patients with ≥300 cells/μl. Age, eosinophilia, exacerbations and FEV1 were associated with greater resource utilization and costs for the health system.
Introduction
Asthma is a chronic inflammatory disease of the airways characterized by the intervention of different cells and inflammatory mediators (partly conditioned by genetic factors), with bronchial hyper-responsiveness and variable airflow obstruction that is fully or partially reversible either spontaneously or through the action of drugsCitation1–3. The prevalence of asthma as reported in the literature is approximately 10% of the adult populationCitation2,Citation4.
The objective of the management of severe asthma is to achieve symptom control and lessen the risk of exacerbationsCitation2,Citation3,Citation5. Severe asthma is a heterogeneous syndrome with multiple phenotypes and accounts for approximately 5% of all cases of asthmaCitation2,Citation5,Citation6. In Spain, the estimated prevalence of uncontrolled persistent asthma is 3.9%, and the affected patients have an average of 4.3 exacerbations a yearCitation7. Studies have described phenotypes related to disease severityCitation8–10. Peripheral blood eosinophilia has been regarded as an inflammatory marker of disease exacerbation, with a positive correlation between severe allergic asthma and poor disease controlCitation3,Citation6–10. In general, asthma is one of the most common reasons for medical consultationCitation2,Citation4.
In Spain, the estimated cost of asthma is 1480 million euros a year, with an annual cost per asthmatic patient of about €1726. In patients with severe asthma, this figure may increase to 2635 euros a yearCitation11. Severe asthma is an important economic burden for the National Health System, the affected individuals and their families, and society in generalCitation1–4,Citation11–14. There is only limited evidence on the relationship between eosinophilia, disease exacerbation and their impact on resource use and related costs. In addition, there is a growing need to conduct naturalistic studies under conditions of routine clinical practice (real life scenario). The present study, therefore, may be relevant in this regard. The objective of this study was to assess the proportion of patients with severe asthma and the disease exacerbation rate, and to evaluate the direct and indirect costs (lost productivity), based on blood eosinophil counts, under conditions of routine clinical practice.
Methods
Study design and population
A retrospective multicenter, longitudinal observational study was carried out based on the review of medical records (electronic databases containing dissociated data). The study population was drawn from the electronic records of different primary care (PC) centers pooled within the anonymized database of the RedISS (Red de Investigación en Servicios Sanitarios) Foundation in Catalonia. The data came from electronic medical records and other complementary databases. The population attended by these PC centers was primarily of an urban and industrial nature, with a medium to low socioeconomic level.
Inclusion and exclusion criteria
The study included all patients diagnosed with asthma requiring care between 1 January 2014 and 31 December 2015 (index date/inclusion period) who met the following characteristics: (a) age ≥18 years; (b) guaranteed follow-up (> 2 contacts, except in the event of death); (c) enrolment in the prescriptions program (with documented daily dosage, time interval and duration of each treatment provided); and (d) asthma diagnosed at least 24 months before the inclusion date. The exclusion criteria were: (a) patients transferred to other centers or outside the center recruitment area; (b) permanently institutionalized subjects; (c) patients with a history of chronic obstructive pulmonary disease, emphysema, chronic bronchitis, cystic fibrosis, lung cancer, bronchiectasis or pulmonary fibrosis; (d) patients with actively treated or advanced cancer; and (e) terminally ill or palliative care patients. The study comprises a 2-year period inclusion period (2014 and 2015), as it was planned and approved by the ethics committee in 2017. The baseline-index period 2014–2015 was the most recent with complete data available in 2017.
Study groups and patient follow-up period
Two study groups were distinguished based on blood eosinophil counts (eosinophilia): (a) <300 cells/µl and (b) ≥300 cells/µl. We compiled the eosinophil counts closest to the index date (resulting from the last blood test made). Patients were followed for one year from the index date.
Asthmatic patients, severe asthma, exacerbation and other variables of interest
Patient records were obtained from the International Classification of Primary Care (ICPC-2; R93)Citation15, and/or the International Classification of Diseases (9th edition) – Clinical Modification (ICD-9-CM; 493.x for the disease and/or exacerbations). Severe asthma was classified according to the international ERS/ATS guidelines on the definition, evaluation and treatment of severe asthmaCitation3, and therapy was assessed according to the recommendations of the Spanish Asthma Management Guide (GEMA) steps 5–6Citation2 (inhaled corticosteroids [ICs] and an additional controller medication during the 12 months prior to the inclusion date).
Exacerbation was defined as an event in the natural course of the disease characterized by worsening of the disease identified by a progressive increase in shortness of breath, breathlessness, wheezing, cough and chest tightness, or a combination of all these symptoms, secondary to severe airflow obstruction, and severe exacerbation as hospitalization due to asthma. The electronic medical records yielded the information corresponding to each exacerbation and the time from diagnosis (in years). In addition, we obtained from electronic medical records: body mass index (BMI, kg/m2), lung function (FEV1, %) and blood total immunoglobulin E (IgE) levels. The value closest to the date of inclusion was obtained.
Sociodemographic variables and comorbidity
We recorded patient age and gender, deaths, as well as the personal history obtained from the ICPC-2Citation15 referring to hypertension (K86, K87), diabetes mellitus (T89, T90), dyslipidemia (T93), obesity (T82), active smoking (P17), alcoholism (P15, P16), all types of organ failure (cardiac, hepatic and renal), ischemic heart disease (K74, K97, K75), cerebrovascular accident (K90, K91, K93), dementia or memory disorders (P70, P20), neurological disorders: Parkinson’s disease (N87), epilepsy (N88), multiple sclerosis (N86) and other neurological diseases (N99), depressive syndrome (P76), and malignancies (all types: A79, B72-75, D74-78, F75, H75, K72, L71, L97, N74-76, T71-73, U75-79, W72-73, X75-81, Y77-79). Data on the history of allergic rhinitis and nasal polyposis were also collected. As a general comorbidity summarizing variable for each patient, we recorded: (a) the number of chronic disease diagnoses; (b) the Charlson comorbidity indexCitation16 as an estimator of patient severity; and (c) the individual case index obtained from the Adjusted Clinical Groups (ACGs): a patient classification system based on resource consumptionCitation17. The ACGs in turn yield resource utilization bands (RUBs), allowing each patient to be grouped according to general morbidity into one of 5 mutually exclusive categories (1: healthy users or with very low morbidity; 2: low morbidity; 3: moderate morbidity; 4: high morbidity; and 5: very high morbidity).
Medication administered and adherence/compliance
The medicinal products (drug substances) indicated for the treatment of asthma were compiled according to the ATC (Anatomical Therapeutic Chemical Classification System)Citation18. The information was obtained from drug dispensing records. The choice of medication for a given patient was decided by the supervising physician (clinical practice). The medications prescribed during the follow-up period (one year) were compiled. Compliance (adherence referring to ICS + long-acting beta-2-agonists [LABAs]) was calculated based on the medication possession ratio (MPR)Citation19, which was assessed from first to last prescription and represented the number of days of medication given divided by the number of days of treatment (from the index date). The duration of treatment was defined as the time (in months) without discontinuation of the initial treatment or without switching to another medication for at least 30 days after the initial prescription.
Resource utilization and associated costs
Direct health care asthma costs (direct costs) were regarded as those related to health care activity (medical visits, hospital days, emergencies, diagnostic or therapeutic requests, and medication), while indirect or non-health care costs were those related to lost work productivity (days off work). Costs were expressed as the mean cost per patient (annual cost). The different study concepts and their economic assessment are detailed in (corresponding to the year 2016). Prescriptions were quantified according to the price per package (public retail price + value added tax [VAT]) at the time of prescription. The indirect cost calculations only considered the days off work, which were determined based on the interprofessional minimum wage (source: Spanish National Statistics Institute [INE])Citation20. The study did not include the calculation of direct non‒health care costs (i.e. “out-of-pocket costs” or costs paid by the patient/family), since these data were not recorded in the database, and the study design precluded direct access to the patient. Patient resource use and costs referred only to asthma.
Table 1. Description of the unit costs and work productivity losses (year 2016).
Confidentiality of the information
The confidentiality of the data (anonymized and dissociated) was respected in accordance with Spanish legislation on personal data protection (Act 15/1999, of 13 December). The study was classified by the Spanish Agency for Medicinal Products and Medical Devices (non-post-marketing study [NO-EPA]) and was subsequently approved by the Clinical Research Ethics Committee of the Unión Catalano-Balears de Hospitales de Cataluña (Barcelona).
Statistical analysis
Data validation was performed to ensure the quality of the results. A univariate descriptive statistical analysis was made for the variables of interest. Absolute and relative frequencies were recorded for qualitative data. Proportions and 95% confidence intervals (CIs) for parameters of interest were based on the total number of subjects with no missing data. The mean, standard deviation (SD), median and 25th and 75th percentiles (interquartile range [IQR]) were recorded for quantitative data. Normal data distribution was checked using the Kolmogorov–Smirnov test. Analysis of variance (ANOVA), the chi-squared test and Pearson’s linear correlation test were used for the bivariate analysis. Kaplan–Meier survival analysis (comparison: log-rank test) was performed to assess the time from diagnosis. Multiple linear regression analysis was used to identify the variables associated with healthcare costs and the number of exacerbations (dependent variable; stepwise procedure). Cost comparisons were made according to Thompson and BarberCitation21, based on the analysis of covariance (ANCOVA; generalized linear model [GLM]), with gender, age, RUB, MPR, Charlson index and time from diagnosis as covariates (procedure: estimation of marginal means; Bonferroni correction). The SPSSWIN version 18 statistical package was used, with statistical significance set at p < .05. We also conducted a sensitivity analysis, considering a cut-off blood eosinophil count of ≥400 cells/µl. This cut-off point (high eosinophil counts according to laboratory reference values) was used to quantify the main (exacerbations and total costs) and interim outcome variables.
Results
A total of 75,259 patients out of an initial population of 90,348 people aged ≥18 years were attended during 2014–2015. Of these, 4755 were diagnosed with asthma (prevalence: 5.3%; 95%CI: 3.7–6.9%). In patients with asthma, the prevalence of severe asthma was 6.3% (N = 301; 95%CI: 4.3–8.2%). A total of 268 patients meeting all the inclusion and exclusion criteria, with data available during the study period, were finally analyzed ().
Figure 1. Study flow chart. A retrospective observational design was adopted based on review of medical records (electronic databases containing anonymized and dissociated data) of patients with severe asthma. CI, confidence interval.
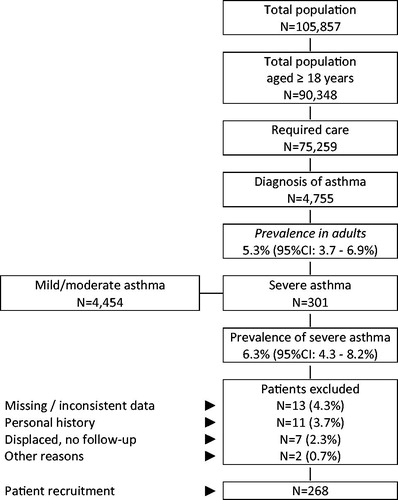
The baseline characteristics according to study group (eosinophilia) are shown in . The mean age was 56.1 years (63.4% female), the RUB was 3.1 points, and the mean Charlson index score was 0.7 points per patient. Allergic rhinitis (42.9%), dyslipidemia (39.6%), hypertension (33.2%), obesity (26.1%), depressive syndrome (25.0%) and diabetes (15.7%) were the most frequent comorbidities. In 58.6% of the patients (N = 157), the blood eosinophilia value was ≥300 cells/µl, while 41.4% (N = 111) had blood eosinophilia values of <300 cells/µl. Six percent of the patients died during the study period; deaths were numerically higher in the subgroup of patients with ≥300 cells/µl; a mortality rate two times higher than that in patients with ≤300 cells/µl. Patients with eosinophilia were older and had more comorbidities.
Table 2. Baseline characteristics of the patients according to study group.
The anthropometric and clinical variables according to study group are detailed in . Patients with ≥300 cells/µl and patients with ≤300 cells/µl presented a similar median time from the diagnosis of asthma (32.2 vs. 31.1 years; p = .074), lower BMI (27.9 vs. 28.2 kg/m2; p = .008) and FEV1 (54.3 vs. 60.7%; p < .001), and higher immunoglobulin E levels (367.9 vs. 247.2 IU/ml; p = .001).
Table 3. Anthropometric and clinical variables according to study groups.
describes the medication administered, adherence to therapy (MPR) and exacerbations during the follow up. All patients in the study were being treated with inhaled corticosteroids (ICs), long-acting beta-2 agonists (LABAs) and short-acting beta-2 agonists (SABAs) as rescue therapy. A total of 57.5% used oral corticosteroids at some point during follow up (30.2% on a chronic or regular basis (defined as at least 6 months of continuous use), 42.9% received leukotriene antagonists and five patients were receiving omalizumab, all in the ≥300 eosinophils/µl group.
Table 4. Medication administered, adherence to therapy and exacerbations during the follow-up period.
Patients with elevated eosinophil counts (≥300 cells/µl) consumed more oral corticosteroids (65.7%), systemic antibiotics (66.2%) and leukotriene antagonists (48.4%). These patients also presented lower adherence to therapy (MPR: 65.6% vs. 77.3%; p < .001); more exacerbations (3.3 vs. 1.9; p < .001) and had more severe exacerbations (hospital admission) 0.7 vs 0.4 p = .001.
In patients with severe asthma (multiple linear regression model) exacerbations were associated with FEV1 (β= −.606), eosinophil count (β = .255), immunoglobulin E level (β = .152) and age (β = .128) (p < .001). The coefficient of determination (R2) of the model was 79.8%.
During the 12-month follow up, patients with eosinophilia used more healthcare resources, particularly with respect to the number of primary care visits (17.5 vs. 10.8; p = .001), days of hospital admission (3.9 vs. 2.1; p < .001) and hospital emergency visits (2.8 vs. 2.0; p < .001) (). The differences in days off work (indirect costs) were inconclusive (21.5 vs. 11.3; p = .193). The total cost of the patients included in the study was 1.5 million euros (), of which 68.2% corresponded to direct healthcare costs and 31.8% to indirect costs – with a mean total annual unit cost of 5493 euros; 37.2% of the total costs occurred in primary care and 31.1% in specialized care. The greatest cost components were lost productivity (31.8%), hospital admissions (18.2%) and medications (16.2%).
Table 5. Resource utilization and associated costs (in euros, 2016 costs).
The mean total annual unit cost of patients with eosinophilia corrected for covariates (gender, age, comorbidity, MPR and time from diagnosis; ANCOVA) was 6222 versus 4152 euros in patients without eosinophilia (p = .016). These differences were maintained for healthcare costs (4226 vs. 2979 euros; p < .001). There were no significant differences in lost work productivity (1996 vs. 1173 euros; p = .318). The mean duration of stay in hospitalized patients was 5.5 (SD: 3.5) days. The total cost in patients diagnosed with asthma in adulthood (last 15 years; late-onset asthma) was comparatively higher (7873 euros; individual groups: 9702 vs. 4336 euros; p < .001).
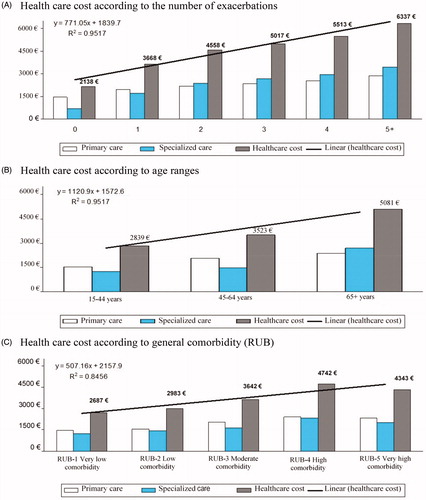
In the binary correlation model, exacerbation correlated moderately with FEV1 (r= −.737), blood eosinophil count (r = .4821) and systemic corticosteroid use (r = .533), while healthcare costs were associated with FEV1 (r= −.440), exacerbations (r = .438), age (r = .358), eosinophil count (r = .314) and comorbidity (RUB: r = .256) (p < .01 in all comparisons). In the multiple linear regression model (stepwise approach), healthcare costs correlated with age (β = .323, t = 4.4), FEV1 (β= −.239, t= −3.2), eosinophil count (β = .177, t = 3.1), and number of exacerbations (β = .158, t = 2.2), (p < .01 in all comparisons). shows the healthcare costs (total and divided into primary care and specialized care) according to the number of exacerbations, patient age ranges, and general comorbidity during the follow up. From the second exacerbation onwards, the cost of specialized care (hospital admissions) exceeded that of primary care.
Figure 2. Health care costs (total; primary care and specialized; in euros) according to number of exacerbations, patient age ranges and general comorbidity during the follow up.
The results of the sensitivity analysis with respect to comorbidity, adherence, exacerbations and total costs with a cut-off point of ≥400 cells/µl showed no significant differences with those obtained with a cut off of ≥300 cells/µl ().
Table 6. Sensitivity analysis according to blood eosinophil counts. Study endpoints.
Discussion
The results of the study show the costs of patients with severe asthma were. Age, eosinophilia, exacerbations and worse lung function were associated with increased use of healthcare resources and costs, and the increases were higher in patients with blood eosinophil counts of ≥300 cells/µl. Available reports state, systematically, that a cut-off point of 300 eosinophils/µL in blood is a good predictor of an increased risk of exacerbations and worse control. This cut off also correlates with lung eosinophilic inflammation to a greater degree than other biomarkers like FeNO or periostin. Current guidelines recommend this cut off as a criterion for the use of new monoclonal antibodies with a mechanism of action of anti IL5 or anti IL5RCitation2. We found few real-life observational studies, and this made comparison of the results difficult. Furthermore, the lack of standardization of the methodologies used means our results should be interpreted with caution, as should the external validity of the findings.
Severe asthma is characterized by the need for multiple drugs and high treatment doses (GEMA steps 5–6)Citation2 and comprises both controlled and uncontrolled patients. Uncontrolled severe asthma is defined as patients receiving high-dose ICS/LABA combination therapy with ≥2 exacerbations in the previous yearCitation3. Two-thirds of the patients in our study met this definition, i.e. most patients with severe asthma can be regarded as having poor control, particularly those with blood eosinophilia ()Citation10. The prevalence of severe asthma was 6.3% in our study. In comparison, in a study conducted in the specialized setting in Spain, the prevalence of poorly controlled severe asthma (according to medical criteria) was 3.9% of all asthmatic subjectsCitation7. The data available on the epidemiology of severe asthma are heterogeneous, particularly in adults, due to the lack of a uniform definition in the literatureCitation6,Citation7. It is generally accepted that severe asthma affects 5-10% of the general asthmatic populationCitation1–3,Citation6. Our data appear to be consistent with those found in the available literatureCitation1–3,Citation6,Citation7,Citation22,Citation23.
Exacerbation was associated with poorer lung function (FEV1), eosinophil count and age. In recent years, various severe asthma phenotypes have been identified, although asthma being seen to constitute a poorly categorized syndrome. This in turn makes the selection of correct treatment difficultCitation2,Citation8–10. At least four severe asthma clinical phenotypes have been identified to date: (a) severe allergic asthma developing in childhood (generally characterized by eosinophilia which appears to be the result of progression of the disease); (b) severe eosinophilic asthma of late onset (manifesting after 20 years of age and associated with rhinitis and nasal polyposis); (c) severe asthma associated with obesity (generally found in women and in adults); and (d) neutrophilic asthma developing in adulthood (with a natural history that has not been well established to date)Citation3,Citation24,Citation25. Systemic corticosteroids were used by 57% of patients in the study observation period but it seems this was not related to baseline blood eosinophils because the majority were in the high-eosinophil group. In patients using chronic oral corticosteroids use, the distribution between patients with low and high eosinophil counts was similar (p .294). It is known that systemic corticosteroid use may mask eosinophilia but, in this study, it seemed not to have a large impact on blood eosinophil levels in patients with uncontrolled severe asthma.
These phenotypes were not directly identified in our study; however, indirectly and with due caution, they may be regarded as being consistent with the data found in the literatureCitation1–3,Citation6. Eosinophilia was present in 58.6% of severe asthma patients, and obesity in 26.1%. The mean time from diagnosis was 33.3 years, and rhinitis and nasal polyposis were present in 42.9% and 13.1% of the cases, respectively and were higher in patients with eosinophilia (). These results are is in line with the Spanish data results of the ENEAS studyCitation26, which included diagnosed uncontrolled severe asthma patients. The analysis found 58.1% of cases of severe eosinophilic asthma of late onset phenotype, defined as disease onset after the age of 12 and blood eosinophils ≥300 cells/µl and 25.7% of obesity associate phenotype.
Severe asthma is associated with high resource consumption and implies an important economic burden for healthcare systems, patients and their familiesCitation2,Citation4. In our study, the mean total annual cost was 5493 euros (healthcare costs: 68.2%; productivity losses: 31.8%). Patients with high eosinophil counts (≥300 cells/µl) had a higher cost (€6222 vs. €4152). The healthcare costs were associated with age, lower FEV1, eosinophilia, and the number of exacerbations. A Canadian reviewCitation12 found asthma was associated with high resource use including hospital admissions, emergencies, medical visits and drug treatments. The economic burden of the disease was high, with a total annual population-level range of 46 million dollars in British Columbia to 141 million in Ontario. The authors emphasized that the indirect costs due to work productivity losses further increase the burden of disease. Other authors have analyzed the cost of severe asthma from the perspective of the Brazilian public health systemCitation27, describing a mean annual cost due to hospital admissions of 764–929 USD. The authors concluded that hospitalizations and drug treatments are the most important costs and underlined that further representative severe asthma cost studies in Brazilian patients are needed. In Europe, a real-life studyCitation28 including data from Spain found the cost per patient with uncontrolled severe asthma to be 2281 euros, with a greater burden in terms of hospitalization costs and indirect costs than in patients with good disease control. Lack of control was the determining factor in total asthma costs, which were higher in uncontrolled patients. A review conducted by GadenneCitation13 emphasized the high methodological variability of the studies analyzed and concluded that, although the data found in the literature are consistent in showing improved disease control to reduce the cost of the disease, there is a growing need for economic studies based on the latest definitions of severe asthma. Another recent study in the United States has shown patients with severe asthma to require more hospital admissions, drugs and medical visitsCitation29. The mean annual asthma-related cost was 5174 USD (in 2013), which was three times higher than the cost in patients with mild to moderate asthma. In addition, patients with two or more exacerbations had a high blood eosinophil count.
In Spain, the AsmaCost studyCitation11 reported the annual cost of an asthmatic patient to be €1726, with an increase in patients over 65 years of age (€3068) and in cases of severe asthma (€2635). The study was performed before the market entry of biological treatments which have increased the cost in the last years; in a recently published study which evaluated the impact of severe asthma in adult Spanish patients, the mean annual cost per severe asthma patient was €8554 from the social perspective, of which €7472 was direct healthcare costsCitation30. Our results are more in line with this study.
Our study has some limitations. These include the categorization of the disease (severe asthma) and possible bias in patient classification and the operational cost measures attributable to the information system used. The study therefore has the limitations inherent to retrospective designs such as, for example, the under-recording of data (exacerbations, clinical course, etc.). In this regard, the possible inaccuracy of diagnostic coding for severe asthma and other comorbidities, and in the definition of exacerbation, as well as the use of resources outside the study sites or the absence of some variables capable of influencing the final outcomes (patient socioeconomic level, environmental/occupational exposure, evolution of the prescribed drug doses, assessment of the inhalation technique or therapy, and/or the distinction of phenotypes) must also be regarded as limitations, as must the external validity of the results (population representativeness) and the evaluation of indirect costs (due only to lost work productivity; we did not estimate the influence of unemployment or retirement on lost production).
In conclusion, blood eosinophil counts were associated with exacerbations. The costs related to patients with severe asthma were high. Age, eosinophilia, exacerbations and the severity of asthma were associated with an increased use of healthcare resources and greater costs for the health system.
Transparency
Declaration of funding
This study was sponsored by AstraZeneca.
Declaration of financial/other relationships
AS received specific funding for the development of this work. JN, GR, MC and MO are employed by AstraZeneca Pharmaceutical Spain S.A. RN declares no conflicts of interest. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the JME policies on sharing data and materials, as detailed online in the guide for authors. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
References
- Olaguibel JM, Quirce S, Juliá B, et al.; on behalf of the MAGIC Study Group. Measurement of asthma control according to Global Initiative for Asthma guidelines: a comparison with the Asthma Control Questionnaire. Respir Res. 2012;13:50.
- Guía española para el manejo del asma (GEMA 4.2). Versión 2017 [cited 2018 Jan]. Available from: https://www.gemasma.com
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373.
- Aumann I, Prenzler A, Welte T, et al. Epidemiology and costs of asthma in Germany - a systematic literature review. Pneumologie. 2014;68:557–567.
- Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902.
- Cisneros Serrano C, Melero Moreno C, Almonacid Sánchez C, et al. Guidelines for severe uncontrolled asthma. Arch Bronconeumol. 2015;51:235–246.
- Quirce S, Plaza V, Picado C, et al. Prevalence of uncontrolled severe persistent asthma in pneumology and allergy hospital units in Spain. J Investig Allergol Clin Immunol. 2011;21:466–471.
- Walsh CJ, Zaihra T, Benedetti A, et al. Exacerbation risk in severe asthma is stratified by inflammatory phenotype using longitudinal measures of sputum eosinophils. Clin Exp Allergy. 2016;46:1291–1302.
- Zeiger RS, Schatz M, Li Q, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2:741–750.
- Chiappori A, De Ferrari L, Folli C, et al. Biomarkers and severe asthma: a critical appraisal. Clin Mol Allergy. 2015;13:20.
- Martínez-Moragón E, Serra-Batllés J, de Diego A, et al. Coste económico del paciente asmático en España (estudio AsmaCost). Arch Bronconeumol. 2009;45:481–486.
- Ismaila AS, Sayani AP, Marin M, et al. Clinical, economic, and humanistic burden of asthma in Canada: a systematic review. BMC Pulm Med. 2013;13:70.
- Gadenne S, Pribil C, Chouaid C, et al. The costs of asthma in France and the economic implications of its level of control. Rev Mal Respir. 2011;28:419–426.
- Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, et al. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20:11–23.
- Lamberts H, Wood M, Hofmans-Okkes ÍM, editors. The International Classification of Primary Care in the European Community. With a multi-language layer. Oxford: Oxford University Press; 1993.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Sicras-Mainar A, Navarro-Artieda R. Adjusted clinicals groups: a patient classification system through risk adjustment. Rev Peru Med Exp Salud Publica. 2013;30:308–314.
- The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD): World Health Organization [cited 2017 Jul 10]. Available from: http://www.who.int/classifications/atcddd/en/
- Benner JS, Glynn RJ, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461.
- Instituto Nacional de Estadística (INE). 2014. Encuesta de costes laborales del año 2014 [cited 2017 Apr]. Available from: http://www.ine.es/infoine
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320:1197–1200.
- Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: clinical impact and management. Respirology. 2017;22:651–661.
- Von Bülow A, Kriegbaum M, Backer V, et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2:759–767.
- Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323.
- Campo P, Rodríguez F, Sánchez-García S, et al. SEAIC Asthma Committee. Severe asthma workgroup; SEAIC asthma committee. Phenotypes and endotypes of uncontrolled severe asthma: new treatments. J Investig Allergol Clin Immunol. 2013;23:76–88.
- Pérez de Llano L, Martínez-Moragón E, Plaza Moral V, et al. Unmet therapeutic goals and potential treatable traits in a population of patients with severe uncontrolled asthma in Spain. ENEAS study. Respir Med. 2019;151:49–54.
- Stirbulov R, Lopes da Silva N, Maia SC, et al. Cost of severe asthma in Brazil-systematic review. J Asthma. 2016;53:1063–1070.
- Accordini S, Corsico AG, Braggion M, et al. Therapy and Health Economics Working Group of the European Community Respiratory Health Survey II. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160:93–101.
- Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. JMCP. 2016;22:848–861.
- Melero Moreno C, Quirce S, Huerta A, et al. Economic impact of severe asthma in Spain: multicentre observational longitudinal study. J Asthma. 2018;9:1–11.
